Introduction
Glioma is one of the most common nervous system
malignancies. According to the Central Brain Tumor Registry of the
United States, 70% of primary malignant brain tumors are gliomas,
for which the annual incidence rate is ~5/100,000, with >14,000
new cases each year (1). Although
the diagnosis and treatment of glioma has progressed, the overall
prognosis of glioma patients remains poor, and the five-year
survival rate is ~25% (2). The
difficulties in treating malignant glioma is partly due to its
malignant biological properties, such as its over-proliferation and
high invasiveness (3). The
occurrence and development of tumors is the integral result of
multiple genes, factors, steps and evolutionary stages. The
activation of oncogenes and inactivation or mutation of tumor
suppressor genes is associated with tumorigenesis (1). As the underlying mechanism of glioma
remains unclear, it is important to investigate the development and
invasive behavior of glioma on the genetic level. An increasing
number of studies are now aiming to identify the key gene targets
of glioma, and to find a mechanism by which to reverse their
malignant behavior (4).
The a disintegrin and metalloproteinase-17 (ADAM)
superfamily consists of a group of transmembrane multi-domain
secretary proteins, which release important ligands, such as tumor
necrosis factor α (TNF-α) and epidermal growth factor (EGF),
thereby promoting the formation and progression of tumors (5,6).
ADAM17, a member of the ADAM family, is also known as tumor
necrosis factor-α converting enzyme (TACE) (7). The primary function of ADAM17 is to
hydrolyze and release protein precursor molecules on the cell
surface, resulting in lateral activation of cell surface molecules
in cell signaling pathways, thereby altering signal transduction
(8). Recent studies have shown that
ADAM17 is highly expressed in non-small cell lung cancer, breast
cancer and other malignant tumors, and is associated with the
degree of malignancy (9–11). Additionally, ADAM17 has been shown
to function as an oncogene, promoting U87 glioblastoma stem cell
migration and invasion (12).
However, whether ADAM17 is highly expressed in glioma is not known.
The present study analyzed ADAM17 expression in normal and glioma
brain tissue, and investigated the association between the ADAM17
expression level and the malignancy and prognosis observed in
glioma patients.
In order to study the role of ADAM17 expression in
glioma, the expression of the protein was analyzed in 60 patients
with glioma and in eight control cases (patients with traumatic
brain injury) by western blotting and immunohistochemistry (IHC).
The association between ADAM17, the malignancy of the glioma and
the clinicopathological factors were determined. The association
between ADAM17 and prognosis was also analyzed using the
Kaplan-Meier method.
Materials and methods
Tumor specimens
A total of 60 glioma patients who were treated in
the Second Affiliated Hospital of Nantong University (Nantong,
China) between 2006 and 2013 were included in the present study.
The cohort consisted of 36 males and 24 females, with a mean age of
45 years (5–85 years). In 18 cases, the tumor was located in the
frontal lobe, in 23 cases it was located in the temporal lobe and
in 19 cases it was located in other regions. The tumor diameter was
measured during surgery. All patients underwent a subtotal tumor
removal without pre-operative radiotherapy, and then underwent
post-operative radiotherapy (60 Gy for one month with single doses
of 1.8–2.0 Gy) and chemotherapy with Semustine (200–225
mg/m2 every six weeks for six months). Patients that
succumbed to other diseases or accidents were excluded from the
study. All patients underwent a pre-operative functional status
assessment according to the Karnofsky score (KPS) for accurate
scoring. A total of 33 cases presented with a KPS score of <80,
while 27 cases presented with a score of >80, indicating their
inability to perform normal activities. According to the 2007 World
Health Organization (WHO) classification of tumors of the central
nervous system (13), all gliomas
were subjected to histological grading as follows: 25 cases formed
the low-grade malignant carcinoma group (grades I and II) and 35
cases formed the high-grade malignant carcinoma group (grades III
and IV). All nine grade I tumors were pilocytic astrocytoma. The 16
grade II cases were divided into 12 cases of diffuse astrocytoma,
three cases of oligodendrocyte cell tumors and another one case of
ependymoma. A total of 19 grade III tumors were anaplastic
astrocytomas, and the 16 grade IV tumors were glioblastoma
multiforme tumors. The surgically resected brain tissue of eight
trauma patients admitted to the hospital within the same period was
used as a control group; all cases were pathologically confirmed to
be without gliosis.
