Introduction
As the paratesticular region contains various
structures, including the epididymis, spermatic cord, tunica
vaginalis and strong fat-ligament-muscle supporting tissues, it may
give rise to a number tumor types with various behaviors (1). Tumor variability may also be due to
the Wolffian duct origin of the testis appendages, including the
spermatic cord. The most significant feature of the paratesticular
region is that it is the origin of a small number of tumors with
rich diversity.
The majority of the masses within the scrotum in
adults are of testicular origin. Paratesticular masses account for
2–3% and sarcomas account for ~30% of all scrotal masses (1–4). The
most common type of sarcoma is liposarcoma, followed by
leiomyosarcoma (LMS), rhabdomyosarcoma (RMS), undifferentiated
pleomorphic sarcoma and fibrosarcoma (1–6). To
the best of our knowledge, only one instance of low-grade
fibromyxoid sarcoma in a paratesticular location has been
previously reported (7).
Determining the association between the
paratesticular mass and the testicle, and differentiation between
benign and malignant masses using radiology is challenging,
therefore the lesions are usually considered to be malignant and
radical orchiectomy with high ligation is used. The prognosis is
often poor, as recurrence and metastasis are common, and the
mechanism and outcome of regional lymph node resection,
radiotherapy and chemotherapy is unclear. The present study reports
seven cases of paratesticular sarcoma and emphasizes the
significant clinical and histological features.
Case reports
Seven cases of paratesticular sarcoma diagnosed at
the Pathology Department of the Istanbul Education and Research
Hospital (Istanbul, Turkey) are retrospectively investigated.
Hematoxylin and eosin and immunohistochemical staining of the cases
were reevaluated and accurately diagnosed according to the recent
World Health Organization classification (8). The clinical information of the
patients was obtained from the patient files. Written informed
