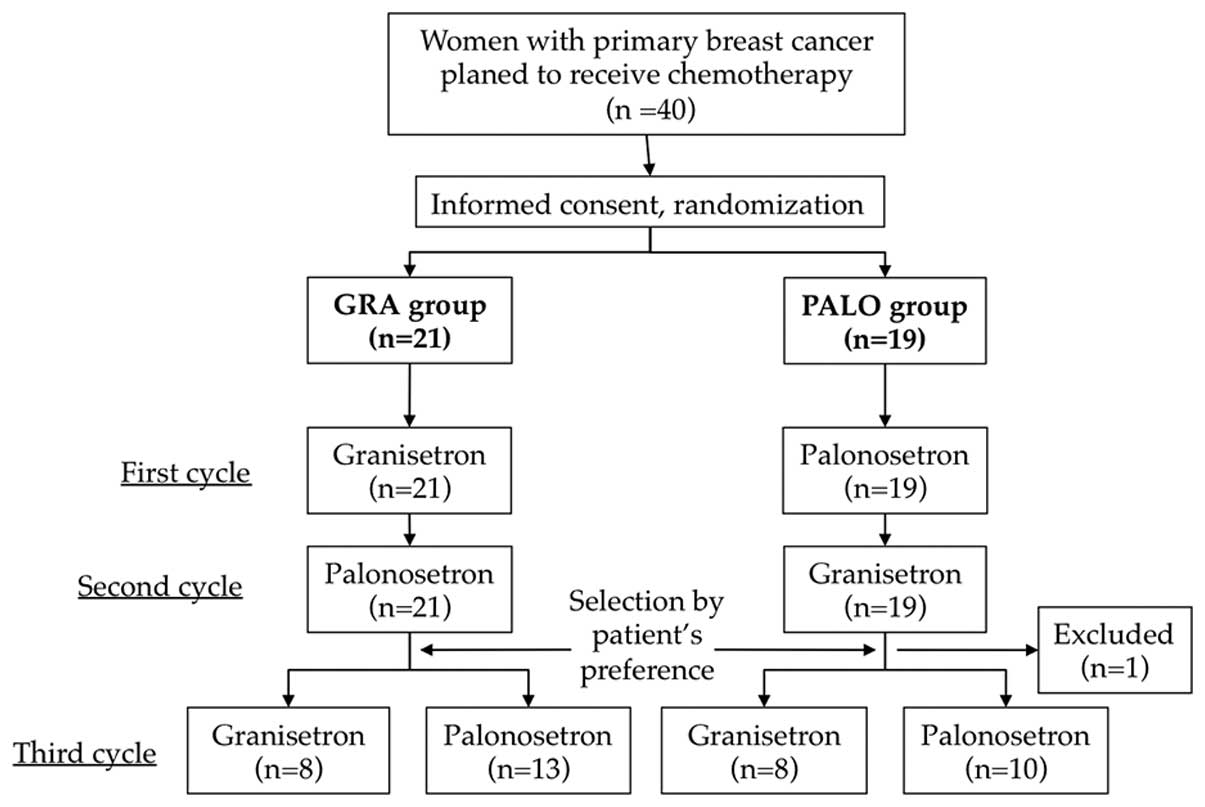
Introduction
Combination chemotherapy regimens for breast cancer,
which include anthracycline drugs and cyclophosphamide [doxorubicin
plus cyclophosphamide (AC); epirubicin plus cyclophosphamide (EC);
and fluorouracil, epirubicin plus cyclophosphamide (FEC)], are
classified as exhibiting a high risk of emesis by the National
Comprehensive Cancer Network in 2012 and American Society of
Clinical Oncology guidelines (1,2). It is
recommended in these guidelines to use a combination of three drugs
[5-hydroxytryptamine (5-HT3) receptor antagonist,
aprepitant (APR) and dexamethasone (DEX)] for antiemetic treatment
(1,2). Recently, a novel 5-HT3
receptor antagonist, palonosetron (PALO), has been identified. PALO
has demonstrated effectiveness against delayed emetic events
(3–5). PALO and APR excel in the prevention of
delayed nausea and vomiting. However, no studies regarding the
comparative efficacy of PALO and the conventional 5-HT3
receptor antagonists used in combination with APR have been
reported.