All specimens were divided into two parts; one part
was fixed with 10% neutral formalin and embedded, from which
paraffin sections were cut to a thickness of 5 μm, while the other
part was immediately frozen at −80°C. Informed consent for use of
the specimens was provided by the family members of the patients or
the patients themselves. All experimental procedures were approved
by the Hospital Ethics Committee of the Second Affiliated Hospital
of Nantong University (Nantong, China).
Reagents
The ADAM17 monoclonal mouse anti-human antibody was
provided by Abcam (Cambridge, UK), and the horseradish
peroxidase-conjugated donkey anti-mouse immunoglobulin G (IgG, H+L)
and Polymerization Peroxidase-labeled Goat anti-Mouse IgG Western
Blot kit were purchased from the Beyotime Institute of
Biotechnology (Jiangsu, China). The anti-β-actin monoclonal
antibody and Biotinylated Rat Anti-mouse IgG IHC kit was obtained
from Boster Biological Technology, Ltd. (Wuhan, China), and TRIzol
was obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
The reverse transcription and quantitative polymerase chain
reaction (PCR) kits were obtained from Fermentas (Waltham, MA,
USA).
Methods
Western blotting
Tissue lysates were homogenized and centrifuged at
13,000 × g for 15 min at 4°C. The supernatants were collected, and
the protein concentrations were determined using a Bicinchoninic
Acid Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). An equal amount (10 μg) of each protein sample was loaded
onto 10% SDS-PAGE gels, transferred to pure nitrocellulose
membranes (PerkinElmer Life Sciences, Boston, MA, USA), and blocked
with 5% skimmed milk in Tris-buffered saline Tween-20 solution. The
membranes were split and incubated with rabbit polyclonal
anti-human ADAM17 primary antibody (1:1,000) at 4°C overnight. The
membranes were subsequently incubated with anti-rabbit secondary
antibodies (1:4,000) at room temperature for 1 h. Enhanced
chemiluminescence (ECL) was performed using an ECL Western Blotting
Detection kit (Pierce Biotechnology, Inc.). The western blotting
band density was analyzed using Quantity One software (Bio-Rad,
Hercules, CA, USA), and then normalized to the values obtained for
β-actin.
IHC
Paraffin sections were dipped twice into xylene for
