Introduction
Rectal cancer is one of the most common malignancies
of the digestive tract, ranking first in the worldwide incidence of
malignant tumours. Furthermore, the incidence of rectal cancer in
China shows an increasing trend, and individuals with the disease
under the age of 40 years account for >30% (1). The five-year survival rate following
radical surgery for rectal cancer is ~60%, with five-year survival
rates of 80–90% for early rectal cancer (2). The early diagnosis, accurate staging
and early treatment of rectal cancer are key to improving the
five-year survival rate, particularly for the systematic screening
of high-risk groups. Therefore, the early detection of rectal
cancer is extremely important.
Magnetic resonance imaging (MRI) has the advantages
of multidimensional imaging and clear display of the association
between the rectum and its surrounding tissues, including the
bladder, uterus and prostate. These advantages make MRI an
important means of analysis for the preoperative evaluation of
pelvic diseases. In addition, the use of MRI in staging rectal
cancer has previously been reported (3,4),
however, the majority of the studies have been performed using
single screening technologies only. The simultaneous use of three
types of cancer screening technologies, specifically enhanced,
diffusion and water imaging, for the evaluation of local invasion
and lymph node metastasis has not yet been reported. Therefore, the
aim of the present study was to evaluate the efficiency of the
combined application of three MRI examination techniques in the
staging of advanced rectal cancer preoperatively. The results were
compared with the surgical and pathological observations.
Material and methods
Clinical data
The clinical data of 72 patients (40 males and 32
females) with rectal cancer, aged between 17 and 79 years (mean
age, 58.4 years), were collected between January 2008 and February
2012. The major patient clinical presentations included
haematochezia, mucus bloody stool, changes in defecation habits,
increasing stool frequency and abdominal pain. Following the MRI
examinations, tumours were resected by local excision (tumours of
stage T1 and partial stage T2), or total excision of the rectum and
total mesorectal excision (tumours of stages T3 and T4 or patients
with lymph node metastasis). This study was conducted in accordance
with the declaration of Helsinki and approval was obtained from the
Ethics Committee of the People’s Hospital of Langfang City
(Langfang, China). Written informed consent was obtained from all
participants.
MRI examination methods
Phenolphthalein or magnesium sulphate was orally
administered to the patients following food the evening prior to
the MRI examination to clean the intestinal tract. For the MRI,
500–1,000 ml of warm water was infused into the rectum. Patients
were placed in the supine position and the centre of the magnetic
field was located on the iliac crest. The Avanto 1.5T MRI scanner
purchased from Siemens AG (Berlin, Germany) was used for
examination.
The diffusion-weighted imaging (DWI) technique with
two b values (b=0 and 1,000) was used to measure the apparent
diffusion coefficient (ADC) values of the normal intestinal and
tumour tissues, as well as enlarged lymph nodes.
In addition, the water imaging techniques were
performed using a turbo spin-echo (SE) sequence and the collected
repetitive T2-weighted images were reconstructed using
three-dimensional (3D) maximum intensity projection (MIP) to obtain
3D images.
Finally, enhanced scanning was performed using 3D
volumetric interpolated breath-hold examination sequences following
simple scanning. The acquisition matrix, field of view and slice
thickness were the same as those used for simple scanning,
specifically with a repetition time (TR) of 3.37 ms, echo time (TE)
of 1.66 ms and Aver 1, respectively. Next, 15–20 ml of
gadolinium-dimeglumine was used as the contrast agent through
antecubital intravenous injection using a high-pressure syringe
with a flow rate of 3 ml/sec. MRI scans were immediately performed
following bolus with axial imaging, complemented by sagittal and
coronal images.
Specimen processing
The specimens were fixed with formaldehyde for 24 h
and successive cross sections (5 mm thick) were performed and
staged in accordance with the pathological criteria for tumour node
metastasis (TNM) staging (5).
Image analysis
All the MRI image data of the patients were divided
into two groups of 72 patients each. One group (the control group)
was analysed only with simple enhanced images and conventional
T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI)
sequences; whereas the other group (the experimental group) was
analysed based on enhanced images, DWI images and water images with
conventional T1WI and T2WI sequences. The invasion of the rectal
wall layers and surrounding organs, as well as pelvic lymph node
size were observed. Lymph nodes of >5 mm in diameter, or iliac
blood vessels along the distribution of lymph nodes with diameters
of >1 cm were used as a diagnostic standard of lymph node
metastasis. Accurate staging was performed according to the TNM
staging method of the Union for International Cancer Control to
guide the determination of clinical treatment programmes (5).
