Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an
aggressive cancer with limited therapeutic options. At present,
surgical resection is the only potential curative treatment for
PDAC. However, only 15–20% of patients with PDAC are eligible for
lesion resection (1). Portal vein
invasion is a significant factor that often precludes the resection
of pancreatic tumors (2). In cases
of portal vein infiltration, total resection of the pancreas and
resection of the superior mesenteric-portal vein (SMPV) may
increase the resectability. However, certain studies have suggested
that SMPV involvement should be a contraindication for resection
(3), as the combined resection
approach may not improve overall survival. In the early 1950s,
total pancreatectomy (TP) was introduced to prevent
anastomosis-related complications (3), which contribute to the majority of
hospital-related mortalities in PDAC patients. The high local
recurrence rates following partial pancreaticoduodenectomies
supported the notion that extended radical resections would improve
long-term survival in cases of pancreatic malignancies. However,
the use of TP to reduce hospital-related mortalities and improve
patient outcomes remains controversial (4). Opponents of TP state that severe
malabsorption and insulin-dependent diabetes mellitus are
post-operative complications that may negatively affect quality of
life following pancreatic resection (5). The present study investigated a case
of PDAC in the pancreatic neck of a male patient. The patient
underwent a TP, combined with an SMVP resection, for a
margin-negative resection, and continues to survive six years after
the surgery.
Case report
On April 5, 2007, a 56-year-old male was admitted to
Zhongshan Hospital (Fudan University, Shanghai, China) with a
primary complaint of weight loss over the previous month. No
abdominal or back pain was reported. Upon admission, the patient’s
body weight was 58 kg, with a reported loss of 6 kg over the
previous month. The patient denied alcohol and cigarette use.
Jaundice was noticeable on the skin, but there was no sign of
ascites. The patient was hospitalized with the suspicion of a
malignant pancreatic neck tumor, without tenderness or a palpable
mass in his abdomen, and without signs of diabetes mellitus.
The serum levels of total bilirubin and direct
bilirubin were 82.1 μmol/l (normal range, 0.0–17.0 μmol/l) and 54.4