10 min to remove the paraffin. The xylene was then removed with a
graded alcohol series (100, 95 and 70%). The sections was rinsed
with deionized water for 5 min followed by treatment with 3%
H2O2 for inactivation of endogenous
peroxidases. Next, the sections were subjected to heat-induced
antigen retrieval with 0.01 mm/l phosphate-buffered saline (PBS),
and stained using the avidin-biotin-peroxidase complex method with
diaminobenzidine as the substrate. The sections were then subjected
to hematoxylin and eosin counter staining. Microscopic observations
were performed at ×400 magnification in five fields, from which a
total of 500 tumor cells were counted. PBS was used, instead of
primary antibody, as a negative control. Positive expression was
observed as cytoplasmic yellow-, brown- or tan-colored staining. A
semi-quantitative analysis was conducted for the staining
intensity: No positive cells or <15% positive cells was defined
as negative (−); 15 to 25% positive cells, where a pale yellow
color was observed, were defined as weakly positive (+); 25 to 50%
positive cells, where a brown-yellow color was observed, were
defined as moderately positive (++); and >50% positive cells,
with a dark brown, yellow or brown color, were defined as strongly
positive (+++).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Differences in the IHC results were assessed using a
χ2 test and a rank correlation analysis. Western
blotting results were assessed using a one-way analysis of
variance, a post-hoc analysis and a rank correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
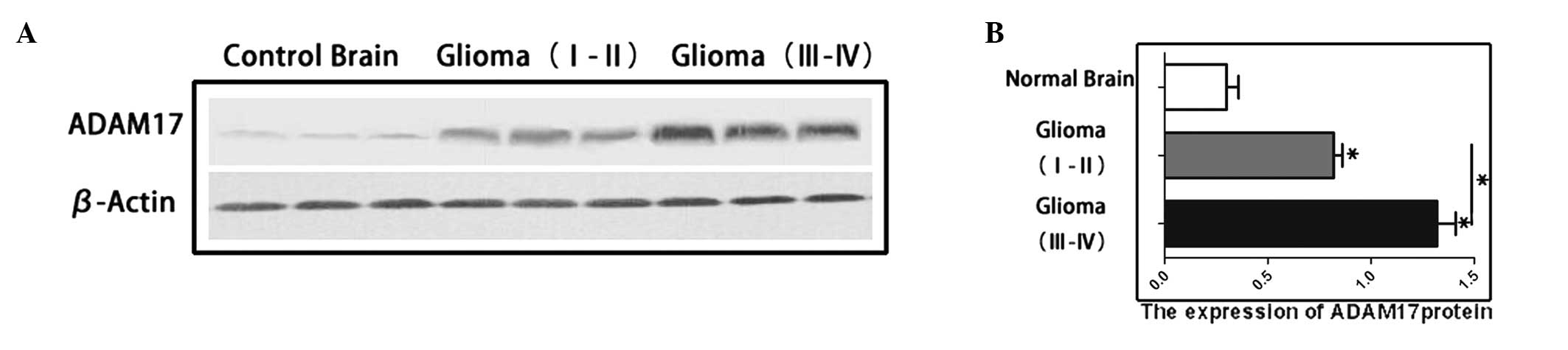
Western blot analysis of ADAM17 protein
expression in glioma tissues of different grades
Western blotting showed that the expression of
ADAM17 in the high-grade (WHO III–IV; 1.292±0.140) and low-grade
(WHO I–II; 0.823±0.101) glioma groups were significantly higher
compared with the control brain tissue (0.325±0.068). The
expression level of ADAM17 in the high- and low-grade gliomas was
significantly higher compared with that of the control brain tissue
(P<0.05) (Fig. 1).
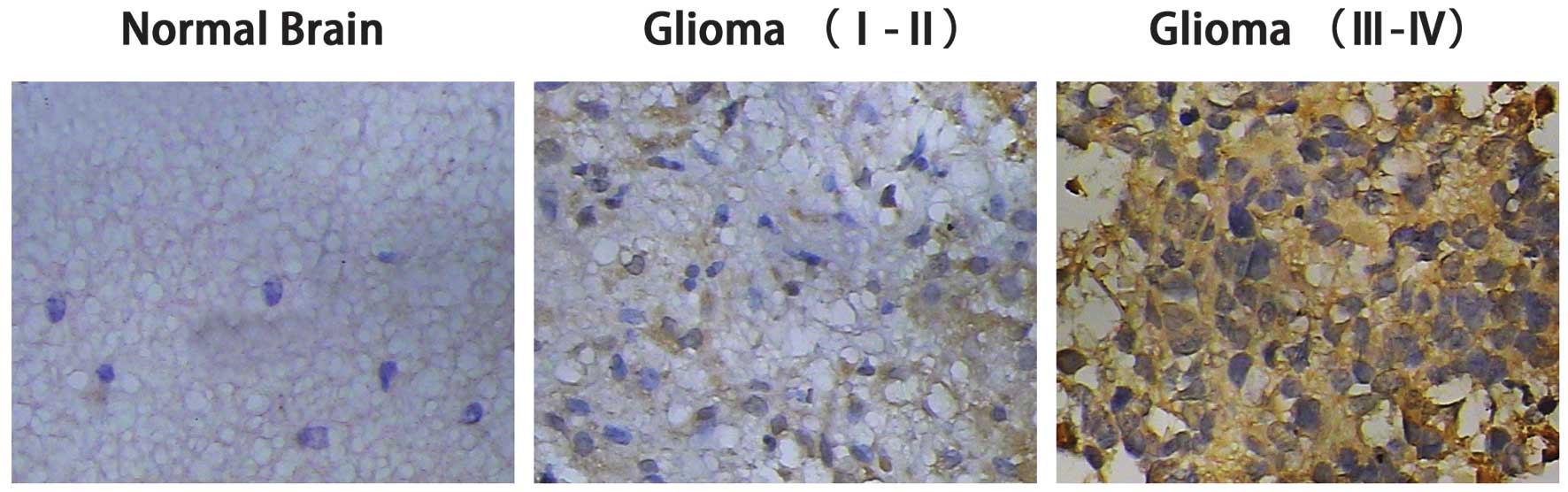
IHC staining of ADAM17 in glioma tissues
of different grades
The ADAM17 protein expression in the eight control
and 60 glioma cases was detected by IHC staining. The results
