Introduction
A primary intraosseous odontogenic carcinoma, which
is a term that was recommended by the World Health Organization
(WHO) in 1972 (1) is a type of
squamous cell carcinoma (SCC) arising within the jawbone that
purportedly develops from remnants of odontogenic epithelium. In
2005, the WHO classified these lesions as odontogenic carcinomas,
termed primary intraosseous SCC (PIOSCC), and divided them into
three types: solid type; keratocystic odontogenic cyst-derived; and
odontogenic cyst-derived (2). A
definitive diagnosis of PIOSCC is difficult as the lesion must be
distinguished from tumors that have metastasized to the jawbone
from distant sites, from alveolar carcinomas that have invaded the
bone from the surface and from tumors of maxillary origin (3,4).
Odontogenic cysts are true cysts that arise from the
dental epithelium, which is associated with tooth formation. The
epithelial lining of odontogenic cysts has the potential to
transform into various types of odontogenic tumor (5,6).
However, transformation from an odontogenic cyst to a malignant
tumor is rare (5,6).
The current study presents a case of PIOSCC of the
maxilla that, based on the results of computed tomography (CT) and
the clinical course, was hypothesized to originate from an infected
residual cyst. Written informed consent was obtained from the
patient.
Case report
In September 2006, a 45-year-old male underwent
extraction of the upper left, first and second premolars at a
dental clinic (Takamatsu, Japan). In March 2012, the patient
identified a gingival swelling in the upper left premolar region
and was referred to another general dental practitioner (Takamatsu,
Japan). At that clinic, the patient underwent incision and drainage
of the lesion following clinical diagnosis of a dental infection.
However, there was no improvement following the treatment and thus
repeat curettage of the lesion was performed. Following these
treatments, the lesion continued to grow gradually. In July 2012,
the patient was referred to Kagawa Prefectural Central Hospital
(Takamatsu, Japan) with swelling and mild pain in the upper
maxilla. The patient had no medical or surgical history. The
patient smoked 20 cigarettes a day and has consumed two alcoholic
beverages per week for the past 15 years. The patient’s family
history was noncontributory.
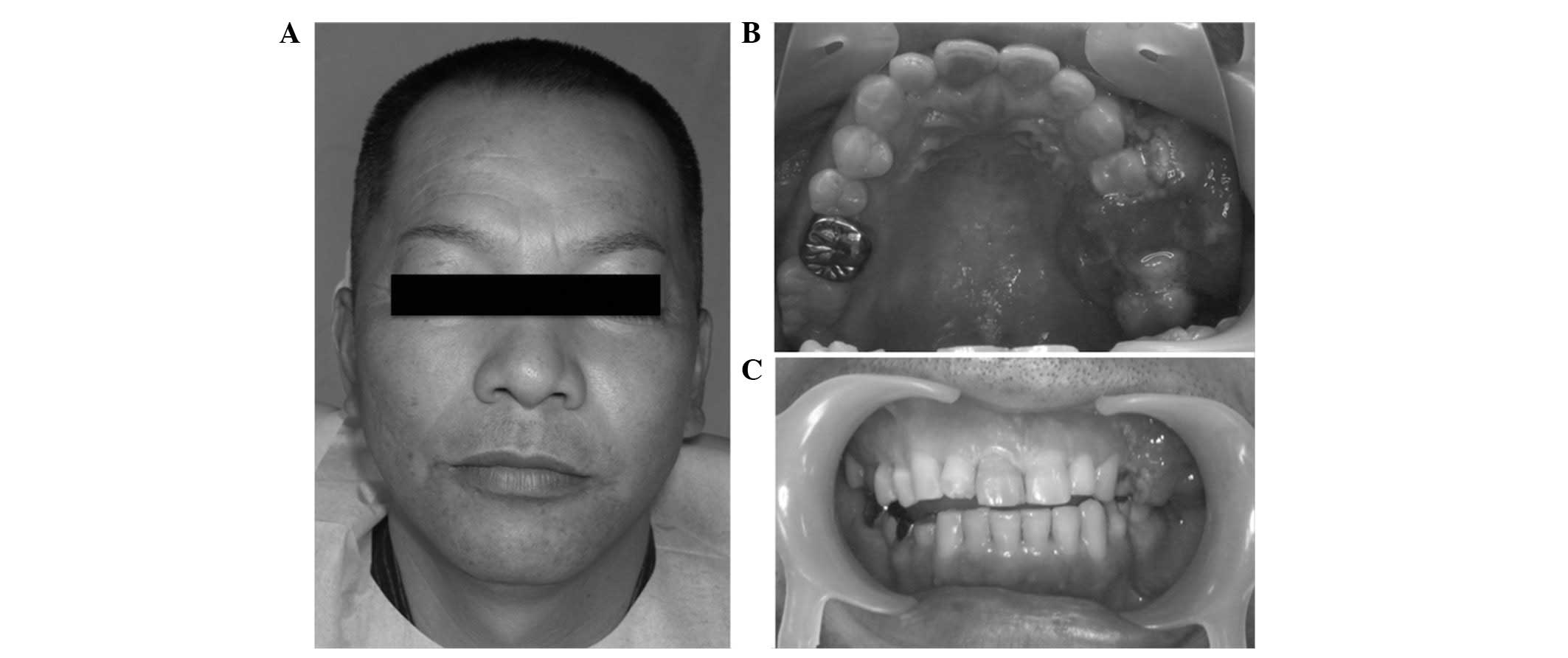
Extraoral examination revealed marginal left-sided
facial asymmetry and tenderness (Fig.