In the present study, the efficacy of the novel
5-HT3 receptor antagonist, PALO, was compared with that
of the conventional drug granisetron (GRA) for the antiemetic
treatment of breast cancer patients treated with highly emetic
therapeutic regimens that involved anthracyclines and
cyclophosphamide. A crossover administration method was used, with
the administration of two cycles of antiemetic agents. Furthermore,
no studies have investigated the efficacy of such drugs, following
the second cycle and, thus, in the present study, the efficacy of
the drugs were also evaluated following the second cycle.
Materials and methods
Patients
This study was approved by the ethics committee of
Jichi Medical University (B10–68; Tochigi, Japan) and written
informed consent was obtained from all patients. This investigation
was a prospective, stratified randomization, non-blinded, crossover
comparative study. Eligible patients were females (≥20 years; age
range, 35–75 years) with histologically confirmed breast cancer,
who were scheduled to receive chemotherapy including anthracycline
drugs and cyclophosphamide at the Department of Breast Surgery,
Jichi Medical University Hospital. Prior to the first cycle of
chemotherapy, 40 patients were assigned to two groups treated with
PALO or GRA first. The group assignment was performed by simple
randomization using a table of random numbers and patients were
informed of which group they were assigned.
Treatment and evaluation
Chemotherapy was administered every three weeks as
follows: AC treatment, adriamycin (60 mg/m2) and
cyclophosphamide (600 mg/m2); EC treatment, epirubicin
(90 mg/m2) and cyclophosphamide (600 mg/m2);
FEC treatment, 5-fluorouracil (500 mg/m2), epirubicin
(100 mg/m2) and cyclophosphamide (500 mg/m2).
Patients were assigned to the PALO or GRA group in the first cycle,
as described above. For the second cycle of treatment, patients
switched to the other medication (GRA followed by PALO or PALO
followed by GRA). Prior to beginning the third cycle, patients
selected GRA or PALO based on their preferences, and chemotherapy
was continued (Fig. 1).
As an antiemetic treatment prior to chemotherapy,
APR (125 mg) was orally administered 1 h prior to treatment, and
PALO (0.75 mg) or GRA (3 mg) was administered in addition to DEX
(13.2 mg) 30 min prior to chemotherapy by intravenous infusion.
Chemotherapy was then administered. APR (80 mg) was orally
administered on days two and three following chemotherapy, and DEX
(8 mg) was administered orally on days two, three and four
following chemotherapy (Table I).
When additional antiemetic treatment was required, metoclopramide
was administered orally, or additional APR was administered orally
on the fourth and fifth days following chemotherapy.
 | Table ISchedule for administration of
antiemetic drugs. |
Table I
Schedule for administration of
antiemetic drugs.
| Antiemetic
regimen | Drug (administration
method) | Day 1 | Day 2 | Day 3 | Day 4 |
|---|
| Palonosetron | Palonosetron | i.v. | 0.75 mg | | | |
| Aprepitant | p.o. | 125 mg | 80 mg | 80 mg | |
| Dexamethasone | i.v. | 13.2 mg | | | |
| Dexamethasone | p.o. | | 8 mg | 8 mg | 8 mg |
| Granisetron | Granisetron | i.v. | 3 mg | | | |
| Aprepitant | p.o. | 125 mg | 80 mg | 80 mg | |
| Dexamethasone | i.v. | 13.2 mg | | | |
| Dexamethasone | p.o. | | 8 mg | 8 mg | 8 mg |
To evaluate instances of nausea and vomiting,
patients were asked to complete a questionnaire on antiemetics, as
well as a patient log. Adverse effects and blood tests were
evaluated prior to each cycle of chemotherapy, and attending
physicians decided whether to continue chemotherapy in accordance
with the criteria used for usual care.
The antiemetic efficacy of the drugs was evaluated
until the third cycle of chemotherapy was completed. The efficacy
was rated on the basis of complete control of acute and delayed
vomiting (complete response; CR) and complete control of emetic
events (complete control; CC). CR was defined as no emetic episode
and no additional antiemetic treatment. CC was defined as no emetic
episode, no additional antiemetic treatment, and no more than mild
nausea.