showed that ADAM17 was predominantly located in the cytoplasm and
was rarely expressed in the nucleus (Fig. 2). The positive rate of ADAM17
expression in the glioma patients of all grades was 88.33%, whereas
it was only 37.5% in the control group. The strong positive
expression rate of ADAM17 in the control, low-grade glioma and
high-grade glioma groups was 0, 0.4 and 51.43%, respectively. The
negative expression rate of ADAM17 in the control group was 62.5%,
whereas it was only 0.29% in the high-grade glioma group (Table I). These results indicated that
ADAM17 expression was significantly higher in the high grade glioma
group (WHO III–IV) compared with the low-grade glioma (WHO I–II)
and control groups.
 | Table IExpression of ADAM17 in glioma tissue
and control brain tissue cases. |
Table I
Expression of ADAM17 in glioma tissue
and control brain tissue cases.
| Group | Negative | Weakly-positive |
Moderately-positive |
Strongly-positive | Total |
|---|
| Control, n | 5 | 2 | 1 | 0 | 8 |
| Glioma (I–II), n | 2 | 9 | 13 | 1 | 25 |
| Glioma (III–IV),
n | 1 | 3 | 13 | 18 | 35 |
Correlation analysis between ADAM17
expression and glioma clinicopathological factors and
prognosis
To further determine the association between ADAM17
expression and the clinical prognosis of patients with glioma, the
clinical data of 60 glioma patients were analyzed (Table II). The glioma patients were
divided into two subgroups, a high expression group (>45%
positive cells) and a low expression group (<45% positive
cells), according to the IHC ADAM17 expression data. The results
showed that ADAM17 expression was not correlated with gender, age,
tumor size, location or necrosis. However, ADAM17 expression was
significantly associated with the glioma WHO histological grade
(P<0.05) (Table II). The
prognosis was significantly different in the patients with high
ADAM17 expression compared with the patients with low ADAM17
expression. Survival analysis showed that the patients with low
ADAM17 expression longer survival times compared with the patients
with high ADAM17 expression (P<0.005) (Fig. 3).
 | Table IIAssociation between ADAM17 expression
and clinicopathological characteristics of gliomas. |
Table II
Association between ADAM17 expression
and clinicopathological characteristics of gliomas.
