Introduction
Gastric cancer is the fourth most prevalent
malignant disease and the second leading cause of cancer-related
mortality worldwide (1,2). Gastric cancer is characterized by a
high mortality rate and a short median survival time, since it is
too late for treatment when the diagnosis is made, in part due to
the asymptomatic nature in the early stages of disease (3). According to the data from the 2008
World Health Organization (4)
statistics, there were 988,000 new cases of gastric cancer
worldwide and 736,000 mortalities, with ~50% of the cases occurring
in China. Currently, gastric cancer remains a formidable disease to
resolve, although great progress has been made in its treatment.
Currently, the main therapeutic modalities involve surgery,
radiation and chemotherapy.
The induction of cell cycle arrest and apoptosis has
been demonstrated to induce cancer cell death and may be considered
as a strategy to deal with gastric cancer (5). Currently, the cell cycle has been
investigated widely and the cyclin-dependent kinase 1 (CDK1)/cyclin
B complex has been found to play a significant role in the
regulation of the G2/M phase (6). Apoptosis is a form of cell death that
can be activated through at least two signaling pathways, the
caspase-dependent and caspase-independent pathways, and several
involve the mitochondria and B-cell lymphoma-2 (Bcl-2) family
proteins (7).
Cantharidin (CTD) is an effective component
extracted from blister beetles and is one of numerous natural
products used in traditional Chinese medicine (8,9). The
molecular formula of CTD is
C10H12O4 and the molecular weight
is 196.2. CTD has been reported to possess antibiotic and antiviral
activities (10). Also, it has been
used as an abortifacient and as a treatment for edema and warts
(10,11). Recently, CTD has been demonstrated
to exhibit potent anticancer activities on numerous types of human
cancer cells, including pancreatic (12), bladder (13), breast (14) and hepatocellular cancer (15). It has also been reported that the
CTD-induced reduction in cell growth and cell death in COLO 205
cells is due to the induction of cell apoptosis, which is
associated with the death receptor and mitochondrial apoptotic
pathways (8). CTD has also been
suggested to be a novel and potent multidrug resistance reversal
agent and may be a possible adjunctive agent for chemotherapy
(16). In addition, CTD has been
revealed to be a potent inhibitor of PP2A, which may block
anaphase-promoting complex activity (17,18).
However, it remains unclear whether CTD induces cell
cycle arrest and cell apoptosis in gastric cancer cells. Therefore,
the aim of the present study was to investigate the effect of CTD
in human gastric cancer cells and to explore the underlying
mechanisms of this effect.
Materials and methods
Chemicals, reagents and antibodies
CTD was purchased from Sigma-Aldrich (St. Louis, MO,
USA) and high-performance liquid chromatography was used to confirm
that the purity was >99%. CTD was dissolved in cell culture
medium at a stock concentration of 100 mg/ml and stored at −20°C.
The stock solution was freshly diluted in the medium immediately
prior to use in each experiment. The primary antibodies for cyclins
A and B, CDK1, Bcl-2, Bad and poly ADP ribose polymerase (PARP)
were purchased from Abcam Inc. (Cambridge, UK). The p21-specific
antibody was obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA) and the antibodies for caspases-3, -7, -8 and -9 and Bid
were obtained from Cell Signaling Technology (Beverly, MA, USA).
β-actin was purchased from Santa Cruz Biotechnology. Horseradish
peroxidase (HRP)-conjugated secondary goat anti-mouse and goat
anti-rabbit secondary antibodies were purchased from Pierce. MTS
was obtained from Promega (Madison, WI, USA).
Cells and cell culture
The human gastric cancer SGC-7901 and BGC-823 cell
lines were obtained from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China), where they were tested and authenticated. These
procedures included cross-species checks, DNA authentication and
quarantine. All the cell lines used in the present study were in
culture for less than six months, and were maintained in RPMI 1640
medium (Gibco, Grand Island, NY, USA), with a 10% fetal calf serum
and 1% penicillin/streptomycin mixture at 37°C in a humidified
atmosphere of 5% CO2 and 95% air.