Participation in the study was discontinued for the
following reasons: If general and disease status became worse than
that prior to study participation; the attending physician judged
the continuation of chemotherapy to be difficult; the patient
requested to withdraw from the study or withdrew consent; or the
circumstances of the patient made continuation impossible.
Statistical analysis
Statistical analyses were performed using JMP
statistical software, version 10 (SAS, Institute Inc., Cary, NC,
USA). The χ2 test was used for statistical analysis, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients
A total of 19 patients received PALO first and 21
patients received GRA first. Among the PALO-first group, the
third-cycle questionnaire was not obtained from one patient and,
thus, this case was withdrawn from analysis of the third cycle
(Fig. 1). The median ages of the
patients in the GRA-first group were 53 years (range, 40–71 years)
and 53 years (range, 35–75 years) in the GRA and PALO-first groups,
respectively (Table II).
 | Table IIPatients characteristics. |
Table II
Patients characteristics.
| Parameter | GRA group (n=21) | PALO group
(n=19) |
|---|
| Median age, years
(range) | 53 (40–71) | 53 (35–75) |
| Menopause status, n
(%) |
| Premenopause | 10 (47.6) | 10 (52.6) |
| Postmenopause | 11 (52.4) | 9 (47.4) |
| ECOG performance
status, n (%) (6) |
| 0 | 21 (100.0) | 19 (100.0) |
| Chemotherapy regimen,
n (%) |
| FEC | 11 (52.4) | 12 (63.2) |
| AC/EC | 10 (47.6) | 7 (36.8) |
| Timing of
chemotherapy, n (%) |
| Neoadjuvant | 19 (90.5) | 16 (84.2) |
| Adjuvant | 2 (9.5) | 3 (15.8) |
Treatment efficacy in the first
cycle
In the first cycle, acute-phase CC was observed in
47.6% and 57.9% of patients of the GRA-first group and PALO-first