Evaluation indices
The normal structure of the intestinal walls using
MRI can be divided into three layers (6). On T2WI, the high signals in the
innermost walls present the mucosa and submucosa (these signals
were not distinguished in the current study), while the moderately
low signals present the muscularis propria. Furthermore, the high
signals in the outer intestinal walls present the fat surrounding
the intestines. These layers were all surrounded by line-like low
signal structures, which present the mesorectal fascia.
The criteria for the diagnosis of rectal cancer
using MRI were determined according to Brown et al (7). The intestine demonstrated irregular
filling defect and irregular valve walls. In this study the the
lesions in the distal end showed ‘cuff’ or ‘truncated’
characteristics on MR water imaging (8). The diagnostic criteria of DWI
(9) were the high signal intensity
of the rectal walls and tumours, or rectal wall thickening in
conventional sequences.
The current diagnostic criteria for T staging using
MRI was recommended by the Union for International Cancer Control
(UICC) and the American Joint Committee on Cancer (10). Additionally, the diagnostic criteria
for N staging using MRI were determined according to Brown et
al (11). Furthermore, the
staging criteria developed by the UICC in 2009 was used as the
pathological staging criteria for TN (5).
Statistical analysis
SSPS 11.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis and the sensitivity,
specificity and accuracy of rectal cancer stages were analysed by
the simple scanning inspection method for MRI. Combining the
sensitivity, specificity and accuracy of DWI and water imaging
ultimately verified the diagnostic values of the various methods.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Pathological typing and location
The postoperative pathology for the 72 cases of
rectal cancer were as follows: 61 cases of adenocarcinoma [five
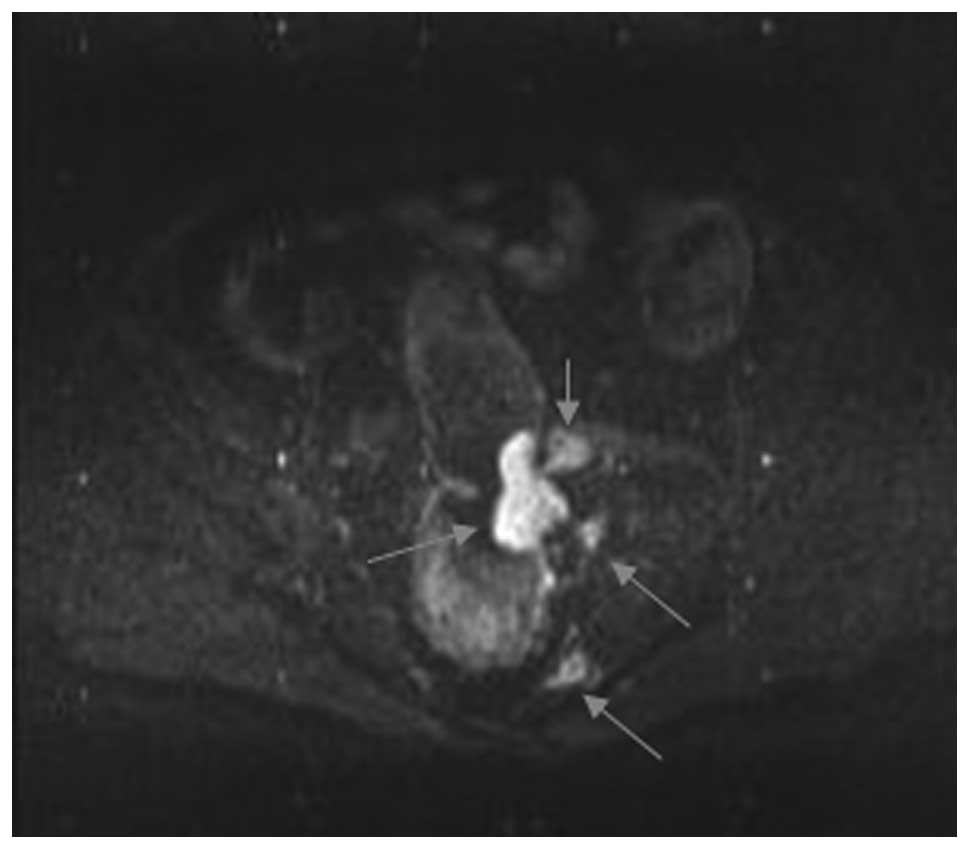
highly differentiated, 41 moderately differentiated (Fig. 1), 13 moderately and poorly
differentiated and two poorly differentiated adenocarcinomas];
seven cases of mucous gland cancer; two cases of carcinoid; one
case of adenosquamous carcinoma; and one case of malignant
melanoma. Of these cases, 35 cases had tumours located in the upper
rectum (10–15 cm from the anal margin), 26 cases had tumours in the
rectal midpiece (5–10 cm from anal margin) and 11 cases had tumours
in the lower segment of the rectum (<5 cm from the anal
margin).