consent was obtained from all patients. All patients had been
referred to the Urology clinic at Istanbul Education and Research
Hospital with a growing scrotal mass. Excisional biopsy and simple
orchiectomy were performed in cases three and four and the two
patients were subsequently diagnosed with fibromyxoid sarcoma.
Radical orchiectomy was performed in the other five cases. Cases
one, two and three did not receive any additional treatment,
whereas chemotherapy and radiotherapy was adminstered to case five.
In cases four, six and seven, re-excision was performed due to
recurrence, and chemotherapy and radiotherapy were adminsitered
following re-excision.
The clinical features are summarized in Table I. The macroscopic and histological
characteristics of the cases were as follows: The lesions in cases
one and two consisted of large yellow (lipomatous) and
well-delineated areas, with occasional tan-gray colored
(leiomyosarcomatous) areas. The diameter of the leiomyosarcomatous
area was 8 cm in case one and 5 cm in case two. In addition, a few
gray-colored nodules, the largest with a 1 cm diameter, were
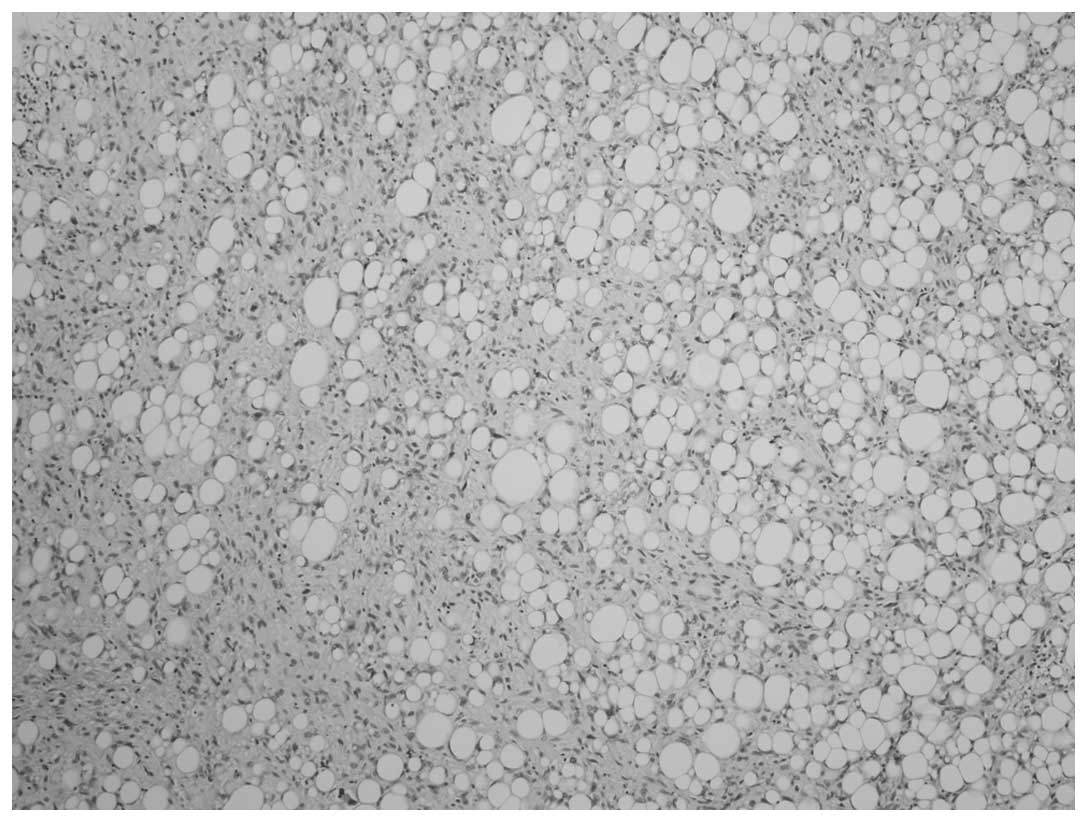
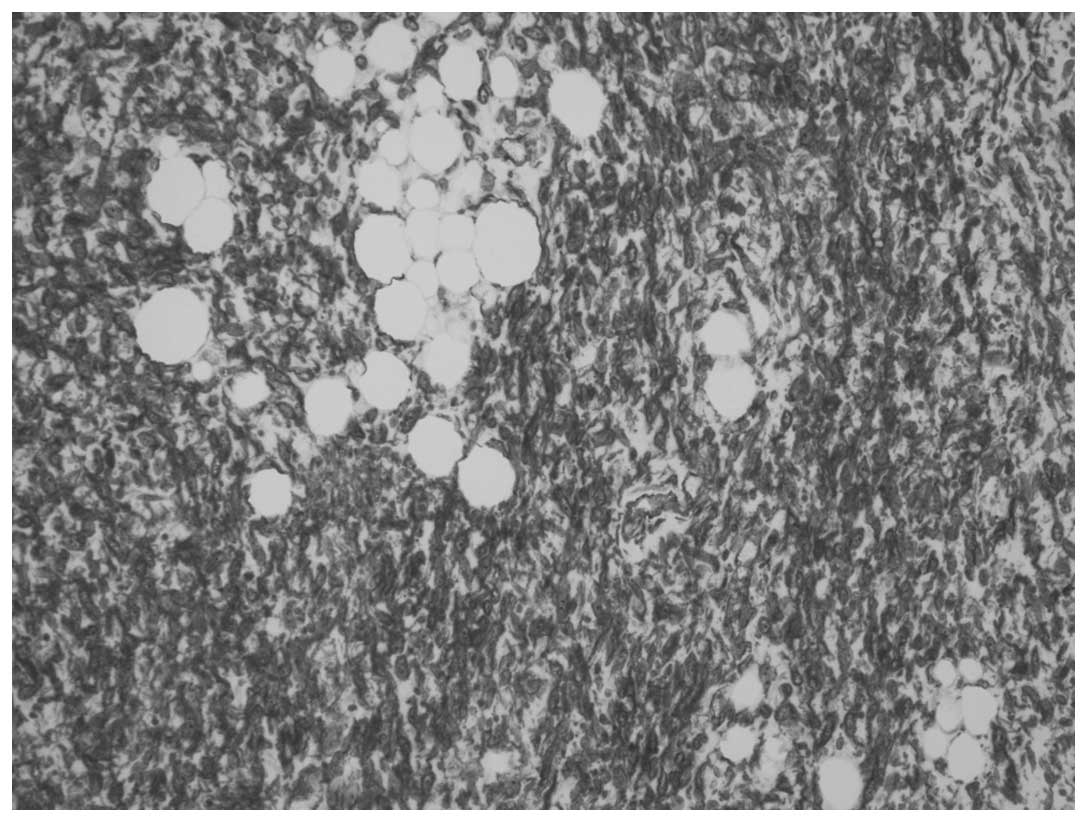
present in case two. A homologous pattern consisting of
well-differentiated liposarcoma, comprising predominantly spindle
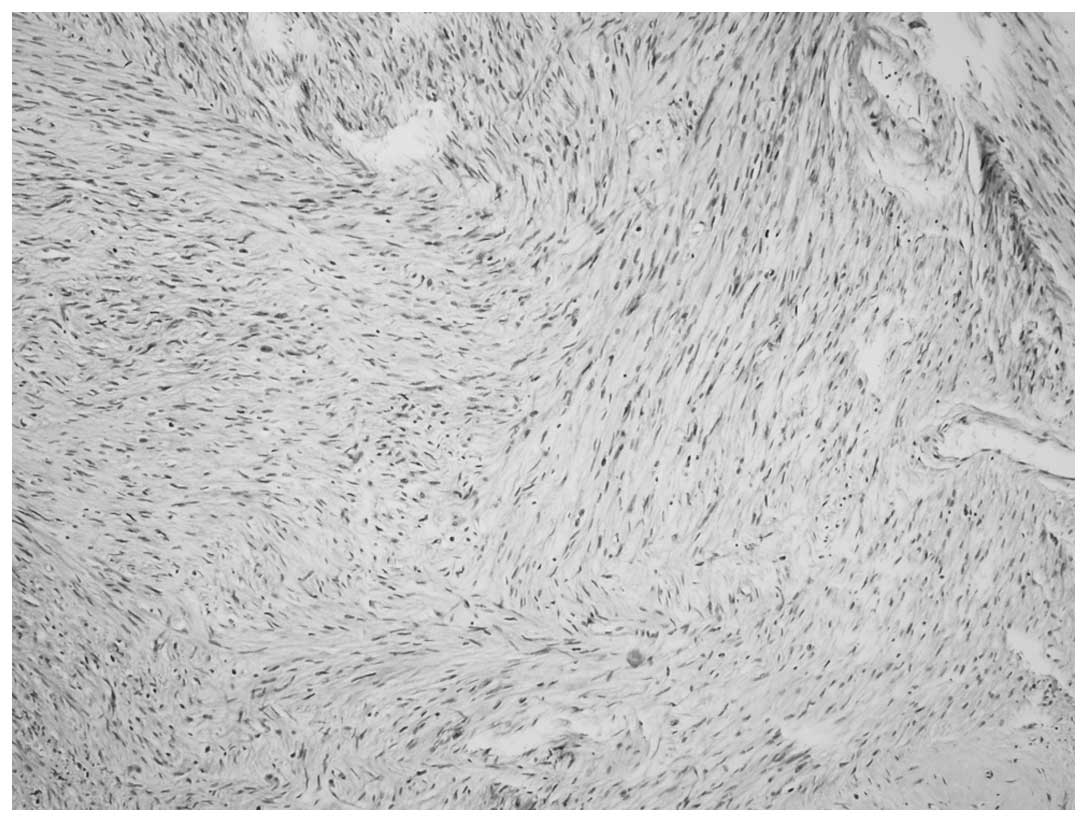
cells, was present in each case (Fig.
1). The heterologous pattern consisted of a LMS and
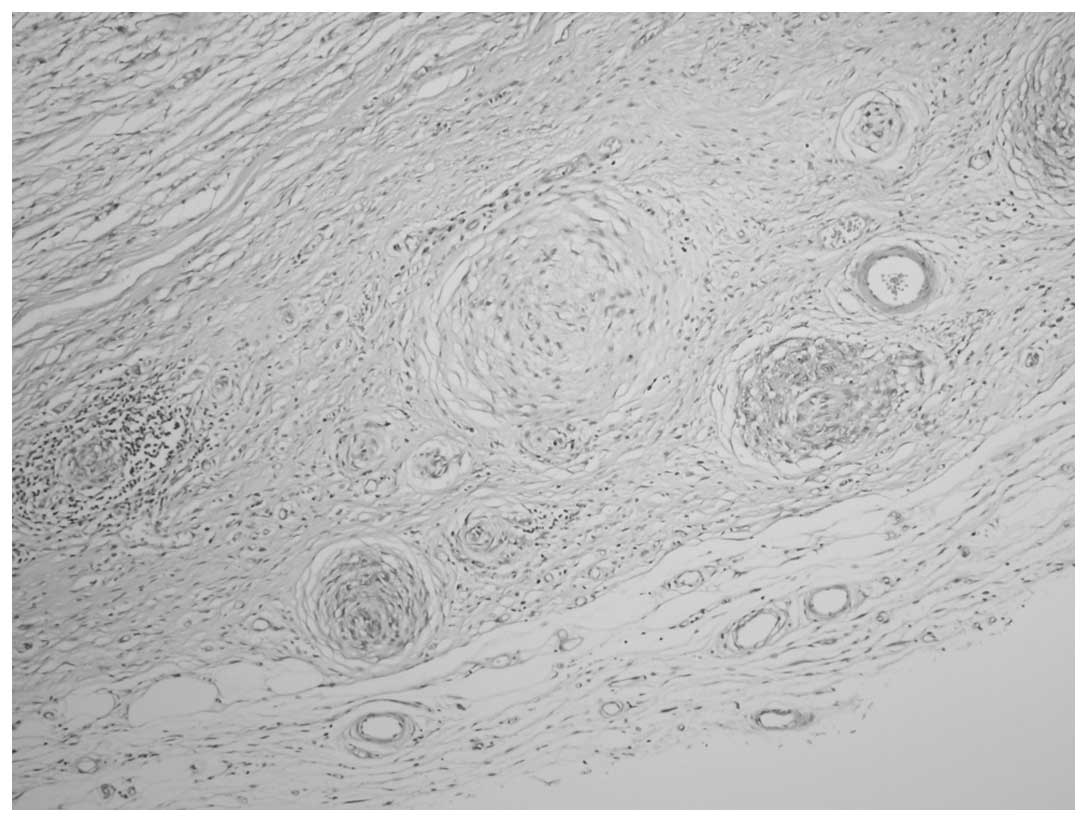
meningothelial-like whorl component in each case and a low-grade
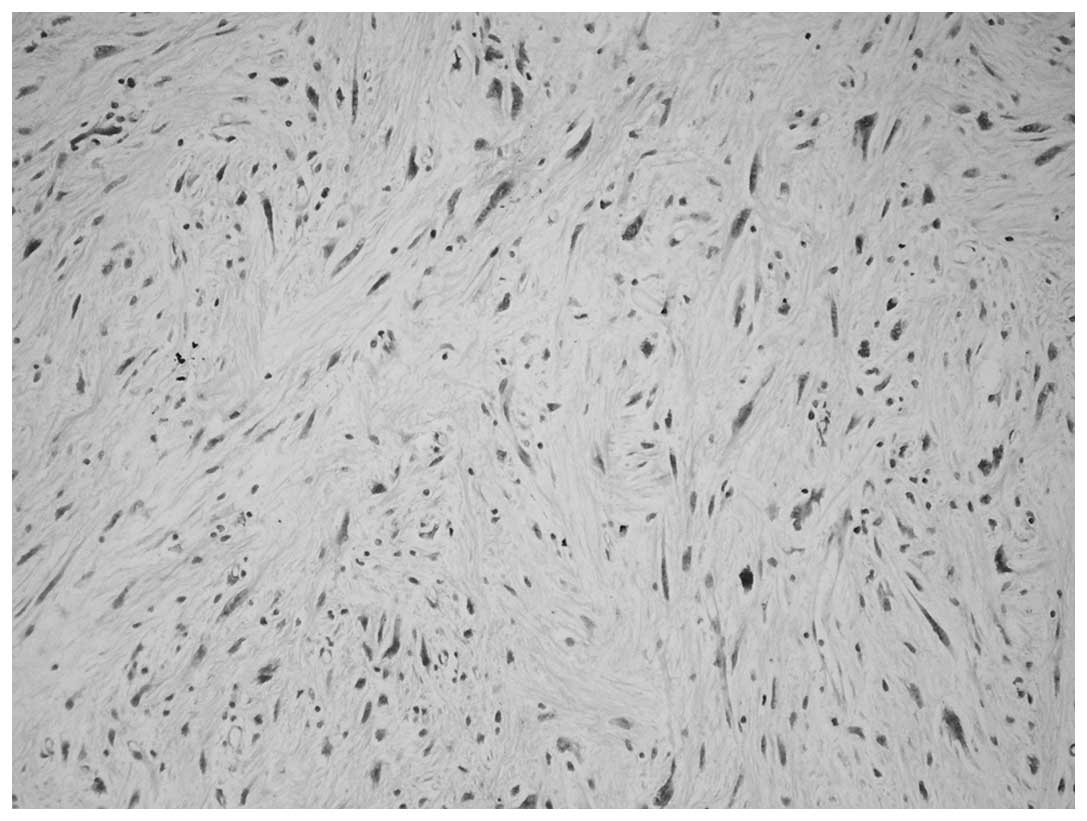
chondrosarcoma component in case one was also present (Figs. 2 and 3). Immunohistochemical staining of the
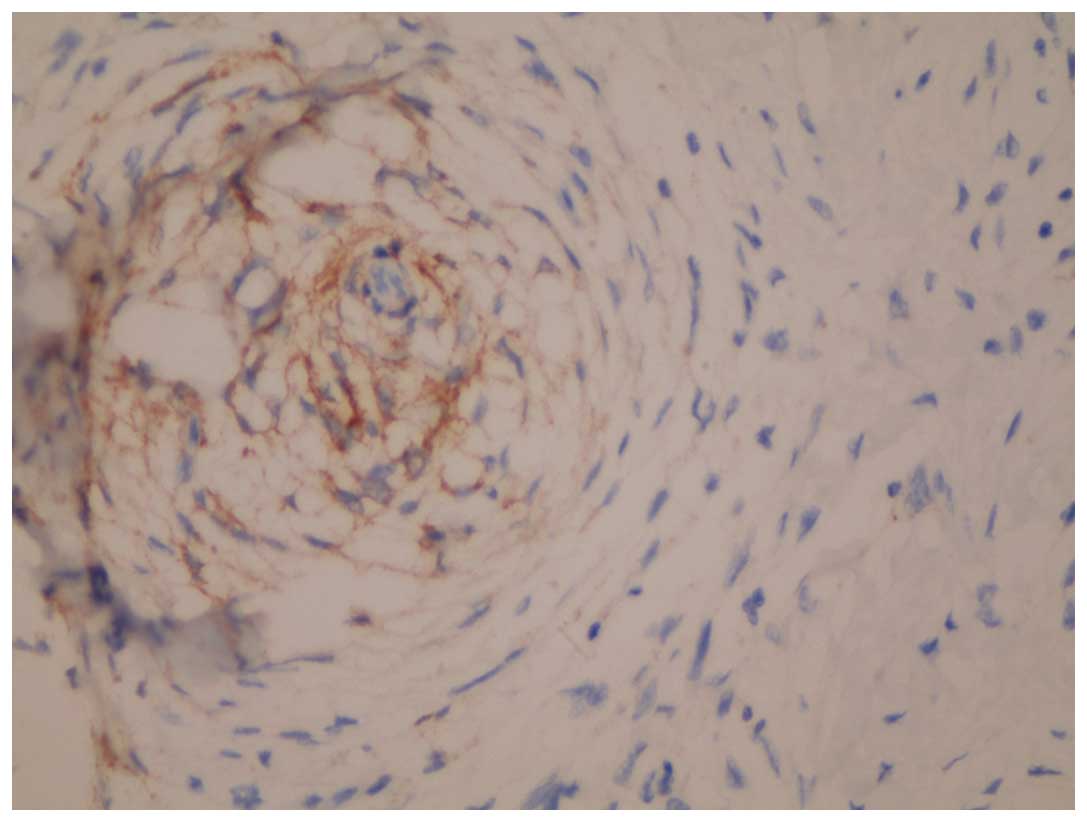
cells revealed that the whorl pattern area was positive for
epithelial membrane antigen (EMA; Fig.
4). A strong positive result for cluster of differentiation
(CD)34 was observed in the spindle cells within the spindle cell
liposarcoma areas, regarded as the homologous component (Fig. 5). The two cases were diagnosed as