1A); however, the patient experienced no abnormal sensation in
the left buccal area. The left submandibular and upper jugular
lymph nodes were palpable and tender. The intraoral examination
revealed a mass, 25×35-mm in diameter, located in the buccal and
palatal aspect of the edentulous alveolus of the left maxilla, in
the area between the second premolar and the first molar (Fig. 1B and C). The mucosal surface of the
mass was rough and covered with small and protruding hemorrhagic
papules, which were pink-red in color. On palpation, the mucosa
surrounding the mass appeared to be normal and was not indurated.
However, tenderness and bleeding were identified.
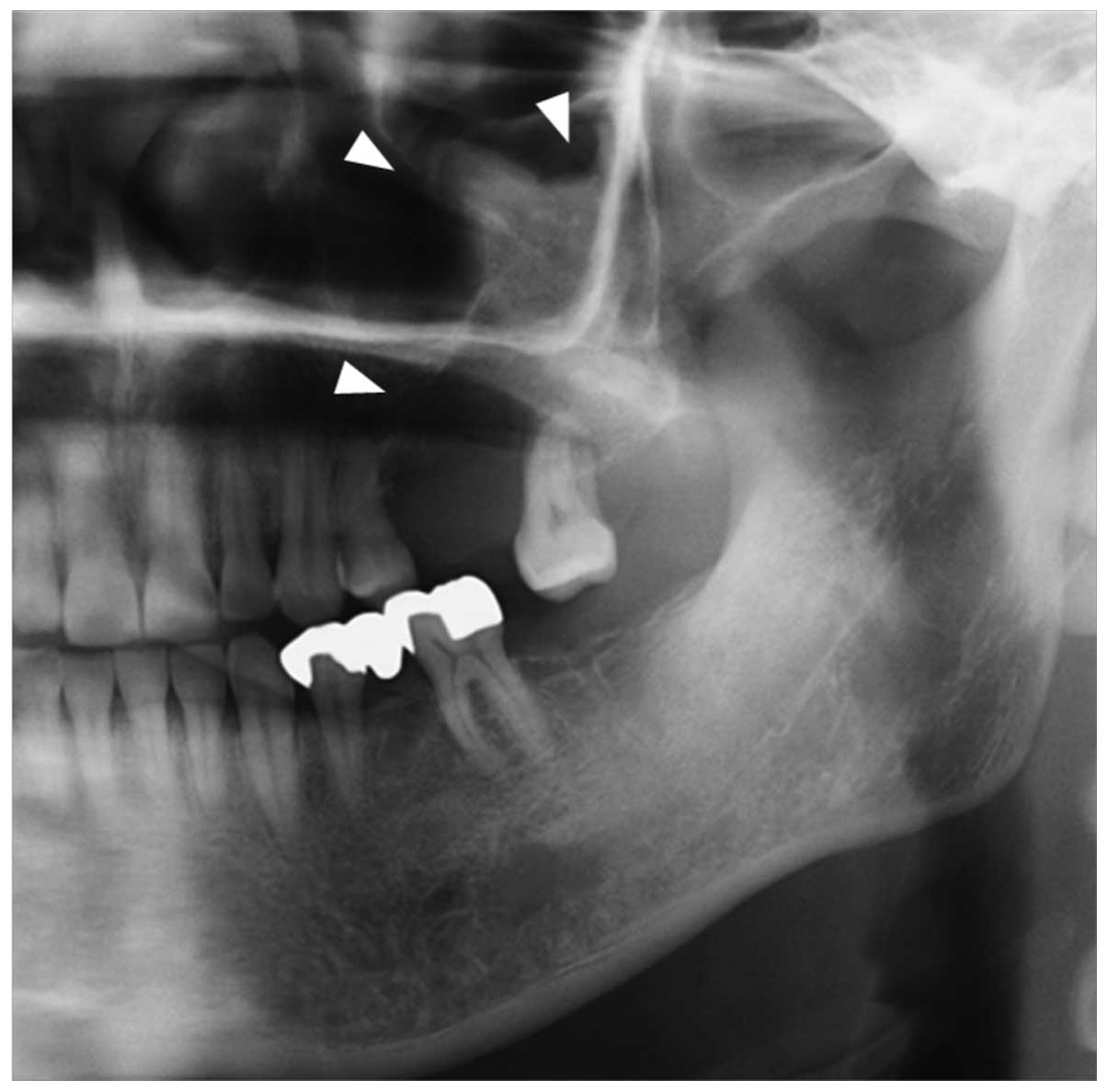
A panoramic radiograph revealed a dome-shaped
radiopaque mass with well-defined margins extending from the left
maxilla to the maxillary sinus. The lesion caused the floor of the
antrum to be elevated (Fig. 2). CT
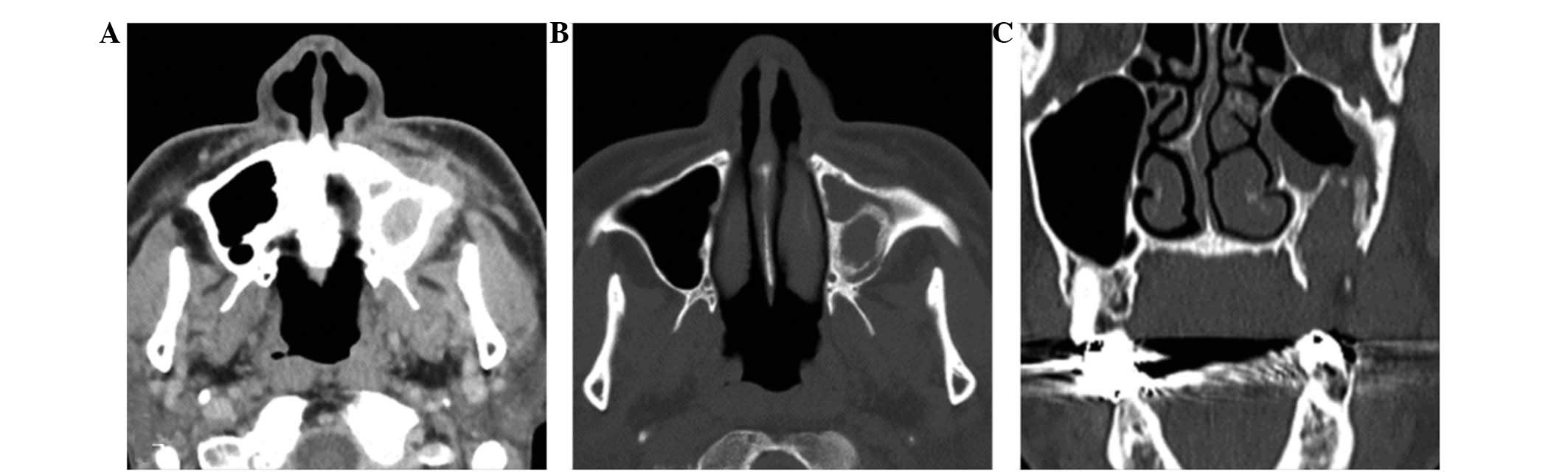
revealed a round cystic lesion, 30×40 mm in dimateter, which
extended from the left maxillary alveolar region to the maxillary
sinus (Fig. 3A and B). The floor of
antrum was elevated by the cystic lesion and its margins were
thickened (Fig. 3B and C). A
section of the elevated sinus floor had been destroyed (Fig. 3C).
