Introduction
The management of endometrial cancer (EC) has
significantly changed over the past 25 years. In 1988, EC staging
was developed from a clinical to a comprehensive surgical staging
system (1). Twenty years later, the
International Federation of Gynaecology and Obstetrics (FIGO)
committee revised the staging criteria based upon survival
similarities or disparities among particular substages (2). Surgical staging provides pathological
and prognostic data, and identifies those patients that require
adjuvant treatment. However, FIGO did not define the precise or
optimal anatomical borders for the performance of
lymphadenectomies, nor the adequate number of lymph nodes (LN) that
require removal for the comprehensive completion of the procedure.
Due to a lack of consistency among recommendations, the extent of
lymphadenectomy for EC in current practice worldwide varies from
the limited procedure of LN sampling alone to combined pelvic and
para-aortic lymphadenectomy (PPaLND) up to the renal vessels.
An additional indicator, which may be adopted for
surgical staging is the potential therapeutic effect of a
lymphadenectomy. Previous studies have assessed the survival effect
of a lymphadenectomy in EC patients, with the majority of
retrospective analyses demonstrating a survival benefit,
particularly in patients presenting with node-positive disease
(3–5), although two large randomized
controlled trials (RCTs) revealed no evidence of survival benefit
in patients with presumed early-stage disease (6,7).
However, all of the previous studies are heterogeneous with regard
to the tumor histology.
In the present study, clinical data were evaluated
to determine whether combining para-aortic lymphadenectomy with
pelvic lymphadenectomy (PLND) improves survival in patients
exhibiting endometrioid type EC. To the best of our knowledge, this
is the first study that addresses the survival effect of PPaLND in
a single type of tumor histology.
Patients and methods
Study design and patients
A total of 276 patients with EC who underwent
surgery at Akdeniz University Hospital (Antalya, Turkey) between
January 2005 and August 2012 were included in the current
retrospective study. In this study, tumor grading was performed
according to the World Health Organization grading system (8) and tumor staging was performed
according to the FIGO 2009 criteria (2). Demographic, clinicopathological and
survival data, as well as information regarding the age at surgery,
date and type of surgical procedure, histological type, tumor size,
tumor grade, depth of myometrial invasion, lympho-vascular space
invasion (LVSI), number of LNs removed, LN involvement, disease
stage, coexistence of primary synchronous malignancy, adjuvant
treatment, disease recurrence or progression, survival status, and
the date of last follow-up were extracted from the institutional
database, surgery notes and patient charts following approval from
the ethics committee of Akdeniz University Hospital. Written
informed consent was obtained from all patients.
The inclusion criteria were as follows: i)
Endometrioid tumor histological type and ii) a surgical procedure
that was performed via laparoscopy or laparotomy, which included a
total hysterectomy and bilateral salpingo-oophorectomy, as well as
either systematic PLND or PPaLND. Patients exhibiting a
non-endometrioid histology, carcinosarcoma or primary synchronous
malignancy, or that had not undergone LN dissection or had no
survival data were excluded. Selective LN sampling was not
considered to be LN dissection.
Procedures
Systematic PLND is performed in all EC patients as a
routine procedure at Akdeniz University Hospital, regardless of any
predefined surgical risk factors. The dissected lymphatic basin
sites during PLND were the external iliac, obturatory, internal
iliac and inferior common iliac regions. The upper dissection
margin was 2–3 cm above the iliac bifurcation. The decision to
perform a para-aortic dissection was at the discretion of the
surgeons. Systematic PPaLND included PLND plus removal of all LNs
from the superior common iliac, pre-sacral, para-caval, pre-caval,
inter aorta-caval, pre-aortic and para-aortic areas up to the renal
vessels. The type or extent of systematic lymphadenectomy (PLND vs.
PPaLND) varied among the practitioners over the study period even
in the same individuals. The superior margin of the PPaLND was
taken as the inferior mesenteric artery for certain patients, due
to technical complications, including adhesions resulting from
previous abdominal surgery, the type of incision made (transverse
vs. midline), anatomical variation or morbid obesity.