leiomyosarcomatosis and dedifferentiated liposarcoma (DDLS)
containing whorl-pattern areas.
 | Table IClinical findings and diagnosis of
paratesticular sarcoma. |
Table I
Clinical findings and diagnosis of
paratesticular sarcoma.
| Case | Age, years | Diagnosis | Tumor size, cm | Treatment | Additional
treatment | Follow-up time,
months | Disease outcome |
|---|
| 1 | 70 | Dedifferentiated
liposarcoma | 13.0 | Radical orchiectomy
with high cord ligation | None | 14 | Survival |
| 2 | 38 | Dedifferentiated
liposarcoma | 13.0 | Radical orchiectomy
with high cord ligation | None | 8 | Survival |
| 3 | 72 | Fibromyxoid
sarcoma | 7.0 | Excisional
biopsy | None | 22 | Mortality |
| 4 | 63 | Fibromyxoid
sarcoma | 9.0 | Simple
orchiectomy | Re-resection, CTh +
RTh | 43 | Mortality, recurrence
with lung metastasis |
| 5 | 64 | Leiomyosarcoma | 6.5 | Radical orchiectomy
with high cord ligation | CTh + RTh | 21 | Mortality, recurrence
with lung metastasis |
| 6 | 68 | Leiomyosarcoma | 7.4 | Radical orchiectomy