Pathological T staging
Overall, one case was determined as stage T1, 43
cases were determined as stage T2, 26 cases were determined as
stage T3 and two cases were determined as stage T4. In addition, a
total of 543 lymph nodes were found in 62 cases, including 427
benign lymph nodes in 60 cases (34 stage N0 cases and 26 cases with
mixed benign and malignant lymph nodes). Malignant lymph nodes were
present in 116 cases (28 cases with mixed benign and malignant
lymph nodes and two cases with malignant lymph nodes, including 18
stage N1 and 12 stage N2 cases) (Fig.
1).
The mean ADC value of the tumour tissues was
1.1076±0.3289×10−3 mm2/sec, whereas the
average ADC value of the normal intestinal walls was
1.7056±0.2217×10−3 mm2/sec.
T staging results of MRI examination
The sequences of the simple enhanced scanning plus
diffusion combined with water imaging for the the T staging of the
experimental group were as follows: Overall sensitivity of 98.5%
(65/66), total specificity of 66.7% (4/6) and overall accuracy of
95.8% (69/72). Two cases were overestimated and three cases were
underestimated, while one stage T1 patient was correctly staged.
The observation of a small tumour (~1 cm), with pathological
invasion to the shallow muscle layer resulted in the
underestimation of one stage T2 case as stage T1. In stage T2, the
sensitivity was 94.7% (36/38), specificity was 80% (4/5) and
accuracy was 93% (40/43). line strips of signal shadow in the
lesion edge, uneven signals from the surrounding fat-free areas
(mixed signals including the equisignal and the high-phase signal)
and the observation of small nodules was the result of two stage T2
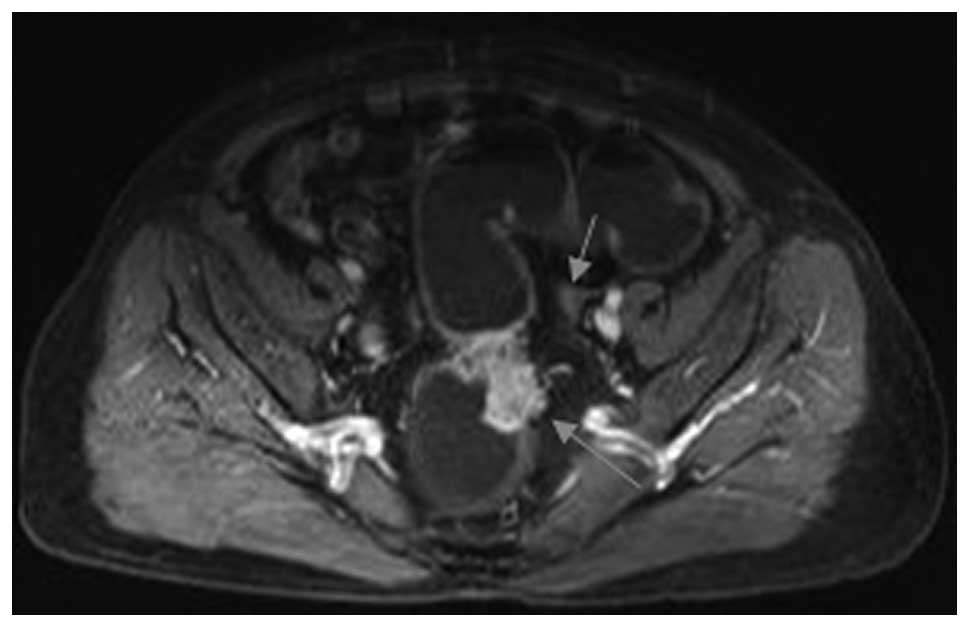
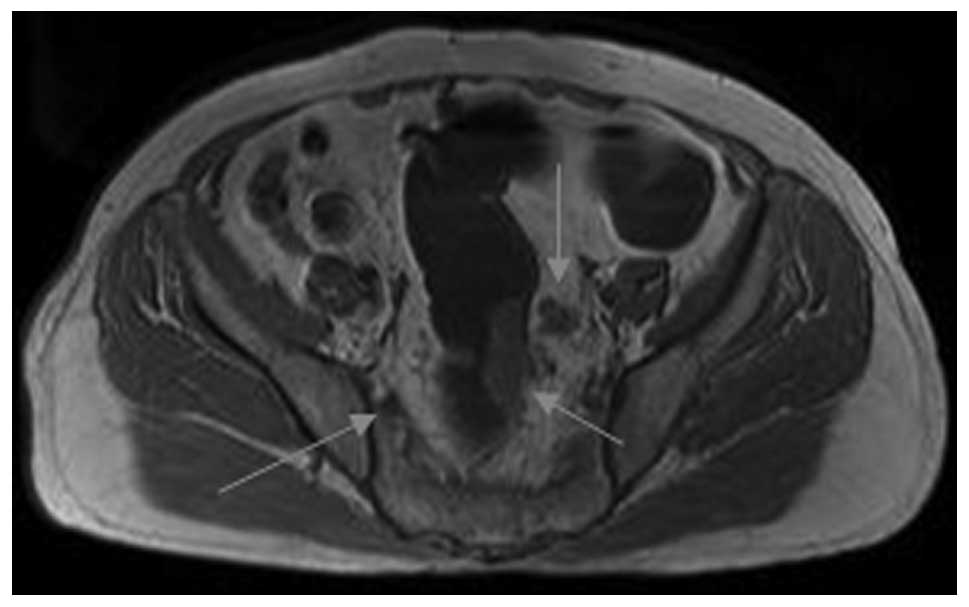
cases being overestimated as stage T3. In stage T3 (Figs. 2 and 3), the sensitivity was 94.7% (36/38),