Cell proliferation analysis by MTS
assay
MTS assays were employed to examine the viability of
the human gastric cancer SGC-7901 and BGC-823 cells treated with
CTD. In brief, the cells were seeded at a concentration of
4–8×103 cells/well on 96-well plates, with a total
volume of 200 μl medium, and then cultured for 24 h to allow for
attachment. The cells were treated with various concentrations of
CTD (0–80 μM) for various periods of time (0–72 h). Two hours prior
to the end of incubation, MTS (20 μl in 100 μl RPMI-1640 medium)
was added to each well and the cells were incubated for 2 h at
37°C. The results were obtained by measuring the absorbance of each
well at 490 nm and the half maximal inhibitory concentration
(IC50) values were calculated using probit analysis.
Cell cycle analysis by flow
cytometry
Human gastric cancer SGC-7901 and BGC-823 cells were
seeded at a concentration of 10×105 cells/well on 6-well
plates and were incubated with a 0–20-μM graded concentration of
CTD for 24 h. The cells were then harvested and washed by
centrifugation. For the determination of the cell cycle, the cells
were fixed with 70% ethanol at −20°C overnight, then the cell
pellet was re-suspended in phosphate-buffered saline (PBS)
containing 40 μg/ml propidium iodide (PI) and 100 μg/ml
ribonuclease A, and was incubated in the dark at room temperature
for 30 min. The cell cycle distributions were determined by flow
cytometry (BD FACSCaliburTM; Becton-Dickinson, Franklin
Lakes, NJ, USA).
Apoptosis analysis by flow cytometry
An annexin V-fluorescein isothiocyanate (FITC)/PI
double-fluorescence apoptosis detection kit (Kaiji Biotech,
Nanjing, China) was employed to quantify the apoptosis of the human
gastric cancer SGC-7901 and BGC-823 cells treated with CTD. In
brief, the cells were exposed to CTD (0–80 μM) on 6-well plates
(2×105 cells/ml) for 24 h. Next, using the Annexin
V-FITC/PI double-fluorescence apoptosis detection kit according to
the manufacturer’s instructions, the cells were stained. The
results were obtained by analyzing the samples using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA) within 1 h
post-staining.
Western blot analysis
SGC-7901 and BGC-823 cells in the exponential growth
phase were treated with a graded concentration (0, 20, 40 and 80
μM) of CTD for 24 h. The cells were harvested and proteins were
extracted from the cells at a density of 1×105 cells/ml
for 30 min in a radioimmunoprecipitation assay buffer containing 50
mmol/l TrisHCl, 150 mmol/l NaCl, 2 mmol/l EDTA, 2 mmol/l ethylene
glycol-bis(β-aminoethylether), 25 mmol/l NaF, 25 mmol/l
β-glycerophosphate, 0.1 mmol/l Na orthovanadate, 5 mg/ml leupeptin,
0.1 mmol/l phenylmethylsulfonyl fluoride, 0.2% Triton-X100 and 0.3%
NP-40. The lysed solution was centrifuged at 13,000 × g for 20 min
at 4°C. The protein levels were quantified using a bicinchoninic
acid protein assay kit (Pierce, Rockford, IL, USA), following the
manufacturer’s instructions. Equivalent amounts of protein were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred to polyvinylidine difluoride
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked in PBS containing 5% w/v skimmed dry milk and incubated at
4°C overnight with antibodies against cyclins A and B, CDK1, p21,
caspases-3, -7, -8 and -9, Bcl-2, Bad, Bid and PARP at the
recommended dilution, and then were finally incubated with
HRP-conjugated goat anti-mouse and goat anti-rabbit secondary
antibodies at room temperature for 1 h using enhanced
chemiluminescence reagents (Cell Signaling Technology) and exposed
to X-ray film.
Statistical analysis
All the data were obtained from at least three
independent experiments. The results were expressed as the mean ±
standard deviation. Statistically significant differences between
the experimental and control groups were identified by one-way
analysis of variance. The IC50 values and 95% confidence
intervals were calculated from the MTS assay data by probit
regression. All statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The P-values were
two-tailed and a P≤0.05 was considered to indicate a statistically
significant difference.