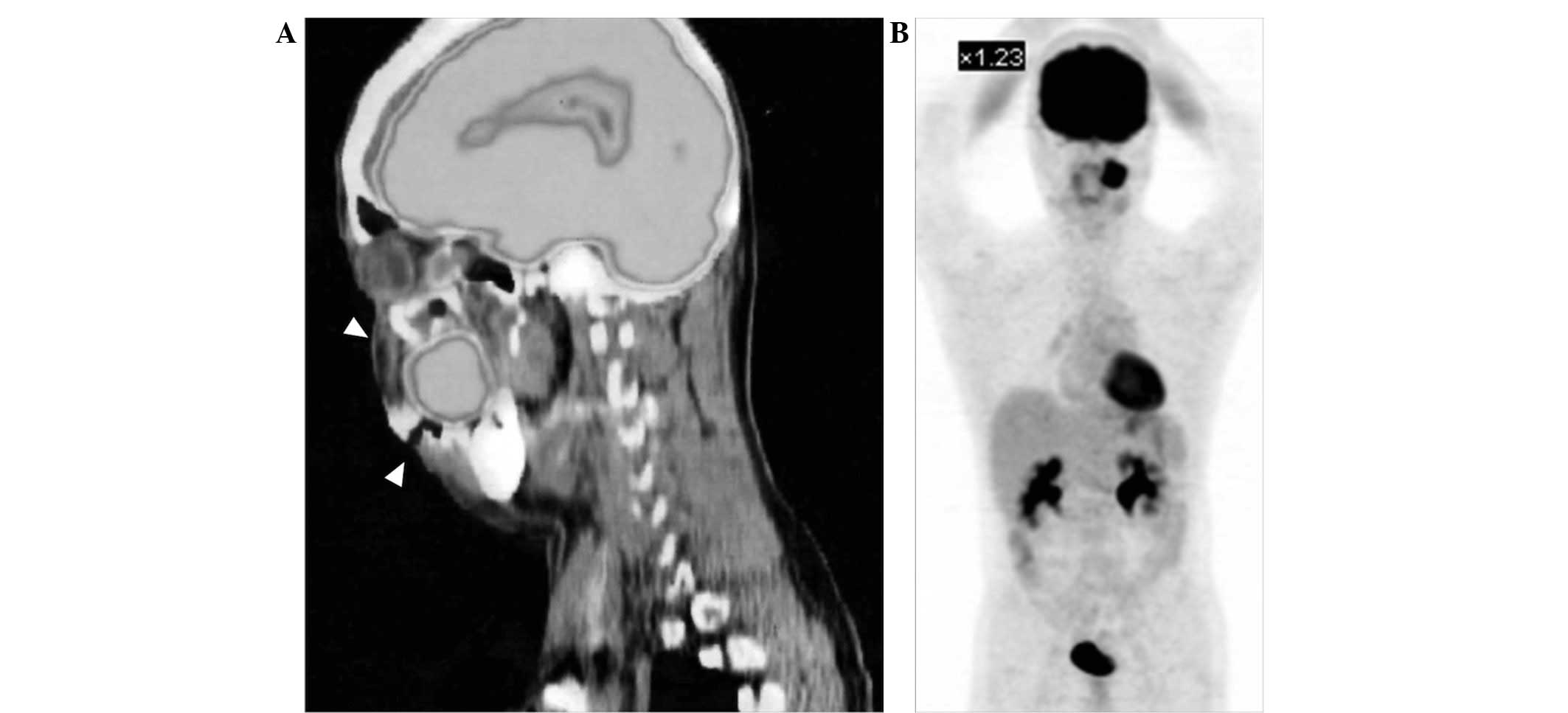
18F-fluorodeoxyglucose-positron emission tomography
(FDG-PET) detected FDG uptake in the left maxilla [maximum
standardized uptake value, (SUVmax), 12.4; Fig. 4A] and in two submandibular lymph
nodes (SUVmax, 2.2). No abnormal FDG uptake that would
have been indicative of another primary tumor or distant metastasis
was detected on the FDG-PET images (Fig. 4B).