μmol/l (normal range, 0.0–9.0 μmol/l), respectively. In addition,
the patient demonstrated elevated levels of several other
laboratory markers, including 366 U/l aspartate aminotransferase
(normal range, 0–75 U/l), 583 U/l alanine aminotransferase (normal
range, 0–75 U/l), 649 U/l alkaline phosphatase (normal range,
15–115 U/l) and 333 U/l lactic dehydrogenase (normal range, 109–245
U/l). The amylase levels were 20 U/l (normal range, 0–200 U/l) and
the carcinomatous biomarker, carbohydrate antigen 19-9 (CA19-9),
was also elevated at 51 U/ml (reference range, 0–37 U/ml). The
serum total protein, albumin and electrolytes levels were normal.
The full blood counts were also normal. Computed tomography (CT)
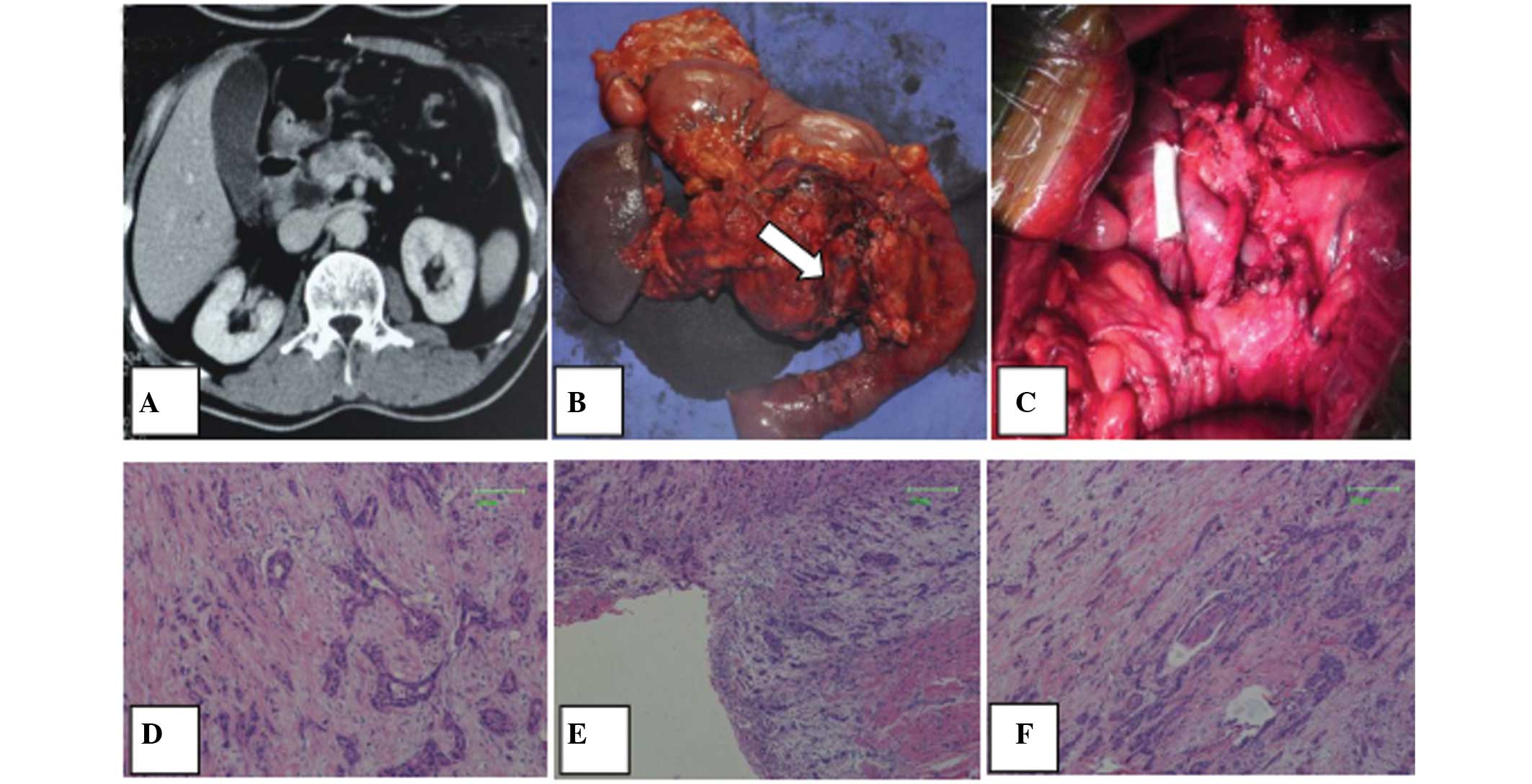
images revealed a low-attenuated mass that was 3 cm in diameter
located in the neck of the pancreas. The tumor was adhered to the
portal vein, but there was no evidence of distant metastasis
(Fig. 1A). Based on these findings,
the tumor was diagnosed as a pancreatic carcinoma involving the
pancreatic neck, with SMPV invasion. Considering the conditions, it
was decided that a TP, with a possible SMPV confluence resection,
would be performed.
On April 12, 2007, the patient underwent a TP with
splenectomy. Upon resection, it was revealed that the tumor had
invaded the SMPV confluence. Therefore, a 4-cm segment of the SMPV
(Fig. 1B) was resected for a
margin-negative resection. The vascular reconstruction was
performed using a 4-cm long vascular graft (GORE-TEX; diameter, 0.8
cm; Gore Medical, Newark, DE, USA; Fig.
1C). The total surgery time was five hours, and the total blood
loss was 620 ml. The patient returned to an oral diet on the sixth
post-operative day. The post-operative course was uneventful, and
the patient was discharged on day 12 in a generally good condition.
The final pathology report revealed a moderately-differentiated
invasive ductal adenocarcinoma, with invasion to the muscle layer
of the portal vein wall, the peripancreatic nerve perineurium and
the anterior pancreatic capsule. The 12 lymph nodes examined were
negative for any histological evidence of regional lymph node
metastasis (Fig. 1D–F).
Post-operatively, the patient received four cycles of gemcitabine
(1,000 mg/m2d, days 1 and 8, every 3 weeks) as adjuvant
chemotherapy. On July 7, 2007, an abdominal CT scan was performed
for suspected lymph node metastasis in the retroperitoneal region.