specificity was 75% (3/4) and accuracy was 92.3% (24/26). Two stage
T3 cases were underestimated to be stage T2. Finally, in stage T4,
the sensitivity, specificity and accuracy were all 100% (2/2).
The sequences of the simple enhanced scanning for
the T staging of the control group were as follows: Overall
sensitivity of 85.7% (42/49), total specificity of 78.3% (18/23)
and overall accuracy of 83.3% (60/72). In total, five cases were
overestimated and seven cases were underestimated. In stage T1, two
stage T2 patients were underestimated as stage T1. In stage T2, the
sensitivity was 86.7% (26/30), specificity was 84.6% (11/13) and
accuracy was 86% (37/43). Two stage T2 patients were underestimated
to be stage T1, whereas four cases were overestimated as stage T3.
In stage T3 (Figs. 4 and 5), the sensitivity was 78.6% (11/14),
specificity was 75% (9/12) and accuracy was 76.9% (20/26). Three
stage T2 patients were underestimated to be stage T2, whereas three
cases were overestimated as stage T4. Finally, the sensitivity,
specificity and accuracy of stage T4 were all 100% (2/2) (Tables I and II).
 | Table IMRI and pathological T staging results
for rectal cancer. |
Table I
MRI and pathological T staging results
for rectal cancer.
| Experimental MRI
group, n | Control MRI group,
n |
|---|
|
|
|
|---|
| Pathological T
staging | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 |
|---|
| T1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| T2 | 1 | 40 | 2 | 0 | 2 | 33 | 8 | 0 |
| T3 | 0 | 2 | 24 | 0 | 0 | 10 | 16 | 0 |
| T4 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Total | 2 | 42 | 26 | 2 | 3 | 43 | 24 | 2 |
| Accuracy (%) | 50 | 88.9a | 85.7a | 100 | 33.3 | 62.3 | 47 | 100 |
 | Table IIStatistical analysis of the T staging
results of the two groups. |
Table II
Statistical analysis of the T staging
results of the two groups.