From these imaging results, the lesion was diagnosed
as a primary malignant tumor arising from the left maxilla. The
tumor was clinically staged as T4aN2bM0 in accordance with the 2009
Union for International Cancer Control system (7). An incisional biopsy was performed and
indicated that the lesion was a PIOSSC. The patient underwent a
subtotal maxillectomy of the left maxilla, and left radical neck
dissection under general anesthesia and the diagnosis was
histopathologically determined following surgery.
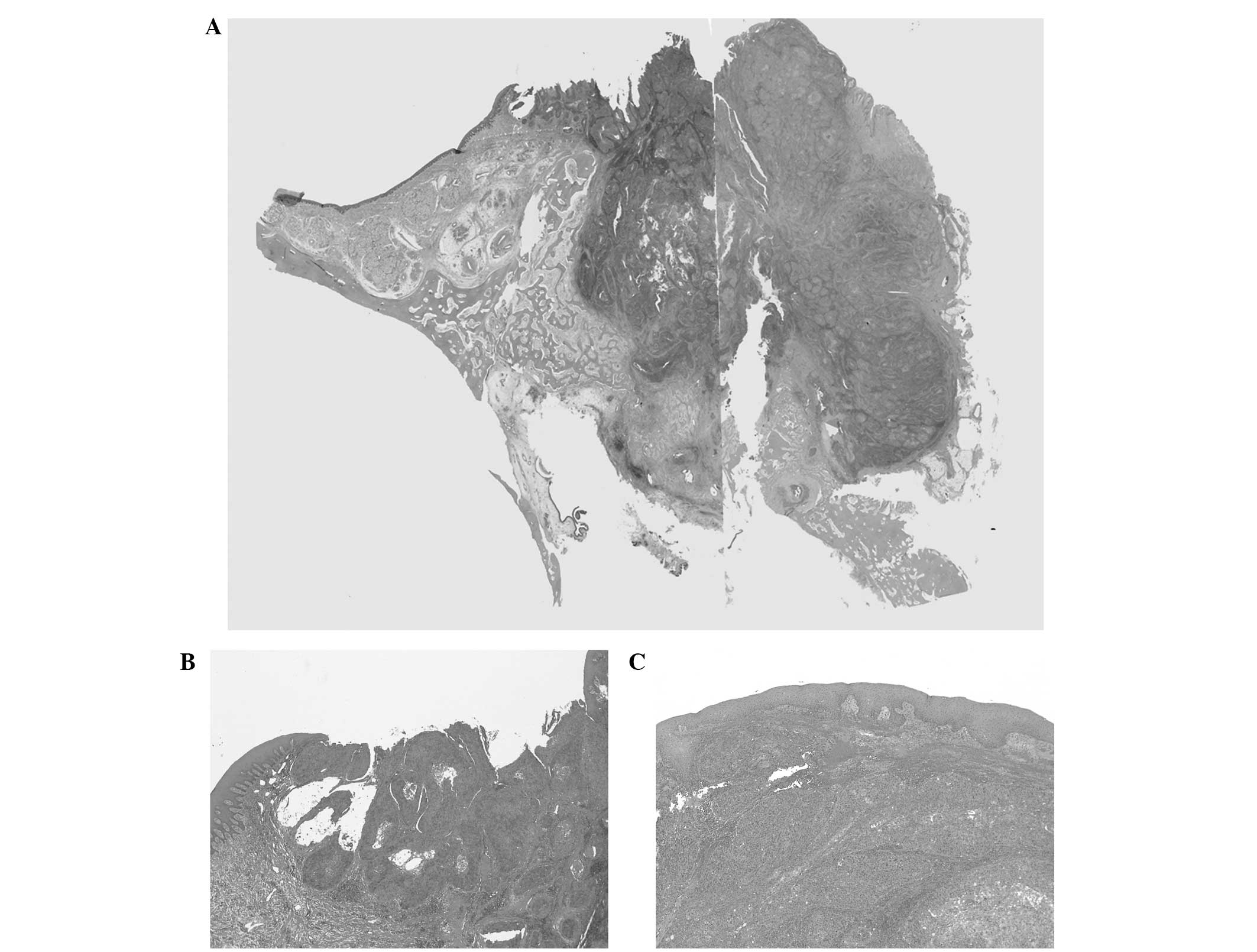
The histopathological examination of the excised
specimen revealed tumor cells consisting of atypical squamous
epithelial cells with enlarged nuclei, which had invaded the
submucosal connective tissue and bone (Fig. 5A–C). The tumor was accompanied by
necrosis inside the tumor nests and scattered mitotic figures
(Fig. 5D and E). These features
indicated a poorly differentiated SCC. Inside the lesion, there was
no clear evidence of the presence of cysts. However, the tumor had
progressed to the surface from deep within the tissue, no atypical
cells were observed in the epithelium at the boundary of the ulcer,
and there was no contiguity between the oral mucosal epithelium and
the maxillary sinus mucosa. No histological metastasis to the lymph
nodes was identified. Based on these findings, the lesion was
finally diagnosed as PIOSCC.
Discussion
PIOSCC is a rare odontogenic tumor of the jawbone
arising from residual odontogenic epithelium, initially without
connection to the oral mucosa. In 2005, the WHO (2) categorized PIOSCC into three types:
solid type; keratocystic odontogenic tumor-derived; and odontogenic
cyst-derived.
Gardner (8) and
Hampl and Harrigan (9) reported
that the most important criterion for the diagnosis of primary
intraosseous odontogenic carcinoma is the presence of a transition
zone between the normal and malignant epithelia. In the present
case, a transitional area between the normal oral squamous
epithelium and the SCC was observed and, histopathologically, the
tumor cells were not contiguous with the oral mucosal epithelium or