| | ADAM17
expression | |
|---|
| |
| |
|---|
| Clinicopathological
features | Total, n | Low expression,
n | High expression,
n | P-value |
|---|
| Gender |
| Male | 36 | 17 | 19 | 0.653 |
| Female | 24 | 11 | 13 | |
| Age |
| <45 | 28 | 13 | 15 | 0.589 |
| >45 | 32 | 15 | 17 | |
| Tumor size, cm |
| <4 | 24 | 9 | 15 | 0.185 |
| >4 | 36 | 19 | 17 | |
| Tumor location | | | | 0.789 |
| Frontal | 18 | 8 | 10 | |
| Temporal | 23 | 12 | 11 | |
| Other | 19 | 8 | 11 | |
| Necrosis |
| Absence | 31 | 12 | 19 | 0.154 |
| Presence | 29 | 16 | 13 | |
| KPS |
| <80 | 33 | 14 | 19 | 0.320 |
| >80 | 27 | 14 | 13 | |
| WHO grade |
| I–II | 25 | 18 | 7 | <0.005 |
| III–IV | 35 | 10 | 25 | |
Discussion
Glioma is the most common intracranial malignancy
(14). Despite the rapid
development of treatment options for glioma in recent years,
including minimally invasive neurosurgical techniques, precise
positioning radiotherapy technology and chemotherapy drugs that
target all aspects of the tumor growth cycle, the high recurrence
and low curative rates caused by the invasion of glioma remain
problematic (2). The development of
molecular biology techniques and an improved understanding of tumor
pathogenesis has allowed the use of targeted therapy in the
comprehensive treatment of glioma. Molecular targets have included
receptors, key genes and regulatory molecules (1), and molecular-targeted drugs have
become novel drug treatments for malignant glioma.
ADAMs are a class of membrane-anchored cell surface
proteins, which function in proteolysis, the release of bioactive
cytokines, cell adhesion, integration, migration and signal
transduction (15). ADAM17, a
member of the ADAM gene family, has been shown to be highly
expressed in various human tumors, reflecting the degree of
malignancy, since ADAM17 promotes tumor invasion and metastasis
(16). In a study of ADAM17
expression in breast cancer, McGowan et al (17) found that the mRNA and protein levels
of ADAM17 in breast cancer tissue were positively correlated with
the number of lymph node metastases, suggesting that ADAM17 is
closely associated with the progression of breast cancer. ADAM17
has been shown to shed ligands of EGFR, such as amphiregulin and
TNFα, and subsequently activate EGFR, thereby improving the
proliferation and migration of lung cancer cells (8). Kornfeld et al (18) reported that ADAM17 could activate
EGFR by releasing amphiregulin, thereby enhancing the proliferation
and invasion of head and neck squamous carcinoma cells. EGFR
activation is a key step in the tumor growth of a variety of
carcinomas, and is associated with the malignancy of astrocytoma
(19). EGFR has functions in the
proliferation, migration, invasion and DNA damage repair processes
of glioma cells, and aberrant signal transduction pathways are able
to promote the growth, migration, angiogenesis and apoptosis of the
tumor cells (20). Additionally,
ADAM17 has been shown to function as an oncogene, promoting U87
glioblastoma stem cell migration and invasion (12).
Overall, the present study hypothesized that ADAM17
is highly expressed in glioma, and that its expression is
correlated with malignant glioma. By using western blotting and
IHC, 60 cases of glioma specimens were compared with 10 cases of
control brain tissues to identify the following: i) In the control
group, the expression of ADAM17 protein expression was
non-detectable or low, whereas it was highly expressed in the
glioma groups; the protein expression of ADAM17 increased with an
increase in glioma malignancy. ii) ADAM17 expression was
significantly associated with malignant gliomas. iii) Survival
analysis confirmed that ADAM17 expression was correlated with
patient survival time, such that patients with a high expression of
ADAM17 had a worse prognosis. ADAM17 may be used as an indicator of
glioma prognosis. Given that ADAM17 enhances cell invasion and the
degree of malignancy by activating EGFR in a variety of tumor
cells, we propose that ADAM17 may have the same role as an oncogene
in glioma, however, the underlying mechanism remains to be
investigated.
References
|
1
|
Mercer RW, Tyler MA, Ulasov IV and Lesniak
MS: Targeted therapies for malignant glioma: progress and
potential. Biodrugs. 23:25–35. 2009.
|
|
2
|
Demuth T and Berens ME: Molecular
mechanisms of glioma cell migration and invasion. J Neurooncol.