Subsequently, radiotherapy for the retroperitoneum in the
surgically-resected region was performed. The patient attended
follow-up examinations every six months. On April 7, 2012, a
follow-up positron-emission tomography CT scan revealed a patent
graft and no evidence of tumor recurrence. The tumor marker CA19-9
was also within normal limits at this follow-up. Currently, at the
sixth post-operative year, the patient continues to survive.
The patient requires a regular dose of insulin (20
U/day), and at the last evaluation, the patient’s blood glucose was
108 mg/dl and the glycated hemoglobin was 6.2%. A course of
digestive enzyme replacement (pancreatin enteric-coated capsules;
Solvay, Brussels, Belgium) was initiated subsequent to the surgery,
with a lipase dosage of 10,000 IU/kg/day. The patient has not
complained of diarrhea with the digestive enzyme supplement, and
maintains a stable body weight at 55 kg.
Discussion
PDAC is the most common form of pancreatic neoplasm,
accounting for >85% of pancreatic tumors (1). PDAC largely affects a patient’s
quality of life and results in an extremely poor prognosis. Tumor
resection is the only effective treatment (6). Despite the fact that surgery is the
only potential curative intervention, only 15–20% of patients have
resectable pancreatic tumors. Furthermore, only 20% of patients who
undergo surgery experience survival at five-years post-surgery,
resulting in an average five-year survival rate of 3–5% for all
individuals diagnosed with PDAC (1)
Portal vein invasion is often a preclusive factor
for surgery (2). Although resection
of the portal vein and pancreas can be achieved safely, with little
patient morbidity and mortality (7), the involvement of the SMPV is
frequently considered a contraindication for resection. Certain
studies have demonstrated limited effectiveness and low survival
rates in cases of portal vein infiltration. A study by Allema et
al (8) revealed that the rate
of margin-negative resection was 15% in patients who had undergone
SMPV resection. In addition, the study demonstrated that the
overall one- and three-year survival rates were 59 and 16%,
respectively. However, the mean survival-time was only 5.6 months
in the margin-positive group. These findings emphasize the
importance of negative pathological margins. Allema et al
(8) also compared the survival
rates between those patients with and without histological evidence
of portal vein involvement. There was no significant difference
identified between the patient groups, which suggested that SMPV
invasion does not affect the survival rate of patients with PDAC
(3). However, those patients with
tumors that demonstrate invasion of the tunica intima are more
likely to have a poorer outcome. Overall, no patients with tumor
invasion to the tunica intima survive >6 months (9). Although invasion of the tunica media
appears to be an important prognostic indicator, a diagnosis of
tunica intima involvement prior to surgery is not possible.
TP was first introduced in 1943 by Rockey (10), and the technique was further
described by Ross (11) in 1954.
Over the past several decades, several clinical studies have
supported the use of TP for the surgical management of pancreatic
cancer (12–14). Proponents of this procedure argue
that i) TP allows for a more extensive lymphadenectomy around the
pancreas and leads to greater surgical ‘oncologic radicality’; and
ii) TP decreases R1 and R2 resections, at least at the site of
glandular transection (12). Data
from the Mayo Clinic (13) has
revealed the overall survival for patients with PDAC at one, two
and three years post-TP to be 63, 43 and 34%, respectively. Reddy
et al (14) reported that
the five-year survival rate was 18.9% in 100 patients with PDAC who
had undergone TP. These findings demonstrated that the long-term
survival rate following TP was equivalent to survival rates after
pancreaticoduodenectomy, and that TP should be performed when
oncologically appropriate.
In the present case study, a patient with PDAC in
the pancreatic neck underwent a TP and segmental resection of the
SMPV. Post-operative pathological findings indicated that an
invasive ductal adenocarcinoma had invaded the muscle layer of the
portal-vein wall, the peripancreatic perineurium and the
peripancreatic capsule. However, there was no evidence of regional
lymph node metastasis, and resection margins, including the
retroperitoneal margin, were negative. The patient currently
reports a high quality of life and continues to survive six years
after the surgery.