the maxillary sinus mucosa. As a diagnostic criterion for primary
intraosseous odontogenic carcinoma, previous studies have proposed
the exclusion of other primary tumors (3,4).
FDG-PET is considered to be a useful modality for evaluating
malignant tumors, as well as the primary site, lymph nodes and
occurrence of distant metastases. In the present case, marked FDG
uptake (SUVmax, 12.4), indicating that the tumor was
malignant, was detected only in the maxilla and no other abnormally
high uptake was observed elsewhere. Therefore, the lesion was
diagnosed as a PIOSCC within the jawbone.
The incidence of PIOSCC derived from odontogenic
lesions is complicated to determine. If the disease is not in its
early stages, it is difficult to demonstrate the actual site of
malignant transformation. At later stages, the carcinoma may
destroy the structures of the original lesion (8). In the present case, the existence of
odontogenic epithelium was not revealed histologically. CT
demonstrated elevation of the floor of the antrum, which revealed
destruction that was caused by the lesion. Considering the CT
findings and the clinical course, it was hypothesized that the
carcinoma had developed from an odontogenic cyst or benign tumor in
the maxilla. Therefore, this case was hypothesized to be a PIOSCC
derived from an odontogenic cyst or keratocystic odontogenic
tumor.
The pathogenesis of PIOSCC remains unclear. It has
been hypothesized that the key factor in carcinogenesis is chronic
inflammation from the infection of odontogenic lesions (8,10,11).
Infection and inflammation may contribute to carcinogenesis via
three major factors: i) Formation of reactive oxygen and nitrogen
species by phagocytes that subsequently damage DNA, proteins and
cell membranes; ii) infectious agents may directly transform cells
by inserting oncogenes into the host genome, inhibiting tumor
suppressor genes or stimulating mitosis; and iii) infectious agents
may induce immunosuppression and thereby reduce immunosurveillance
(12,13). PIOSCCs and oral mucosal carcinomas
express a different set of oncogenes and tumor markers, indicating
different genetic pathways (14).