70:217–228. 2004.
|
|
3
|
Warren KE: Diffuse intrinsic pontine
glioma: poised for progress. Front Oncol. 2:2052012.
|
|
4
|
Ortega-Aznar A, Jimenez-Leon P, Martinez E
and Romero-Vidal FJ: Clinico-pathological and molecular aspects of
diagnostic and prognostic value in gliomas. Rev Neurol. 56:161–170.
2013.(In Spanish).
|
|
5
|
Carl-McGrath S, Lendeckel U, Ebert M,
Roessner A and Rocken C: The disintegrin-metalloproteinases ADAM9,
ADAM12, and ADAM15 are upregulated in gastric cancer. Int J Oncol.
26:17–24. 2005.
|
|
6
|
Kanda K, Komekado H, Sawabu T, et al:
Nardilysin and ADAM proteases promote gastric cancer cell growth by
activating intrinsic cytokine signalling via enhanced ectodomain
shedding of TNF-α. EMBO Mol Med. 4:396–411. 2012.
|
|
7
|
Meng P, Yoshida H, Matsumiya T, et al:
Carnosic acid suppresses the production of amyloid-β 1–42 by
inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human
neuroblastoma cells. Neurosci Res. 75:94–102. 2013.
|
|
8
|
Borrell-Pagès M, Rojo F, Albanell J,
Baselga J and Arribas J: TACE is required for the activation of the
EGFR by TGF-alpha in tumors. EMBO J. 22:1114–1124. 2003.
|
|
9
|
Gao MQ, Kim BG, Kang S, et al: Human
breast cancer-associated fibroblasts enhance cancer cell
proliferation through increased TGF-α cleavage by ADAM17. Cancer
Lett. 336:240–246. 2013.
|
|
10
|
Ni SS, Zhang J, Zhao WL, Dong XC and Wang
JL: ADAM17 is overexpressed in non-small cell lung cancer and its
expression correlates with poor patient survival. Tumour Biol.
34:1813–1818. 2013.
|
|
11
|
Shou ZX, Jin X and Zhao ZS: Upregulated
expression of ADAM17 is a prognostic marker for patients with
gastric cancer. Ann Surg. 256:1014–1022. 2012.
|
|
12
|
Chen X, Chen L, Chen J, et al: ADAM17
promotes U87 glioblastoma stem cell migration and invasion. Brain
Res. 1538:151–158. 2013.
|
|
13
|
Alexandru D, Haghighi B and Muhonen MG:
The treatment of angiocentric glioma: case report and literature
review. Perm J. 17:e100–e102. 2013.
|
|
14
|
Ahluwalia MS and Gladson CL: Progress on
antiangiogenic therapy for patients with malignant glioma. J Oncol.
2010:6890182010.
|
|
15
|
Klein T and Bischoff R: Active
metalloproteases of the A Disintegrin and Metalloprotease (ADAM)
family: biological function and structure. J Proteome Res.
10:17–33. 2011.
|
|
16
|
Lu X, Lu D, Scully M and Kakkar V: ADAM
proteins - therapeutic potential in cancer. Curr Cancer Drug
Targets. 8:720–732. 2008.
|
|
17
|
McGowan PM, Ryan BM, Hill AD, et al:
ADAM-17 expression in breast cancer correlates with variables of
tumor progression. Clin Cancer Res. 13:2335–2343. 2007.
|
|
18
|
Kornfeld JW, Meder S, Wohlberg M,
Friedrich RE, et al: Overexpression of TACE and TIMP3 mRNA in head
and neck cancer: association with tumour development and
progression. Br J Cancer. 104:138–145. 2011.
|
|
19
|
Petrás M, Lajtos T, Friedländer E, et al:
Molecular interactions of ErbB1 (EGFR) and integrin-beta1 in
astrocytoma frozen sections predict clinical outcome and correlate
with Akt-mediated in vitro radioresistance. Neuro Oncol.
15:1027–1040. 2013.
|
|
20
|
Huang PH, Xu AM and White FM: Oncogenic
EGFR signaling networks in glioma. Sci Signal. 2:re62009.
|