groups, respectively (P=0.515) (Fig.
2). Acute-phase CR was observed in 71.4 and 73.7% patients of
the GRA-first and PALO-first groups, respectively (P=0.873).
Delayed-phase CC was observed in 57.1 and 71.4% of patients in the
GRA-first and PALO-first groups, respectively (P=0.461).
Delayed-phase CR was observed in 71.4 and 73.7% of patients in the
GRA-first and PALO-first groups, respectively (P=0.873).
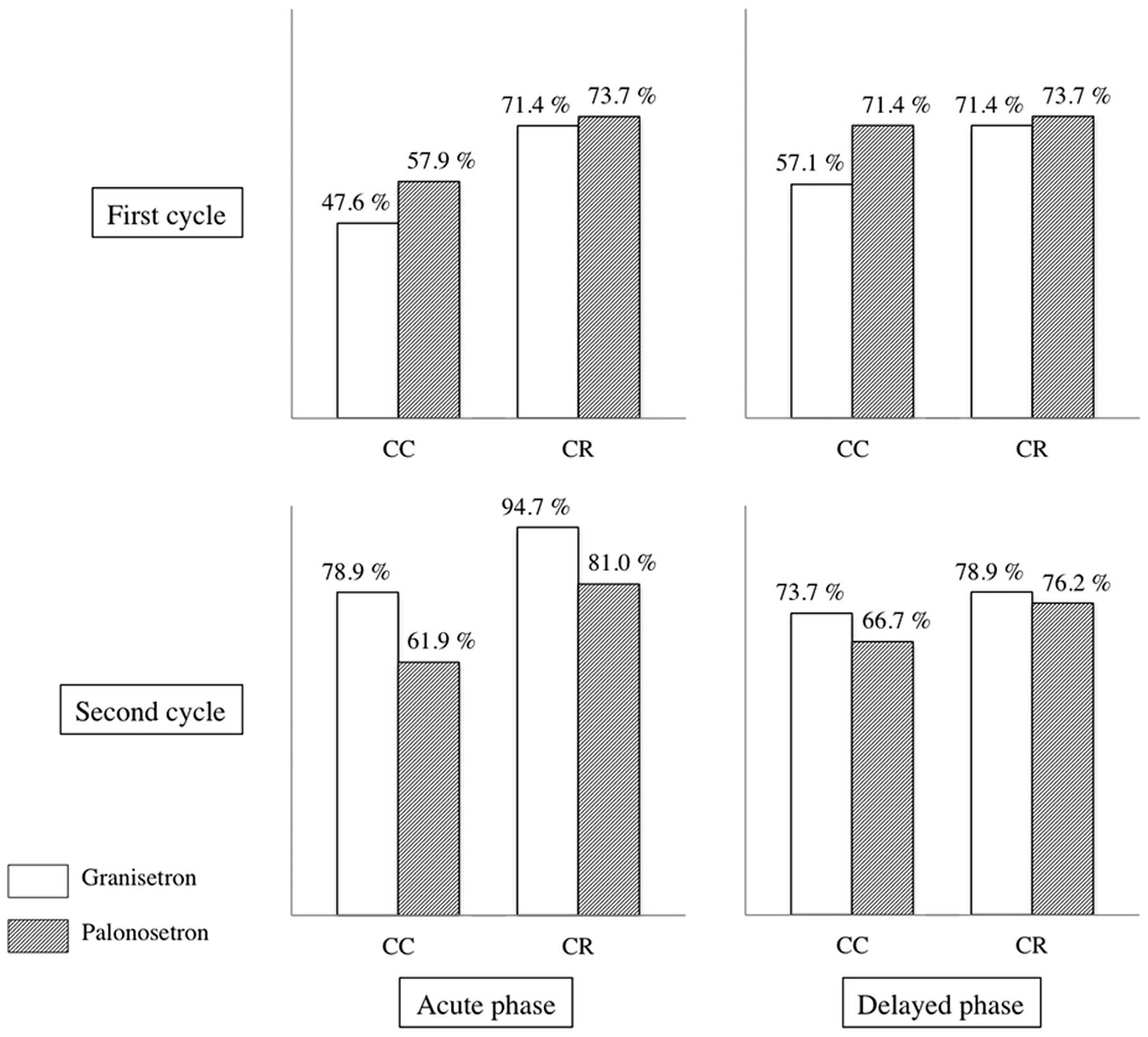 | Figure 2Antiemetic efficacy of granisetron and
palonosetron in the first and second cycles of chemotherapy. The
efficacy was evaluated by CC rate and CR rate. In the first cycle,
granisetron and palonosetron were administered to GRA-first group
and PALO-first group, respectively. In the second cycle, the
antiemetics were switched. Therefore, granisetron and palonosetron
were administered to PALO-first group and GRA-first group,
respectively. No significant differences in CC or CR were
identified between granisetron and palonosetron. CC, complete
control (no emetic episode, no additional antiemetic treatment and
no more than mild nausea); CR, complete response (no emetic episode
and no additional antiemetic treatment); GRA, granisetron; PALO,
palonosetron. |
Treatment efficacy in the second
cycle
In the second cycle, acute-phase CC of emetic events
was observed in 61.9 and 78.9% of patients in the GRA-first and
PALO-first groups, respectively (P=0.240) (Fig. 2). Acute-phase CR was observed in
81.0 and 94.7% of patients in the GRA-first and PALO-first groups,
respectively (P=0.019). Delayed-phase CC was observed in 66.7 and
73.7% of patients in the GRA-first and PALO-first groups,
respectively (P=0.628). Delayed-phase CR was observed in 76.2 and
78.9% of patients in the GRA-first and PALO-first groups,
respectively (P=0.834).