with high cord ligation | Re-resection, CTh +
RTh | 18 | Survival,
recurrence |
| 7 | 46 | Undifferentiated
pleomorphic sarcoma | 4.2 | Radical orchiectomy
with high cord ligation | Re-resection, CTh +
RTh | 44 | Mortality,
recurrence |
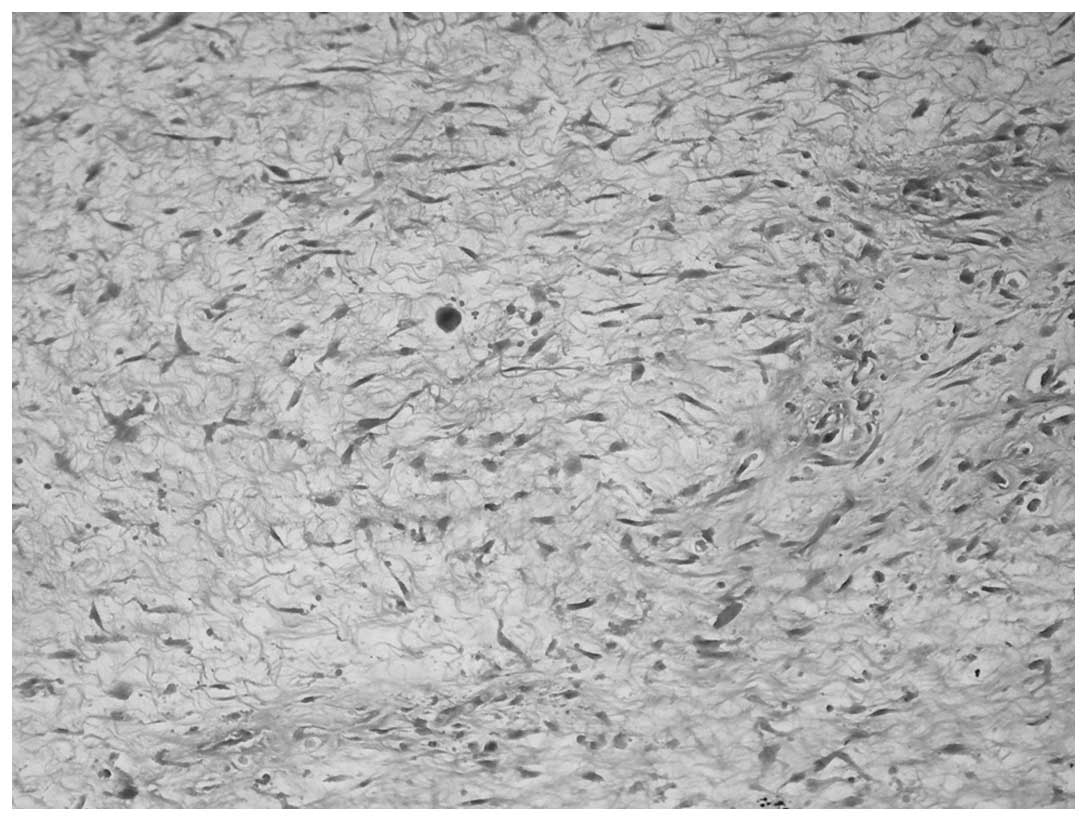
Cases three and four exhibited nodular,
well-delineated tumors with surgical border invasion. Microscopy
revealed the histology to be similar in the two cases, with the
prominent features consisting of a collagen and myxoid zone, mixed
with bland spindle-like fibroblastic cells and a whorl pattern, and
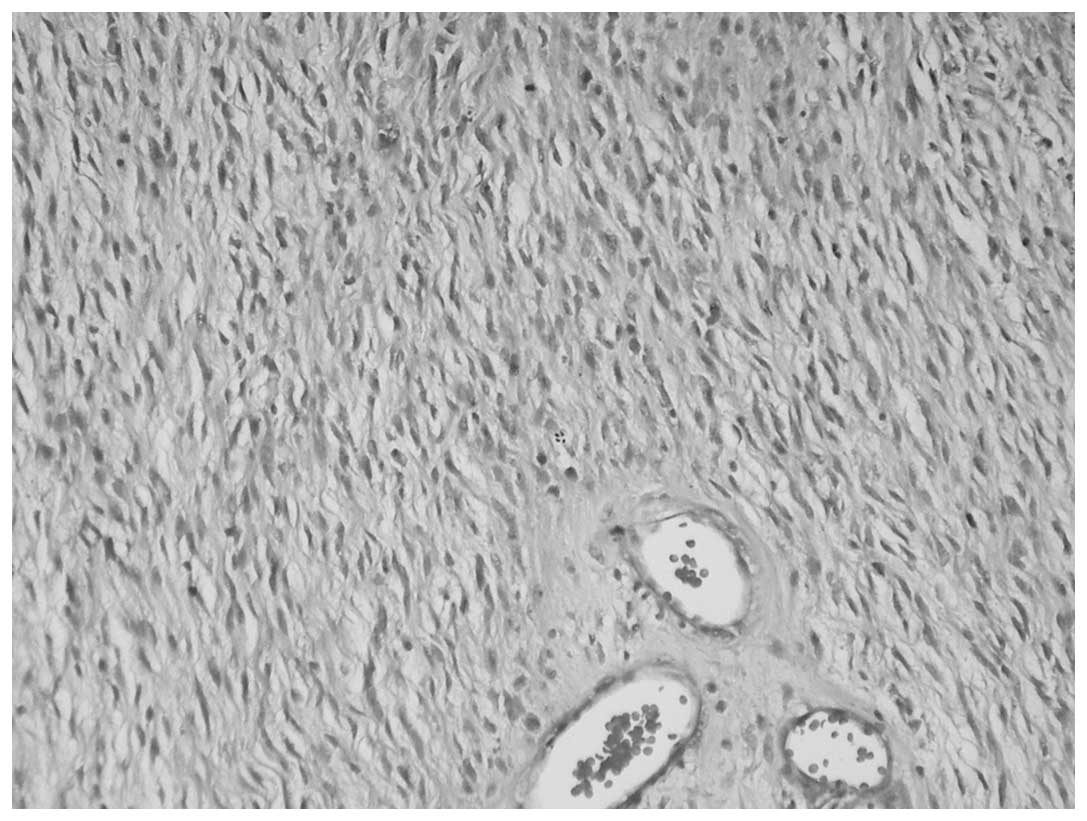
arcades of curvilinear blood vessels (Figs. 6 and 7). The cellularity of the tumors varied
from extremely low to moderate. Pleomorphism and mitosis were
present in the nuclei in a focal area in case four (Fig. 8). Immunohistochemical staining for
vimentin and MUC4 yielded a positive result in the two cases,
together with focal CD34 and EMA positivity. The patients were
diagnosed with low-grade fibomyxoid sarcoma.