In the present case, inflammatory cell (lymphocyte and neutrophil)
infiltration was identified within the stromal components, which
may have been caused by the incision and drainage of the lesion
that had been performed previously. However, these pathological
findings did not indicate the existence of chronic inflammation. CT
revealed bone thickening of the elevated floor of the maxillary
sinus and these bone changes indicated chronic inflammation. In
addition to the abovementioned CT observations, the findings of the
present case indicated the past existence of a lesion that had been
infected for a long period. Therefore, we hypothesized that the
PIOSCC was derived from a radicular (residual) cyst of an
inflammatory cyst, which had the potential for infection.
In the updated WHO (2005) classification (2) odontogenic carcinomas are generally
divided into four categories: ameloblastic carcinoma; PIOSCC; clear
cell odontogenic carcinoma; and ghost cell carcinoma. Of these,
ameloblastic carcinoma is classified as primary- or secondary-type
ameloblastic carcinoma. The primary type of ameloblastic carcinoma
arises de novo. The secondary type, malignant transformation
of ameloblastic carcinoma, is considered to occur from a recurrent
or pre-existing ameloblastoma. Karakida et al (15) proposed that chronic inflammation
following surgical treatment may lead to a malignant
transformation, resulting in the secondary type of ameloblastic
carcinoma. In the current case, it was hypothesized that chronic
inflammation caused the cyst-lining epithelium to undergo malignant
transformation. Therefore, the present case may have occurred
secondary to a residual cyst.
In conclusion, the current study presents a case of
PIOSCC of the maxilla, which, based on the CT findings and its
clinical course, was potentially derived from a residual cyst.
Clinicians must be aware that odontogenic cysts that are subject to
chronic inflammation have the potential to undergo malignant
transformation.
References
|
1
|
Pindborg JJ, Kramer IRH and Torloni H:
International Histological Classification of Tumors. Histological
Typing of Odontogenic Tumours, Jaw Cysts and Allied Disease.
Springer-Verlag; Geneva: pp. 35–36. 1972
|
|
2
|
Sciubba JJ, Eversole LR and Slootweg PJ:
Odontogenic/ameloblastic carcinomas. Pathology and Genetics of Head
and Neck Tumours. Barnes L, Eveson JW, Reichart P and Sidransky D:
IARC Press; Lyon: pp. 287–293. 2005
|
|
3
|
Suei Y, Tanimoto K, Taguchi A and Wada T:
Primary intraosseous carcinoma: review of the literature and
diagnostic criteria. J Oral Maxillofac Surg. 52:580–583. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
To EH, Brown JS, Avery BS and Ward-Booth
RP: Primary intraosseous carcinoma of the jaws. Three new cases and
a review of the literature. Br J Oral Maxillofac Surg. 29:19–25.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoelinga PJ and Bronkhorst FB: The
incidence, multiple presentation and recurrence of aggressive cysts
of the jaws. J Craniomaxillofac Surg. 16:184–195. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eversole LR, Sabes WR and Rovin S:
Aggressive growth and neoplastic potential of odontogenic cysts:
with special reference to central epidermoid and mucoepidermoid
carcinomas. Cancer. 35:270–282. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. 8th edition. Wiley; New
Jersey: 2009
|
|
8
|
Gardner AF: The odontogenic cyst as a
potential carcinoma: a clinicopathologic appraisal. J Am Dent
Assoc. 78:746–755. 1969.PubMed/NCBI
|
|
9
|
Hampl PF and Harrigan WF: Squamous cell
carcinoma possibly arising from an odontogenic cyst: report of
case. J Oral Surg. 31:359–362. 1973.PubMed/NCBI
|
|
10
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar
|
|
12
|
Hold GL and El-Omar EM: Genetic aspects of
inflammation and cancer. Biochem J. 410:225–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuper H, Adami HO and Trichopoulos D:
Infections as a major preventable cause of human cancer. J Intern
Med. 248:171–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alevizos I, Blaeser B, Gallagher G, et al:
Odontogenic carcinoma: a functional genomic comparison with oral
mucosal squamous cell carcinoma. Oral Oncol. 38:504–507. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karakida K, Aoki T, Sakamoto H, et al:
Ameloblastic carcinoma, secondary type: a case report. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 110:e33–e37. 2010.
View Article : Google Scholar : PubMed/NCBI
|