Treatment efficacy in the third
cycle
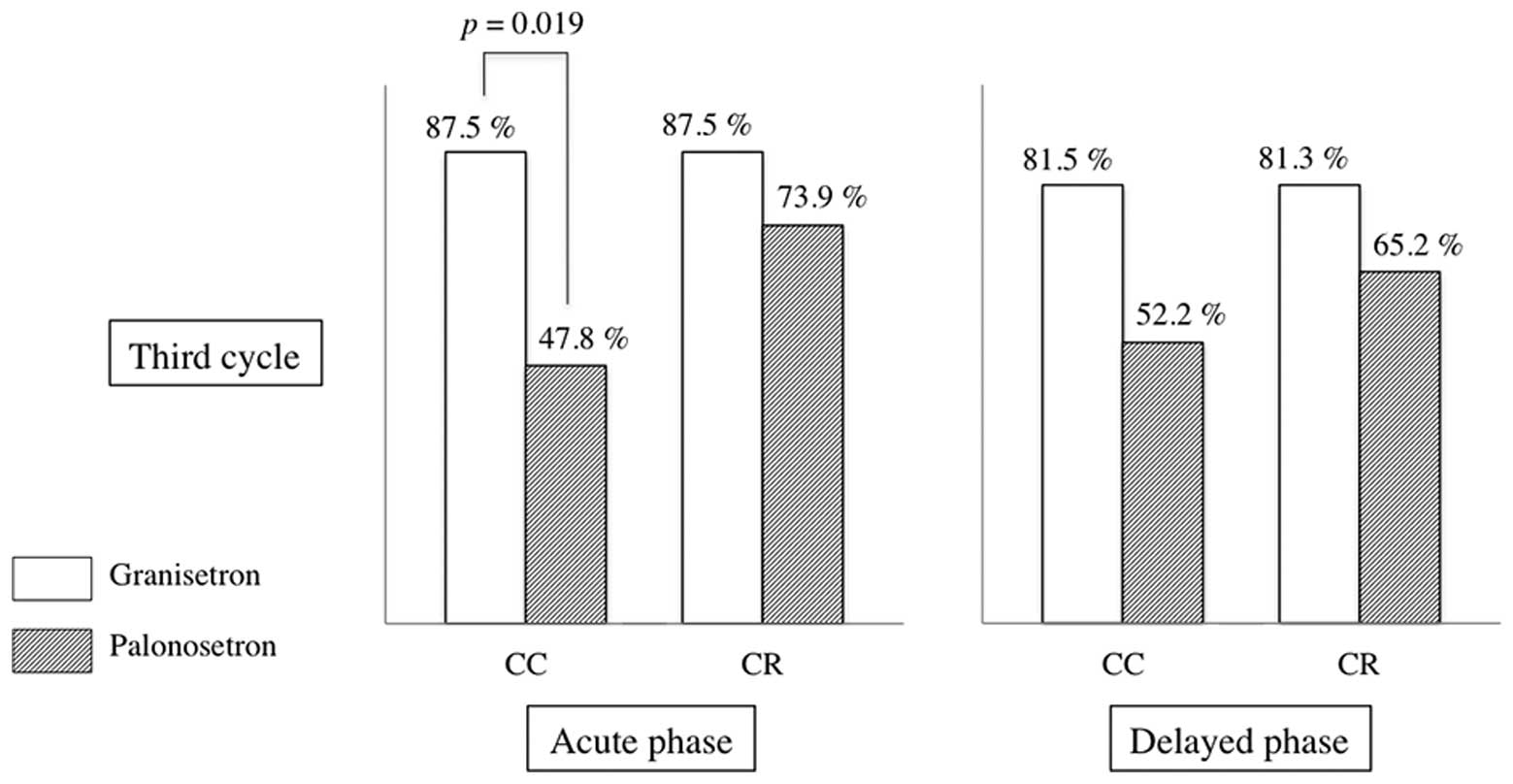
In the third cycle, a total of 46.2% of the patients
(18/39) selected GRA and 53.8% (21/39) selected PALO. In the third
cycle, a significant difference in acute-phase CC of emetic events
was identified between PALO and GRA treatment groups. Acute-phase
CC was observed in 87.5 and 47.8% of patients in the GRA-selection
and PALO-selection groups, respectively (P=0.011) (Fig. 3). Acute-phase CR was observed in
87.5 and 73.9% of patients in the GRA-selection and PALO-selection
groups, respectively (P=0.301). Delayed-phase CC was observed in
81.3 and 52.2% of patients in the GRA-selection and PALO-selection
groups, respectively (P=0.273). Delayed-phase CR was observed in
81.3 and 65.2% of patients in the GRA-selection and PALO-selection
groups, respectively (P=0.273).