Results
CTD inhibits proliferation in SGC-7901
and BGC-823 cells
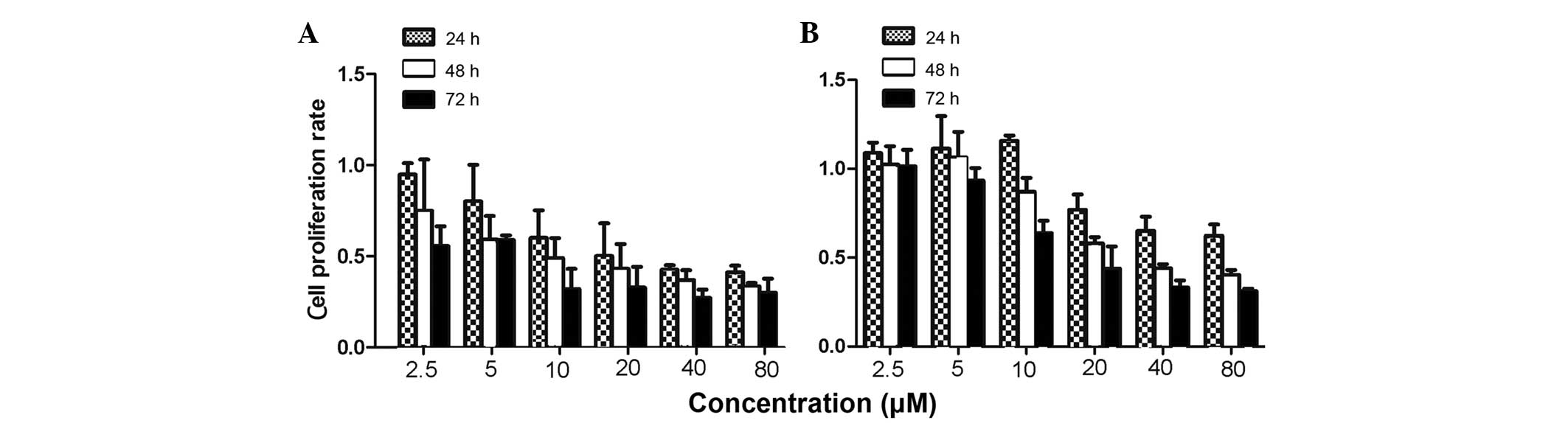
To evaluate the effects of CTD on the proliferation
of human gastric cancer cells, MTS assays were used to measure the
growth of SGC-7901 and BGC-823 cells. The present results revealed
that CTD inhibited the proliferation of the SGC-7901 and BGC-823
cells in a dose- and time-dependent manner (Fig. 1). After 24 h, the IC50
value for CTD was 20.87±3.56 μM in the SGC-7901 cells, while after
48 and 72 h, the values were 12.14±1.28 and 6.45±2.10 μM in the
SGC-7901 cells, and 30.25±0.48 and 18.90±2.51 μM in the BGC-823
cells, respectively.
CTD induces G2/M phase arrest
in SGC-7901 and BGC-823 cells
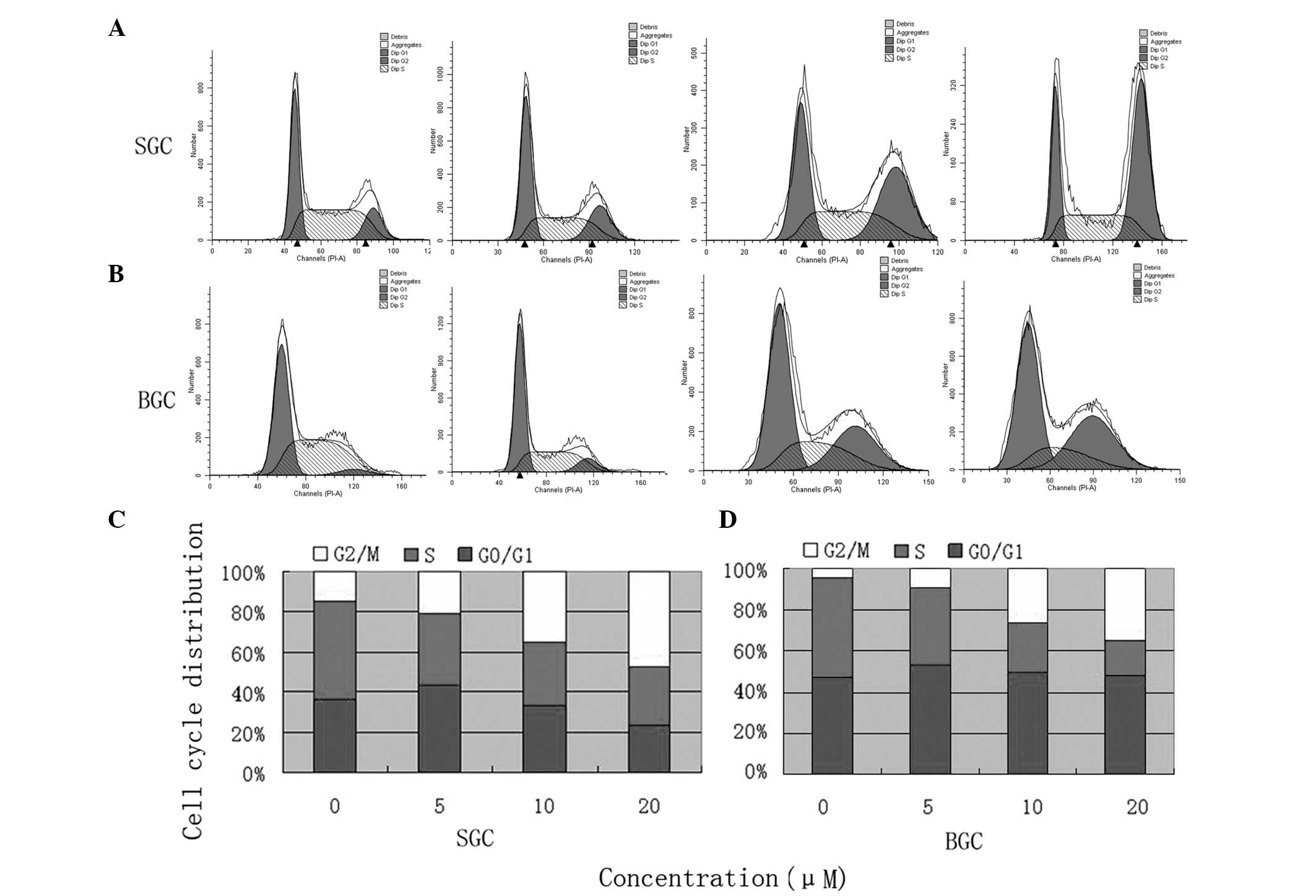
To investigate whether CTD induced inhibition of
SGC-7901 and BGC-823 cell growth via cell cycle-arresting
mechanisms, flow cytometry was used to analyze cell cycle
distribution subsequent to the cells being treated with 0, 5, 10
and 20 μM CTD for 24 h. In the SGC-7901 cells treated with 0, 5, 10
or 20 μM CTD, the ratios of cells in the G2/M phase were
14.67, 20.71, 34.92 and 47.32%, respectively. The ratios for the
BGC-823 cells treated with 0, 5, 10 and 20 μM of CTD were 4.31,
9.66, 26.12 and 34.97%, respectively. The results revealed that CTD