| Groups | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| Experimentala | 98.5 | 66.7 | 95.8 |
| Controlb | 85.7 | 78.3 | 83.3 |
| P-value | <0.05 | <0.05 | <0.05 |
N staging results of MRI examination
The sequences of the simple enhanced scanning plus
diffusion combined with water imaging for the N staging of the
experimental group were as follows. A total of 454 lymph nodes were
found in 57 cases, including 346 benign lymph nodes (with equal DWI
signals or of regular shapes) with an average ADC value of
0.9126±0.1945×1−3 mm2/sec, as well as 108
malignant lymph nodes (with high DWI signal or of irregular shapes)
with an average ADC value of 0.7691±0.1193×10−3
mm2/sec. In total, 30/34 cases were determined as stage
N, 16/18 cases were determined as stage N1 and 11/12 cases were
determined as stage N2. The overall accuracy of the N staging was
89%.
The sequences of the simple enhanced scanning for
the N staging of the control group are as follows. A total of 28/34
cases were determined as stage N0, 5/18 cases were determined as
stage N1 and 6/12 cases were determined as stage N2. The total
accuracy of the N staging was 61% (Table III).
 | Table IIILymph node status of MRI and
pathological examination. |
Table III
Lymph node status of MRI and
pathological examination.
| Experimental MRI
group for, n | Control MRI group,
n |
|---|
|
|
|
|---|
| Pathological N
staging | N0 | N1 | N2 | N0 | N1 | N2 |
|---|
| N0 | 30 | 1 | 0 | 28 | 9 | 4 |
| N1 | 2 | 16 | 1 | 5 | 5 | 6 |
| N2 | 2 | 1 | 11 | 1 | 4 | 2 |
| Total | 34 | 18 | 12 | 34 | 18 | 12 |
| Accuracy (%) | 85.7 | 76.2a | 92.3a | 58.3 | 17.2 | 12.5 |
Discussion
The main treatment for rectal cancer is surgery,
which cures the majority of early-stage patients. However, the
majority of patients have no treatment options at diagnosis. With
the development of novel treatment methods, such as preoperative
radiotherapy, systemic or intra-arterial chemotherapy, surgical
resection of liver metastasis, and preoperative radiotherapy and
chemotherapy, the tumour size can be reduced and thereby improve
the resection rate (12). The aim
of the current study was to provide important references for
formulating preoperative treatment plans using accurate imaging
staging, particularly for patients with tumours that could not be
directly surgically treated, but could be surgically resected
following radiotherapy or chemotherapy.
The imaging examination of rectal cancers includes
endorectal ultrasound, computed tomography (CT) and MRI. The
accuracy of T staging for rectal cancer in the rectal cavity using
endorectal ultrasound is 86%, and the accuracy of N staging is
62–83% (13). Rectal stenosis and
upper rectum diseases are the major limitation factors of
endorectal ultrasound due to inflammatory infiltration of the
tumour edges. In addition, the tumour invasion is difficult to
distinguish and the morphological changes of the outer edges of the
muscular layers caused by muscular contraction may be misdiagnosed
as tumours breaking through the muscular layers. The rectal
tumours, surrounding invasion, distant metastasis and postoperative
recurrence can be displayed on CT. However, CT is unable to
accurately distinguish the intestinal wall layers and is
insensitive to the slight infiltration of fat. Thus, preoperative T
staging by CT has certain limitations and the determination of
lymph node metastasis based on the size and shape of lymph nodes
lacks specificity (13).
The resolution of MRI for soft tissue is high and
axial, sagittal and coronal 3D imaging clearly shows the rectal
mucosa, submucosa, muscularis and serosa of the intestine and
tumours, as well as the surrounding fat tissues and organs. The
accuracy of the T staging of rectal cancer using MRI is between 67
and 86%, whereas that for N staging is between 57 and 65% (14). In the present study, the accuracy of
T staging using only conventionally enhanced sequences was 83.3%,
whereas that for N staging was 61%. Furthermore, the accuracy of T
staging using simple enhanced scanning plus diffusion combined with
water imaging was 95.8%, and that for N staging was 89%, which was
significantly improved. The results indicated that the combined
application of various inspection technologies, such as simple
enhanced scanning, DWI and water imaging, has important effects
that contribute to the correct diagnosis of rectal cancer,
significantly improving the accuracy of TN staging.