Discussion
To the best of our knowledge, this was the first
study to use crossover administration of first- and second-cycle
antiemetic agents in association with chemotherapy for breast
cancer to compare the efficacy of these agents. The majority of
previous studies have evaluated the efficacy of antiemetic agents
following the first cycle of chemotherapy, and few studies
regarding the efficacy from the second cycle onwards have been
performed. In the current study, the efficacy of the drugs
following the second and third cycles was also evaluated. In the
GRA- and PALO-first groups, the prevalence of acute-phase CC
increased between the first to second cycle. In addition, the
prevalence of acute-phase CC in the third cycle decreased in
patients who selected PALO treatment. This result indicated that an
order effect was exhibited in PALO followed by GRA and GRA followed
by PALO patients, and a carry over effect was exhibited in PALO
followed by PALO patients. Considering these effects, antiemetic
treatment in breast cancer chemotherapy requires a refined
administration design for optimal efficacy.
As side effects of breast cancer chemotherapy,
nausea and vomiting are often problematic (1). Emetic events lead to a decrease in
appetite and body weight, reducing the quality of life (1,7). In
addition, the dose of chemotherapy is considered to have an impact
on prognosis. Identifying ways to complete chemotherapy with fewer
side effects is important to improve the treatment outcome. AC, EC
and FEC, representative chemotherapy regimens for perioperative
early-stage breast cancer, are anthracycline-based regimens with a
high emetic risk, which require effective prevention of
chemotherapy-induced nausea and vomiting (CINV). Treatment-related
factors and patient-related factors are associated with CINV.
Treatment-related factors include the type and dose of anti-cancer
drugs and patient-related factors include women aged <50 years
with no history of pregnancy and with no history of alcohol
consumption (8–10).
To date, APR and PALO have been reported to exhibit
effective delayed antiemetic effects (1,3).
However, the efficacy of these agents in combination has not been
investigated. APR primarily affects the vomiting reaction pathway
in the central nervous system (CNS) and has selective neurokinin-1
(NK-1) receptor antagonist actions (1). It is hypothesized to prevent and
control acute and delayed nausea and vomiting. CINV develops when
the vomiting center in the medulla oblongata receives a stimulus.
The two main pathways for this stimulus have been hypothesized to
be the CNS pathway and peripheral pathways. NK-1 receptors, which
bind substance P and 5-HT3 receptors that bind serotonin
are known to be involved in this process. Substance P is
hypothesized to be dominant in the CNS pathway and 5-HT3
is considered to be dominant in the peripheral pathway (11,12).
For acute-phase emesis, the two receptors are associated with
vomiting. However, in the case of delayed emesis, the impact of
substance P is considered to become dominant (12), which is regarded to be a cause for
limited antiemetic action of 5-HT3 receptor antagonists
for delayed vomiting.
PALO and GRA are 5-HT3 receptor
antagonist antiemetic agents. PALO differs from conventional drugs
as it has an extremely long half-life in the blood (~40 h), as well
as high affinity and selectivity for 5-HT3 receptors.
Thus, it has been identified to be efficacious for the treatment of
delayed nausea and vomiting, which occur ≥24 h following
chemotherapy. The delayed effects of PALO are considered to be a
result of its slow release after binding to the receptors, with a
reported continuation of receptor inhibition of >96 h. It has
also been reported that PALO induces internalization of the
receptor on the cell surface, causing allosteric downregulation
(4,13,14)
and that PALO controls substance P independently of serotonin
(15). In the present study, no
significant difference was identified between delayed vomiting in
the PALO-first and GRA-first groups. However, the efficacy of
5-HT3 receptor antagonist antiemetic drugs against
delayed vomiting may be masked by the administration of APR.
A limitation of the present study was the small
patient cohort. Although, by employing the prospective study
design, the cohort was considered to be sufficient. The evaluation
of vomiting and nausea is difficult; however, the evaluation of CC
and CR was possible via the use of patient logs and survey
questionnaires. It has been reported that psychological elements
also have an impact on nausea. Psychological aspects were not
considered in this study; however, these factors may have exhibited
an effect on drug selection for the third cycle or order effects. A
crossover treatment was used in this study. However, each drug had
a short half-life and, thus, in terms of the three-week drug
intervals, it is hypothesized that the effects of the drugs on the
next cycle administered prior to the start of the cycle were
small.