increased the ratio of cells in the G2/M phase in the
SGC-7901 and BGC-823 cells in a dose-dependent manner (Fig. 2).
CTD induces apoptosis in SGC-7901 and
BGC-823 cells
To investigate whether CTD induced inhibition of
SGC-7901 and BGC-823 cell growth via the cell apoptotic pathways,
cell apoptosis was measured by flow cytometry. As shown in Fig. 3, the cells treated with a high
concentration of CTD exhibited much higher rates of apoptosis
compared with the cells treated with a low concentration. The
results revealed that CTD induced the apoptosis of the SGC-7901 and
BGC-823 cells in a dose-dependent manner.
CTD regulates the expression of
G2/M phase- and apoptosis-associated proteins in
SGC-7901 and BGC-823 cells
The possible signaling pathways through which CTD
induces cell cycle arrest and apoptosis in SGC-7901 and BGC-823
cells were investigated. Western blot analysis revealed that the
protein expression levels of CDK1 and cyclins A and B were
decreased following exposure to CTD in the two cell lines, but that
the level of p21 was markedly increased (Fig. 4A), which led to cell cycle arrest
and cell apoptosis. CTD increased the level of caspases-7, -8 and
-9, activated caspase-3, PARP and Bad, but decreased the level of
Bcl-2 and Bid (Fig. 4B).