The primary aim of TP is to avoid a
pancreatic-enteric anastomosis, as the principal cause of
post-operative patient mortality following partial pancreatectomy
is anastomotic leakage. Despite the intentions of this surgical
approach, TP has not reduced hospital-associated mortalities
(4), and there have been additional
concerns regarding severe post-operative metabolic conditions that
may arise with the complete removal of the pancreas (5). However, regardless of these initial
concerns, morbidity and mortality following TP have decreased as a
result of enhanced perioperative care and surgical techniques, and
the presence of high-volume surgical centers (15). A number of centers have revealed
perioperative mortality and morbidity rates of TP that are
equivalent to those using the Whipple procedure (4). In addition, insulin-dependent diabetes
and malabsorption following TP are better controlled with newer
pharmacological interventions (15). In the present case study, the
patient was administered insulin to control diabetes mellitus, and
used a digestive enzyme to improve malabsorption following TP. The
patient’s blood sugar and body weight currently remain stable and
at normal levels.
Given the outcome of the present case, TP combined
with SMPVR may provide PDAC patients the opportunity for improved
long-term survival and a high quality of life. The TP and SMPV
resection increases the surgical ‘oncologic radicality’ through
extensive resection, and allows the surgeons to obtain negative
margins. In conclusion, those patients with PDAC tumors typically
believed to be unresectable based on pre-operative assessment may
benefit from TP and SMPV resection.
References
|
1
|
Hezel AF, Kimmelman AC, Stanger BZ,
Bardeesy N and Depinho RA: Genetics and biology of pancreatic
ductal adenocarcinoma. Genes Dev. 20:1218–1249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poon RT, Fan ST, Lo CM, et al:
Pancreaticoduodenectomy with en bloc portal vein resection for
pancreatic carcinoma with suspected portal vein involvement. World
J Surg. 28:602–608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou GW, Wu WD, Xiao WD, Li HW and Peng
CH: Pancreatectomy combined with superior mesenteric-portal vein
resection: report of 32 cases. Hepatobiliary Pancreat Dis Int.
4:130–134. 2005.PubMed/NCBI
|
|
4
|
Grace PA, Pitt HA, Tompkins RK, DenBesten
L and Longmire WP Jr: Decreased morbidity and mortality after
pancreatoduodenectomy. Am J Surg. 151:141–149. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dresler CM, Fortner JG, McDermott K and
Bajorunas DR: Metabolic consequences of (regional) total
pancreatectomy. Ann Surg. 214:131–140. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama Y, Nimura Y and Nagino M:
Advances in the treatment of pancreatic cancer: limitations of
surgery and evaluation of new therapeutic strategies. Surg Today.
39:466–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aramaki M, Matsumoto T, Etoh T, et al:
Clinical significance of combined pancreas and portal vein
resection in surgery for pancreatic adenocarcinoma.
Hepatogastroenterology. 50:263–266. 2003.PubMed/NCBI
|
|
8
|
Allema JH, Reinders ME, van Gulik TM, et
al: Prognostic factors for survival after pancreaticoduodenectomy
for patients with carcinoma of the pancreatic head region. Cancer.
75:2069–2076. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibata C, Kobari M, Tsuchiya T, et al:
Pancreatectomy combined with superior mesenteric-portal vein
resection for adenocarcinoma in pancreas. World J Surg.
25:1002–1005. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rockey EW: Total pancreatectomy for
carcinoma: case report. Ann Surg. 118:603–611. 1943. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross DE: Cancer of the pancreas; a plea
for total pancreatectomy. Am J Surg. 87:20–33. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
ReMine WH, Priestley JT, Judd ES and King
JN: Total pancreatectomy. Ann Surg. 172:595–604. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stauffer JA, Nguyen JH, Heckman MG, et al:
Patient outcomes after total pancreatectomy: a single centre
contemporary experience. HPB (Oxford). 11:483–492. 2009. View Article : Google Scholar
|
|
14
|
Reddy S, Wolfgang CL, Cameron JL, et al:
Total pancreatectomy for pancreatic adenocarcinoma: evaluation of
morbidity and long-term survival. Ann Surg. 250:282–287. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarr MG, Behrns KE and van Heerden JA:
Total pancreatectomy. An objective analysis of its use in
pancreatic cancer. Hepatogastroenterology. 40:418–421.
1993.PubMed/NCBI
|