The masses in cases five and six were
well-circumscribed and nodular, with long bundles that were
parallel or perpendicular to each other. The cell cytoplasm was
strongly eosinophilic and the nuclei were generally spindle shaped,
with one blunt end. Mitosis was not frequent, with 1–3 mitoses per
10 high power fields. Necrosis was observed in focal areas and
pleomorphism was moderate. Immunohistochemical staining for smooth
muscle actin and desmin yielded a positive result. The two cases
were diagnosed as LMS.
Macroscopically, the mass in case seven had
infiltrative borders, and, microscopically, was rich in spindle
cells, with small bundles and storiform patterns. The tissue also
contained large pleomorphic cells, with large eosinophilic
cytoplasm in certain areas. Immunohistochemical staining yielded a
positive result for vimentin only. This case was diagnosed as
undifferentiated pleomorphic sarcoma.
Discussion
Paratesticular sarcomas are rare and account for ~2%
of all soft tissue sarcomas (9,10). No
clear approach is available regarding their behavior and treatment
due to their rarity. Liposarcoma and LMS are the most common
sarcomas (1–6). RMS is more frequent in younger
patients (6). The development of
various paratesticular neoplasia is due to the differing complex
structures in the region. In addition, embryological development of
the spermatic cord, the most common tumor localization, and the
other testicular adnexal structures from the Wolffian duct may be
responsible for this histological diversity.
The histological features of the three cases
diagnosed with LMS and undifferentiated pleomorphic sarcoma were
typical and created no diagnostic problems. However, the two DDLS
cases exhibited extremely rare histological features.
Although the LMS and whorl patterns observed in the
DDLS cases is not common, they have been described in previous
studies (11–13). Leiomyosarcomatous areas were
observed in each of the DDLS cases. The cases exhibited low-grade
morphology and extremely low rates of mitosis, with mild
pleomorphism in focal areas. The positive immunohistochemical
staining for EMA in the whorl pattern areas was noteworthy and
suggested perineural or meningeal differentiation. Meningothelial
whorl-like morphology is less frequently observed than LMS. EMA was
present in each case and, to the best of our knowledge, these are
the first DDLS cases positive for EMA to be reported.
A further feature of the DDLS cases was the dominant
spindle cell liposarcoma as a homologous component. The
denomination of spindle cell liposarcoma remains controversial;
certain authors use spindle-cell neoplasm or spindle cell
lipoma-like neoplasm (14), and the
recommended name for certain lesions was fibrosarcoma-like lipoid
neoplasm, as determined in a report published by Deylup et
al (15). The authors who
recommend these names do not classify these tumors as liposarcoma,
however, lipoblasts were observed. The two DDLS cases in the
present study were rich in spindle cell lipoma-like (spindle cell
liposarcoma) areas with strong cytoplasmic CD34-positivity, as well
as rich in lipoblasts. These areas, which were the homologous
component for DDLS, should be classified as spindle cell
liposarcoma.
Low-grade fibromyxoid sarcoma is a relatively rare
sarcoma (16–17). The upper extremities and the torso
are the most frequent locations, however, no paratesticular
localization has been previously reported. Low-grade fibromyxoid
sarcoma usually exhibits variable microscopic findings, with bland
fibroblasts, whorls, linear sequencing and less cellular myxoid
sections in certain areas (16).
Mitosis and necrosis are rare. One of the present cases exhibited
typical features of low-grade fibromyxoid sarcoma, while case four
exhibited pleomorphism in focal areas that were also rich in
mitoses.