In conclusion, the GRA-selection group in the third
cycle exhibited a significant difference in acute-phase CC and CR
when compared with the PALO group, and the effect of vomit control
was observed. No significant difference between delayed-phase CC
and CR was identified, and APR and PALO did not affect each other.
These results differ from those reported previously. However, the
effects of PALO may have been inhibited due to the presence of APR.
Hence, considering order or carry over effects, a novel three-drug
antiemetic regimen involving PALO in the first cycle followed by
GRA in later cycles may present a novel treatment for breast cancer
patients.
References
|
1
|
Ettinger DS, Armstrong DK, Barbour S, et
al; National Comprehensive Cancer Network. Antiemesis. J Natl Compr
Canc Netw. 10:456–485. 2012.PubMed/NCBI
|
|
2
|
Basch E, Hesketh PJ, Kris MG, Prestrud AA,
Temin S and Lyman GH: Antiemetics: american society of clinical
oncology clinical practice guideline update. J Oncol Pract.
7:395–398. 2011. View Article : Google Scholar :
|
|
3
|
Botrel TE, Clark OA, Clark L, Paladini L,
Faleiros E and Pegoretti B: Efficacy of palonosetron (PAL) compared
to other serotonin inhibitors (5-HT3R) in preventing
chemotherapy-induced nausea and vomiting (CINV) in patients
receiving moderately or highly emetogenic (MoHE) treatment:
systematic review and meta-analysis. Support Care Cancer.
19:823–832. 2011. View Article : Google Scholar
|
|
4
|
Schwartzberg L, Barbour SY, Morrow GR,
Ballinari G, Thorn MD and Cox D: Pooled analysis of phase III
clinical studies of palonosetron versus ondansetron, dolasetron,
and granisetron in the prevention of chemotherapy-induced nausea
and vomiting (CINV). Support Care Cancer. 22:469–477. 2014.
View Article : Google Scholar :
|
|
5
|
Saito M, Aogi K, Sekine I, et al:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: a double-blind, double-dummy, randomised, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen L, de Moor CA, Eisenberg P, Ming EE
and Hu H: Chemotherapy-induced nausea and vomiting: incidence and
impact on patient quality of life at community oncology settings.
Support Care Cancer. 15:497–503. 2007. View Article : Google Scholar
|
|
8
|
Tonato M, Roila F and Del Favero A:
Methodology of antiemetic trials: a review. Ann Oncol. 2:107–114.
1991.PubMed/NCBI
|
|
9
|
Roila F, Tonato M, Basurto C, et al:
Antiemetic activity of high doses of metoclopramide combined with
methylprednisolone versus metoclopramide alone in cisplatin-treated
cancer patients: a randomized double-blind trial of the Italian
Oncology Group for Clinical Research. J Clin Oncol. 5:141–149.
1987.PubMed/NCBI
|
|
10
|
Sullivan JR, Leyden MJ and Bell R:
Decreased cisplatin-induced nausea and vomiting with chronic
alcohol ingestion. N Engl J Med. 309:7961983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hesketh PJ: Chemotherapy-induced nausea
and vomiting. N Engl J Med. 358:2482–2494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Langford P and Chrisp P: Fosaprepitant and
aprepitant: an update of the evidence for their place in the
prevention of chemotherapy-induced nausea and vomiting. Core Evid.
5:77–90. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rojas C, Thomas AG, Alt J, et al:
Palonosetron triggers 5-HT(3) receptor internalization and causes
prolonged inhibition of receptor function. Eur J Pharmacol.
626:193–199. 2010. View Article : Google Scholar
|
|
14
|
Hothersall JD, Moffat C and Connolly CN:
Prolonged inhibition of 5-HT3 receptors by palonosetron results
from surface receptor inhibition rather than inducing receptor
internalization. Br J Pharmacol. 169:1252–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rojas C, Li Y, Zhang J, et al: The
antiemetic 5-HT3 receptor antagonist Palonosetron inhibits
substance P-mediated responses in vitro and in vivo. J Pharmacol
Exp Ther. 335:362–368. 2010. View Article : Google Scholar : PubMed/NCBI
|