Discussion
In China, gastric cancer is one of the most common
types of cancer and gastric cancer-related mortality accounts for
~23% of all cancer-related mortalities (19). It is therefore vital to solve such a
formidable problem. Chemotherapy is becoming one of the main
therapeutic modalities for the treatment of gastric cancer. The
search for a new drug or therapeutic method is important, since the
current treatments for gastric cancer are not ideal. Certain
traditional Chinese medicines, including CTD, have been
demonstrated to be reasonable and effective treatments for cancer.
It has been reported that CTD has an effect on several types of
human cancer, including pancreatic (12), bladder (13), hepatocellular (15) and colorectal (20) cancer. However, it remains unclear
whether CTD is effective in treating gastric cancer as well.
Therefore, in the present study, the function of CTD in human
gastric cancer cells was examined and the underlying mechanisms
were investigated.
The present study revealed that CTD inhibited the
proliferation of human gastric cancer SGC-7901 and BGC-823 cells
in vitro in a dose-dependent and time-dependent manner. The
SGC-7901 and BGC-823 cells that were treated with CTD revealed
G2/M phase arrest in cell cycle distribution. In
addition, CTD induced apoptosis in the SGC-7901 and BGC-823 cells
in a dose-dependent manner. It was indicated that CTD may inhibit
the proliferation of human gastric cancer cells by inducing
G2/M phase arrest and cell apoptosis.
Distinct protein kinase complexes, including the
cyclins that are necessary for the activity of CDC/CDK kinase, have
been reported to control the cell cycle (21). Cyclin D/CDK2, 4, 5 or 6 play an
important role in the regulation of the G1 phase, and
the cyclin E/CDK2 and cyclin A/CDK2 complexes are associated with
the G1/S and S phases, respectively. By contrast, cyclin
B/CDK1 is pivotal for cell progression through the G2/M
phase (6,22–24).
The activity of CDK1 kinase first depends on the accumulation of
the cyclin B content. The synthesis of cyclin B starts late in the
G1 phase and its content continually increases during S
phase until cyclin B levels reach their maximum level in the
G2 phase. Once cyclin B levels increase to a certain
degree, the activity of CDK1 kinase appears. The activity of CDK1
kinase also increases to a maximum level in late G2
phase and this level is maintained until the middle of M phase
(25). p21, as a potent
cyclin-dependent kinase inhibitor, is a pivotal regulator and can
also inhibit the activity of CDK1 and strengthen G2/M
phase arrest (8,26,27).
The G2/M checkpoint presents a potential target in the
cell cycle for cancer therapy (28). Cyclin A also plays an important role
in the initiation process of the S phase (25).
The present results revealed that CTD decreased the
protein levels of CDK1 and cyclin B and increased the level of p21
in the SGC-7901 and BGC-823 cells, suggesting that CTD may induce
G2/M phase arrest through downregulation of cyclin B and
CDK1 and upregulation of p21. This finding is similar to previous
studies in which CTD was revealed to induce G2/M phase
arrest in the human bladder cancer T24 cell line and in pancreatic
cancer cells (12,29), and to induce
G0/G1 phase arrest in human bladder cancer
TSGH 8301 cells (13). These
findings require further investigation. Additionally, in the
present study, the observation of a decreased level of cyclin A in
the SGC-7901 and BGC-823 cells led to the suggestion that CTD may
inhibit the process of S phase by downregulating cyclin A.
Apoptosis plays a central role in anti-tumorigenesis
and involves two main signaling pathways, the death-receptor
extrinsic pathway and the mitochondrial intrinsic pathway (30). These pathways are triggered by
caspase-8 and -9, respectively. Membrane-associated protein
complexes activate procaspase-8, while procaspase-9 is activated by
mitochondria-associated protein (31). In addition, the two pathways
converge at the point of caspase-3 activation. Following the
activation of caspase-3, certain specific substrates for caspase-3,
including PARP proteins, are cleaved, which is important for the
development of apoptosis (32). The
present results revealed that CTD increased the number of apoptotic
cells and the level of caspase-7, -8 and -9, activated caspase-3
and PARP in the SGC-7901 and BGC-823 cells, suggesting that CTD may
induce apoptosis via activation of a caspase cascade. In addition,
Bcl-2 family proteins play a crucial role in regulating cell life.