The accepted treatment for paratesticular masses is
radical inguinal orchiectomy, including the surrounding soft
tissues. No consensus with regard to regional lymph node excision
has been reached, radiotherapy and chemotherapy. In the current
study, radical orchiectomy with high ligation was performed for the
seven cases. Simple orchiectomy and excisional biopsy were
conducted for the two cases with fibromyxoid sarcoma. The two
patients with fibromyxoid sarcoma succumbed to the disease within
22 and 43 months, respectively, as there was residual mass and the
patients did not accept additional treatment. This emphasizes the
importance of radical surgical treatments. No recurrence or
metastasis was observed in the two cases of liposarcoma and the
improved prognosis of liposarcoma compared with the other sarcomas,
or the short clinical follow-up durations may have had an effect on
this finding. As four of the seven cases succumbed to the disease
and one remains alive with the sarcoma demonstrates the requirement
for a multidisciplinary approach to the treatment of paratesticular
sarcomas.
In conclusion, the paratesticular region consists of
complex structures that can develop various neoplastic formations
and patterns. Sarcomas comprise a significant part of
paratesticular masses and may exhibit an aggressive clinical
course. In older patients, paratesticular sarcomas must be
considered for the differential diagnosis of scrotal masses, which
do not exhibit a clear association with the testes. Furthermore,
clinicians and patients must be informed about the high probability
of local recurrence and distant metastasis in paratesticular
sarcomas.
References
|
1
|
Lioe TF and Biggart JD: Tumours of the
spermatic cord and paratesticular tissue. A clinicopathological
study. Br J Urol. 71:600–606. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varzaneh FE, Verghese M and Shmookler BM:
Paratesticular leiomyosarcoma in an elderly man. Urology.
60:11122002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sogani PC, Grabstald H and Whitmore WF Jr:
Spermatic cord sarcoma in adults. J Urol. 120:301–305.
1978.PubMed/NCBI
|
|
4
|
Russo P, Brady MS, Conlon K, Hajdu SI,
Fair WR, Herr HW and Brennan MF: Adult urological sarcoma. J Urol.
147:1032–1036. 1992.PubMed/NCBI
|
|
5
|
Khoubehi B, Mishra V, Ali M, Motiwala H
and Karim O: Adult paratesticular tumours. BJU Int. 90:707–715.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soosay GN, Parkinson MC, Paradinas J and
Fisher C: Paratesticular sarcomas revisited: a review of cases in
the British Testicular Tumour Panel and Registry. Br J Urol.
77:143–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hansen T, Katenkamp K, Brodhun M and
Katenkamp D: Low-grade fibrosarcoma - report on 39 not otherwise
specified cases and comparison with defined low-grade fibrosarcoma
types. Histopathology. 49:152–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: WHO Classification of Tumours of Soft Tissue and
Bone. 5. 4th edition. IARC Press; Lyon: 2013
|
|
9
|
Stojadinovic A, Leung DH, Allen P, Lewis
JJ, Jaques DP and Brennan MF: Primary adult soft tissue sarcoma:
time-dependent influence of prognostic variables. J Clin Oncol.
20:4344–4352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dotan ZA, Tal R, Golijanin D, Snyder ME,
Antonescu C, Brennan MF and Russo P: Adult genitourinary sarcoma:
the 25-year Memorial Sloan-Kettering experience. J Urol.
176:2033–2038. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pilotti S and Pierotti MA:
Well-differentiated liposarcoma with leiomyomatous differentiation.
Am J Surg Pathol. 26:1643–1644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henricks WH, Chu YC, Goldblum JR and Weiss
SW: Dedifferentiated liposarcoma: a clinicopathological analysis of
155 cases with a proposal for an expanded definition of
dedifferentiation. Am J Surg Pathol. 21:271–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fanburg-Smith JC and Miettinen M:
Liposarcoma with meningothelial-like whorls: a study of 17 cases of
a distinctive histological pattern associated with dedifferentiated
liposarcoma. Histopathology. 33:414–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mentzel T, Palmedo G and Kuhnen C:
Well-differentiated spindle cell liposarcoma (‘atypical spindle
cell lipomatous tumor’) does not belong to the spectrum of atypical
lipomatous tumor but has a close relationship to spindle cell
lipoma: clinicopathologic, immunohistochemical, and molecular
analysis of six cases. Mod Pathol. 23:729–736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deyrup AT, Chibon F, Guillou L, Lagarde P,
Coindre JM and Weiss SW: Fibrosarcoma-like lipomatous neoplasm: a
reappraisal of so-called spindle cell liposarcoma defining a unique
lipomatous tumor unrelated to other liposarcomas. Am J Surg Pathol.
37:1373–1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vernon SE and Bejarano PA: Low-grade
fibromyxoid sarcoma: a brief review. Arch Pathol Lab Med.
130:1358–1360. 2006.PubMed/NCBI
|
|
17
|
Folpe AL, Lane KL, Paull G and Weiss SW:
Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor
with giant rosettes: a clinicopathologic study of 73 cases
supporting their identity and assessing the impact of high-grade
areas. Am J Surg Pathol. 24:1353–1360. 2000. View Article : Google Scholar : PubMed/NCBI
|