In the current study, irregular soft tissue lumps,
localised or diffused intestinal wall thickening and irregular
luminal stenosis in the rectal cavity were shown in the MRI
scanning. In simple scanning, the 72 cases exhibited slightly low
signals or equal signals on T1WI, and mixed low and high signals on
T2WI. In addition, seven cases with mucinous adenocarcinoma
exhibited significantly high signals on T2WI, which is consistent
with the literature (15) and is
the result of a mucus lake formed by the large amounts of mucus
secreted by the tumours. Thus, the prognoses were poorer than those
of the non-mucinous adenocarcinomas (15). Homogeneous or heterogeneous
enhancements were shown in the tumours and the edges were smooth,
nodular or jagged on enhanced scanning.
In rectal cancer staging, the involvement of the
circular muscle is key to identifying stages T1 and T2, and the
invasion of the perirectal fat tissues is key to identifying stages
T2 and T3. Furthermore, the normal rectal circular muscle layers
show circular homogeneous low signals on T2WI. This complete ring
indicates that the cancer has not broken through the myometrium,
which is considered to indicate stages T1 and T2. In addition, no
involvement of the annular muscle is considered to indicate stage
T1, whereas the involvement of the circular muscle that does not
exceed its outer edges and surrounding fat tissue are considered to
indicate stage T2 (16). However,
the identification of lesions of stages T1 and T2 using MRI is
limited, as observed in the two groups of the current study with
the underestimation of stage T2 as stage T1. This observation
indicated that the differentiation between stages T1 and T2 using
DWI and water imaging has no evident significance. The invasion of
the serosa and surrounding fat tissues is the basis for identifying
stages T2 and T3 and is criteria for performing neoadjuvant therapy
(17). Invasion of the muscle
layer, as well as to the surrounding nodules or lumps by the tumour
tissues are considered to indicate stage T3. Furthermore, the
adipose tissues surrounding the intestine are shown well without
fat suppression on T1WI and T2WI sequences, which is beneficial for
identifying stages T2 and T3.
For DWI sequences, one study (18) compared the image quality with the
ADC values obtained with different b values and concluded that
b=1,000 sec/mm2 is a reasonable b value for the DWI of
colorectal cancer. Thus, the above parameters were used in the
present study. Based on the value of b=1,000 sec/mm2,
all tumour lesions showed high signals, with stark contrast to the
intestinal walls, faeces with low signals and significantly
increased diagnostic sensitivity (98.5%) and accuracy (95.8%).
Quantitative measurement of the ADC value is an additional
important tool for the analysis of benign and malignant tumours, as
well as lymph nodes using DWI imaging, which reflects the diffusion
properties of water molecules, with size depending on the imaging
material and spatial distribution of the internal molecules
(19). The ADC value of the
surrounding normal intestinal tissues was significantly higher than
that of the rectal tumour tissues, which is predominantly
attributed to the high content of tumour cells in tissues, dense
cell structures, close and disorder arrangement, free water
reduction in the cell gaps, water diffusion limitations and
decreasing ADC value (20).
Therefore, the ADC value with different large b values may present
an effective tool for rectal tumour diagnosis.
The evaluation of lymph nodes using MRI combined
with enhanced scanning relies only on tumour size. Thus, the
internal structures of the lymph nodes are not shown. Only using
the tumour size as the standard for assessing metastasis has some
limitations, such as the inability to identify whether enlarged
lymph nodes are the result of inflammation, reactive lymph node
hyperplasia or metastasis, and the failure to detect small but
metastasized lymph nodes. The number of perirectal lymph nodes with
diameters of >5 mm or distributed along the iliac vessels and
groin with diameters of >1 cm detected by MRI scanning and
enhanced scanning was less than that observed during surgery. It
was not possible to individually compare the swollen lymph nodes
shown on MRI with those observed during surgery, thus, the
correlation between lymph node metastasis and size could not be
accurately determined. Therefore, N staging by MR scanning combined
with enhanced MRI scanning exhibited more evident limitations. The
water diffusion of the metastasized cancer lesions or the internal
structure of lymph nodes was restricted, similar to that of the
primary tumour. The same high signals were displayed using DWI with
or without irregular margin changes, and the two ADC values were
significantly reduced. Thus, the missing or unclearly displayed
cancer lesions and lymph nodes in the conventional sequences of the
simple scanning combined with enhanced scanning can be observed on
DWI-weighted imaging, which significantly improves the sensitivity
and accuracy of N staging. A previous study (21) reported that the evaluation of lymph
node metastasis for rectal cancer using DWI sequences was
significant and that the differentiation between benign and
malignant lymph nodes may be determined through the ADC value of
the lymph nodes. The study further confirmed this hypothesis and
the accuracy of N staging was improved from 61 to 89%.