Three subfamilies of Bcl-2 family proteins have been identified in
the apoptotic response, including the Bcl-2 subfamily, which
includes Bcl-2 and Bcl-XL. The Bcl-2 subfamily is associated with
the inhibition of apoptosis, whereas the Bax subfamily, which
includes Bax, Bak and Bcl-Xs, and the BH3-only subfamily, which
includes Bid and Bad, each promote apoptosis (33). The present study found that CTD
upregulated Bad and downregulated Bcl-2 and Bid, suggesting that
CTD may induce the apoptosis of SGC-7901 and BGC-823 cells by
regulating Bcl-2 family proteins. Previous studies revealed that
CTD induced apoptosis through the death receptor and mitochondrial
apoptotic pathways in COLO 205 cells, while apoptosis was mediated
through the JAK/STAT pathway in myeloma cells and through
mitochondria-dependent signal pathways in human bladder cancer TSGH
8301 cells (8,13,34).
It was indicated that the pathways may vary between cell lines.
However, the tissue specificity of CTD requires further
investigation.
In conclusion, the present results indicate that CTD
may inhibit the proliferation of human gastric cancer SGC-7901 and
BGC-823 cells in vitro by inducing G2/M phase
arrest and cell apoptosis. CTD may induce G2/M phase
cell-cycle arrest by downregulating cyclin A and B and CDK1, and
upregulating p21. Apoptosis may be induced by CTD by the activation
of a caspase cascade through the upregulation of caspases-7, -8 and
-9, activated caspase-3 and PARP. In addition, CTD regulates Bcl-2
family proteins through the upregulation of Bad and the
downregulation of Bcl-2 and Bid, which may also induce
apoptosis.
Acknowledgements
This study was supported by grants from the National
Health Key Special Fund (no. 200802112), the Health Department Fund
(no. 2007A093 and 2008A094), the Traditional Chinese Medicine
Bureau Fund (nos. 2007ZA019 and 2008ZA011), the Natural Science
Fund of Zhejiang Province (nos. Y208001, Y12H160121 and Z2080514)
and the Key Project of Zhejiang Province (no. 2009C03012-5).
References
|
1
|
Dai J, Shen J, Pan W, Shen S and Das UN:
Effects of polyunsaturated fatty acid on the growth of gastric
cancer cells in vitro. Lipids Health Dis. 12:712013.
|
|
2
|
Rasul A, Khan M, Yu B, Ma T and Yang H:
Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in
human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer
Prev. 12:1219–1223. 2011.
|
|
3
|
Zhang C, Xu W, Pan W, et al:
Selenium-binding protein 1 may decrease gastric cellular
proliferation and migration. Int J Oncol. 42:1620–1629. 2013.
|
|
4
|
Ferlay J, Shi HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917
|
|
5
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
|
|
6
|
Jin CY, Choi YH, Moon DO, et al: Induction
of G2/M arrest and apoptosis in human gastric epithelial AGS cells
by aqueous extract of Agaricus blazei. Oncol Rep.
16:1349–1355. 2006.
|
|
7
|
Wang X, Zhu S, Drozda M, et al:
Minocycline inhibits caspase-independent and -dependent
mitochondrial cell death pathways in models of Huntington’s
disease. Proc Natl Acad Sci USA. 100:10483–10487. 2003.
|
|
8
|
Huang WW, Ko SW, Tasi HY, et al:
Cantharidin induces G2/M phase arrest and apoptosis in human
colorectal cancer colo 205 cells through inhibition of CDK1
activity and caspase-dependent signaling pathways. Int J Oncol.
38:1067–1073. 2011.
|
|
9
|
Rauh R, Kahl S, Boechzelt H, Bauer R,
Kaina B and Efferth T: Molecular biology of cantharidin in cancer
cells. Chin Med. 2:82007.
|
|
10
|
Dorn DC, Kou CA, Png KJ and Moore MA: The
effect of cantharidins on leukemic stem cells. Int J Cancer.
124:2186–2199. 2009.
|
|
11
|
Moed L, Shwayder TA and Chang MW:
Cantharidin revisited: a blistering defense of an ancient medicine.
Arch Dermatol. 137:1357–1360. 2001.
|
|
12
|
Li W, Xie L, Chen Z, et al: Cantharidin, a
potent and selective PP2A inhibitor, induces an oxidative
stress-independent growth inhibition of pancreatic cancer cells
through G2/M cell-cycle arrest and apoptosis. Cancer Sci.
101:1226–1233. 2010.
|
|
13
|
Kuo JH, Chu YL, Yang JS, et al:
Cantharidin induces apoptosis in human bladder cancer TSGH 8301
cells through mitochondria-dependent signal pathways. Int J Oncol.
37:1243–1250. 2010.
|
|
14
|
Williams LA, Möller W, Merisor E, Kraus W
and Rösner H: In vitro anti-proliferation/cytotoxic activity
of cantharidin (Spanish fly) and related derivatives. West Indian
Med J. 52:10–13. 2003.
|
|
15
|
Wang CC, Wu CH, Hsieh KJ, Yen KY and Yang
LL: Cytotoxic effects of cantharidin on the growth of normal and
carcinoma cells. Toxicology. 147:77–87. 2000.
|
|
16
|
Zheng LH, Bao YL, Wu Y, Yu CL, Meng X and
Li YX: Cantharidin reverses multidrug resistance of human hepatoma
HepG2/ADM cells via down-regulation of P-glycoprotein expression.
Cancer Lett. 272:102–109. 2008.
|
|
17
|
Honkanen RE: Cantharidin, another natural
toxin that inhibits the activity of serine/threonine protein
phosphatases types 1 and 2A. FEBS Lett. 330:283–286. 1993.
|
|
18
|
Fang G, Yu H and Kirschner MW: Control of
mitotic transitions by the anaphase-promoting complex. Philos Trans
R Soc Lond B Biol Sci. 354:1583–1590. 1999.
|
|
19
|
Shi WT, Wei L, Xiang J, et al: Chinese
patients with gastric cancer need targeted adjuvant chemotherapy
schemes. Asian Pac J Cancer Prev. 13:5263–5272. 2012.
|
|
20
|
Liu B, Gao HC, Xu JW, Cao H, Fang XD, Gao
HM and Qiao SX: Apoptosis of colorectal cancer UTC116 cells induced
by cantharidinate. Asian Pac J Cancer Prev. 13:3705–3708. 2012.
|
|
21
|
Hartwell LH and Weinert TA: Checkpoints:
controls that ensure the order of cell cycle events. Science.
246:629–634. 1989.
|
|
22
|
King RW, Jackson PK and Kirschner MW:
Mitosis in transition. Cell. 79:563–571. 1994.
|
|
23
|
Jackman M, Lindon C, Nigg EA and Pines J:
Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat
Cell Biol. 5:143–148. 2003.
|
|
24
|
Malumbres M and Barbacid M: To cycle or
not to cycle: a critical decision in cancer. Nat Rev Cancer.
1:222–231. 2001.
|
|
25
|
Pines J: Cyclins, CDKs and cancer. Semin
Cancer Biol. 6:63–72. 1995.
|
|
26
|
van den Heuvel S: Cell-cycle regulation.
WormBook. 21:1–16. 2005.
|
|
27
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006.
|
|
28
|
Lee DH, Park KI, Park HS, et al:
Flavonoids isolated from Korea citrus aurantium L. induce G2/M
phase arrest and apoptosis in human gastric cancer AGS cells. Evid
Based Complement Alternat Med. 2012:5159012012.
|
|
29
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006.
|
|
30
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001.
|
|
31
|
Launay S, Hermine O, Fontenay M, Kroemer
G, Solary E and Garrido C: Vital functions for lethal caspases.
Oncogene. 24:5137–5148. 2005.
|
|
32
|
Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY,
Ko CH and Tseng SW: Emodin induces apoptosis in human
promyeloleukemic HL-60 cells accompanied by activation of caspase 3
cascade but independent of reactive oxygen species production.
Biochem Pharmacol. 64:1713–1724. 2002.
|
|
33
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000.
|
|
34
|
Sagawa M, Nakazato T, Uchida H, Ikeda Y
and Kizaki M: Cantharidin induces apoptosis of human multiple
myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci.
99:1820–1826. 2008.
|