On MRI combined with water scanning, the appearence
of the rectum is a hollow organ with a specific anatomical
location. MR water imaging was used with a long TR (>3,000 ms)
and extremely long TE (>150 ms) to obtain the repeat T2WI
effect, as well as to image the excretory organs. The rectum was
filled with water prior to examination and significant signal
reduction was observed in the lesions of the internal rectal cavity
or thickened intestinal walls, as well as the surrounding
structures. The signals of the aqueous rectal cavity were clearly
displayed and the narrow characteristics and extent of the lesion
in the intestinal cavity were clearly displayed through 3D or 2D
MIP reconstruction. The scope of the surgical resection and the
distance of the lesion from the anus can also be visually
displayed. Compared with barium enema, it is possible to display
tumour invasion of the proximal segment for the intestinal
obstruction and to identify invasion of the tissues and organs
outside the intestinal cavity by MRI (15,16).
However, DWI and water imaging have poorer qualities
than the SE sequences and the spatial resolution is lower. Thus, TN
staging for tumours using only DWI and water imaging is infeasible.
However, the combined application of dynamic contrast-enhanced MRI,
DWI and water imaging result in more accurate images for the TN
staging of tumours than those obtained by enhanced MRI only
(22). In the current study, the
location of the tumours determined using the combined application
of DWI and water imaging was clearer and the dynamic
contrast-enhanced MRI images compensated for the lower image
resolution of the DWI and water imaging.
MRI can be used with multiple parameters and
sequences for good resolution of soft tissues and a wide scan range
without space restrictions. In addition, the thickness of the
intestinal walls, anatomical structures and neighbouring structures
are clearly shown. Lumps in the intestinal wall and their size, as
well as adjacent organ invasion can be exhibited at cancer
diagnosis. Furthermore, lymph node swelling and abdominopelvic
cavity metastasis may be observed (23). In the current study, the signal
changes of the MRI were quite specific, which has high clinical
value in disease diagnosis, staging, assessment of resectability
and other aspects. The sensitivity and accuracy of the diagnosis
for elevated lesions of >10 mm in size were all >95%, and the
ADC values provided a reasonable basis for the identification of
benign and malignant rectal lesions and aid in the selection of a
suitable chemotherapy and chemoradiotherapy.
Limitations of the current study included the
following: i) The positive lymph nodes detected by imaging did not
correspond to those observed during surgery, which has a certain
influence on the evaluation of the positive lymph nodes; and ii)
the capabilities identified for stage T1 and T2 lesions were
limited, and patients of stage T2 were underestimated as stage T1
in the two groups. This observation indicated that no method is
superior to the other in terms of the differentiation between
stages T1 and T2.
In conclusion, the multi-sequencing combined
application of MRI is important for the early diagnosis and
accurate staging of rectal cancer, which may improve the
formulation of clinical treatment plans. In addition, DWI imaging
is important for improving the sensitivity of T staging and
contributes to the enhanced accuracy of N staging combined with the
determination of the ADC value. However, the application of this
method alone is not suitable for cancer diagnosis and staging. In
addition, the application of water imaging alone, which is
important for clearing the range of tumour imaging, is also not
recommended for cancer diagnosis and staging.
References
|
1
|
Kirkham A, Brown G, Williams GT, et al:
High-resolution MRI of the anatomy important in total mesorectal
excision of the rectum. AJR Am J Roentgenol. 182:431–439. 2011.
|
|
2
|
You W, Jin F, Gridley G, et al: Trends in
colorectal cancer rate, in urban shanghai, 1972–1996, in relation
to dietary changes. Ann Epidemiol. 10:4692000.
|
|
3
|
Zhang YL, Zhang ZS, Wu BP and Zhou DY:
Early diagnosis for colorectal Cancer in China. World J
Gastroenterol. 8:21–25. 2008.
|
|
4
|
Berlin JW, Gore RM, Yaghmai V, Newmark GM
and Miller FH: Staging of colorectal cancer. Semin Roentgenol.
35:370–384. 2009.
|
|
5
|
Low RN, McCue M, Barone R, Saleh F and
Song T: MR staging of primary colorectal carcinoma: comparison with
surgical and histopathologic findings. Abdom Imaging. 28:784–793.
2009.
|
|
6
|
Greene FL, Page DL, Fleming I, et al: AJCC
Cancer Staging Manual. 6th edition. Springer-Verlag; New York, NY:
2002
|
|
7
|
Brown G, Radcliffe AG, Newcombe RG,
Dallimore NS, Bourne MW and Williams GT: Preoperative assessment of
prognostic factors in rectal cancer using high-resolution magnetic
resonance imaging. Br J Surg. 90:355–364. 2011.
|
|
8
|
Kim NK, Kim MJ, Park JK, Park SI and Min
JS: Preoperative staging of rectal cancer with MRI accuracy and
clinical usefulness. Ann Surg Oncol. 7:732–737. 2009.
|
|
9
|
Klessen C, Rogalla P and Taupitz M: Local
staging of rectal cancer: the current role of MRI. Eur Radiol.
17:379–389. 2007.
|
|
10
|
Blomqvist L: Preoperative staging of
colorectal cancer-computed tomography and magnetic resonance
imaging. Scand J Surg. 92:35–43. 2003.
|
|
11
|
Brown G, Richards CJ, Bourne MW, et al:
Morphologic predictors of lymph node status in rectal cancer with
use of high-spatial-resolution MR imaging with histopathologic
comparison. Radiology. 227:371–337. 2008.
|
|
12
|
Havenga K, Enker WE, Norstein J, et al:
Improved survival and local control after totalmesorectal excision
or D3 lymphadenectomy in the treatment of primary rectal cancer: an
international analysis of 1411 patients. Eur J Surg Oncol.
25:368–374. 2009.
|
|
13
|
Gualdi GF, Casciani E, Guadalaxara A, et
al: Local staging of rectal cancer with transrectal ultrasound and
endorectal magnetic resonance imaging: Comparison with histologic
findings. Dis Colon Rectum. 43:338–345. 2010.
|
|
14
|
Kim NK, Kim MJ, Park JK, Park SI and Min
JS: Preoperative staging of rectal cancer with MRI: accuracy and
clinical usefulness. An Surg Oncol. 7:732–737. 2008.
|
|
15
|
Kim MJ, Park JS, Park SI, et al: Accuracy
in differentiation of mucinous and nonmucinous rectal carcinoma on
MR imaging. J Comput Assist Tomogr. 27:48–55. 2007.
|
|
16
|
Beets-Tan RG, Beets GL, Vliegen RF, et al:
Accuracy of magnetic resonance imaging in prediction of tumour-free
resection margin in rectal cancer surgery. Lancet. 357:497–504.
2010.
|
|
17
|
Tsushima Y, Takano A, Taketomi-Takahashi A
and Endo K: Body diffusion-weighted MR imaging using high b-value
for malignant tumor screening: usefulness and necessity of
referring to T2-weighted images and creating fusion images. Acad
Radiol. 14:643–650. 2007.
|
|
18
|
Sugita R, Yamazaki T, Furuta A, Itoh K,
Fujita N and Takahashi S: High b-value diffusion-weighted MRI for
detecting gallbladder carcinoma: preliminary study and results. Eur
Radiol. 19:1794–1798. 2009.
|
|
19
|
Hosonuma T, Tozaki M, Ichiba N, et al:
Clinical usefulness of diffusion-weighted imaging using low and
high b-values to detect rectal cancer. Magn Reson Med Sci.
5:173–177. 2006.
|
|
20
|
Rao SX, Zeng MS, Chen CZ, et al: The value
of diffusion-weighted imaging in combination with T2-weighted
imaging for rectal cancer detection. Eur J Radiol. 65:299–303.
2008.
|
|
21
|
Ichikawa T, Erturk SM, Motosugi U, Sou H,
Iino H, Araki T and Fujii H: High-B-value diffusion-weighted MRI in
colorectal cancer. AJR Am J Roentgenol. 187:181–184. 2006.
|
|
22
|
Tu XH, Li CJ, Ma M, et al: Application of
magnetic resonance hydrography in diagnosis of rectal carcinoma.
Zhonghua Xiao Hua Wai Ke Za Zhi. 5:330–332. 2007.
|
|
23
|
Pegios W, Vogl J, Mack MG, et al: MRI
diagnosis and staging of rectal carcinoma. Abdom Imaging.
21:211–218. 1996.
|