The patients who underwent surgery prior to 2009
were restaged according to the FIGO 2009 criteria (2). The postoperative risk of recurrence
stratification was determined by the following standards determined
by Gynecologic Oncology Group (GOG)-249 protocol (9): Low risk (LR; stage IA, grade 1,
LVSI-negative); low-intermediate risk (LIR; stages IA, IB and II
that do not meet the LR or high-intermediate risk criteria);
high-intermediate risk (HIR; stages IA, IB and II, with the
following risk factors: i) Grade 2 or grade 3; ii) LVSI-positive;
iii) outer half myometrial invasion; (iv) patient aged ≥70 years
with one other risk factor; (v) patient aged ≥50 years with two
other risk factors; (vi) any age with all three risk factors); and
high risk (HR; stages III and IV).
According to institutional procedure, the EC
postoperative adjuvant treatment strategy was as follows:
Observation for LR patients, high-dose rate (HDR) brachytherapy
alone for LIR patients, external pelvic irradiation alone for HIR
patients and chemotherapy alone for HR-stage IVB patients. These
procedures were consistent between the two lymphadenectomy
groups.
In the PPaLND group, the patients with HR-stage IIIA
to IIIC1 disease were treated with pelvic irradiation
with or without chemotherapy, and patients with HR-stage
IIIC2 disease were treated with extended field
irradiation of the pelvic and para-aortic regions with or without
chemotherapy. In the PLND group, the patients with HR-stage III
disease were offered a comparible treatment strategy to those with
HR-stage IIIC2 disease in the PPaLND group. The decision
to administer chemotherapy depended on the preference of the
patient.
The dose of HDR brachytherapy used in LR patients
was 2,100 cGy to a 5-mm depth in three fractions (700 cGy per
fraction). A dose of 1,500 cGy was applied in three fractions for
HR patients following external beam irradiation. Patients with HIR
were treated with external pelvic irradiation at a dose of
4,500–5,040 cGy in 25–28 fractions. A dose of 4,500 cGy was
administered in 25 fractions simultaneously with pelvic irradiation
in patients who received extended field irradiation to the
para-aortic region. In HR patients with stage III disease, adjuvant
chemotherapy consisted of three to four cycles of carboplatin (area
under the curve, 5) and paclitaxel (175 mg/m2)
administered every three weeks. The same regimen was provided for
six cycles in patients with stage IVB disease.
The standard surveillance practice in Akdeniz
University Hospital was to follow up patients, who achieved
complete remission or no evidence of disease following initial
treatment, every three months for two years, every six months for
the next three years, and then annually. The patients will continue
to be followed-up until the disease recurs or mortality occurs.
Statistical analysis
The primary outcome was progression-free survival
(PFS). The secondary outcomes were overall survival (OS) and time
to progression (TTP). PFS was determined to be the time period
between the date of surgery and the date of disease progression, or
relapse or mortality from any cause. TTP was calculated as the time
period between surgery and disease progression/recurrence, or
fatality caused by EC or complications associated with the surgery.
OS was determined by the time period between the date of surgery
and the date of mortality from any cause. The surviving patients
that were not exhibiting progression or relapse were censored at
the date they were last known to be alive according to the PFS and
TTP data. Patients that continued to live, regardless of whether
they exhibited progression or relapse, were censored at the date
they were last known to be alive according to the OS analysis
(10). The log-rank test was used
to compare the Kaplan-Meier curves for OS, TTP and PFS. The Cox
proportional hazards model was used to obtain the hazard ratio, for
the treatment comparison, and the 95% confidence interval (CI),
unadjusted or adjusted, for all factors. The data are expressed as
the median and range for continuous variables. Binary variables are
presented as counts and percentages. When appropriate, groups were
compared with either a Mann-Whitney U test or a χ2 test.
All P-values were two-sided and P<0.05 was considered to
indicate a statistically significant difference. For statistical
analysis, the Stata software package (Special Edition v11.2 for
Macintosh OSX; StataCorp, College Station, TX, USA) was used.
Results
Patient characteristics
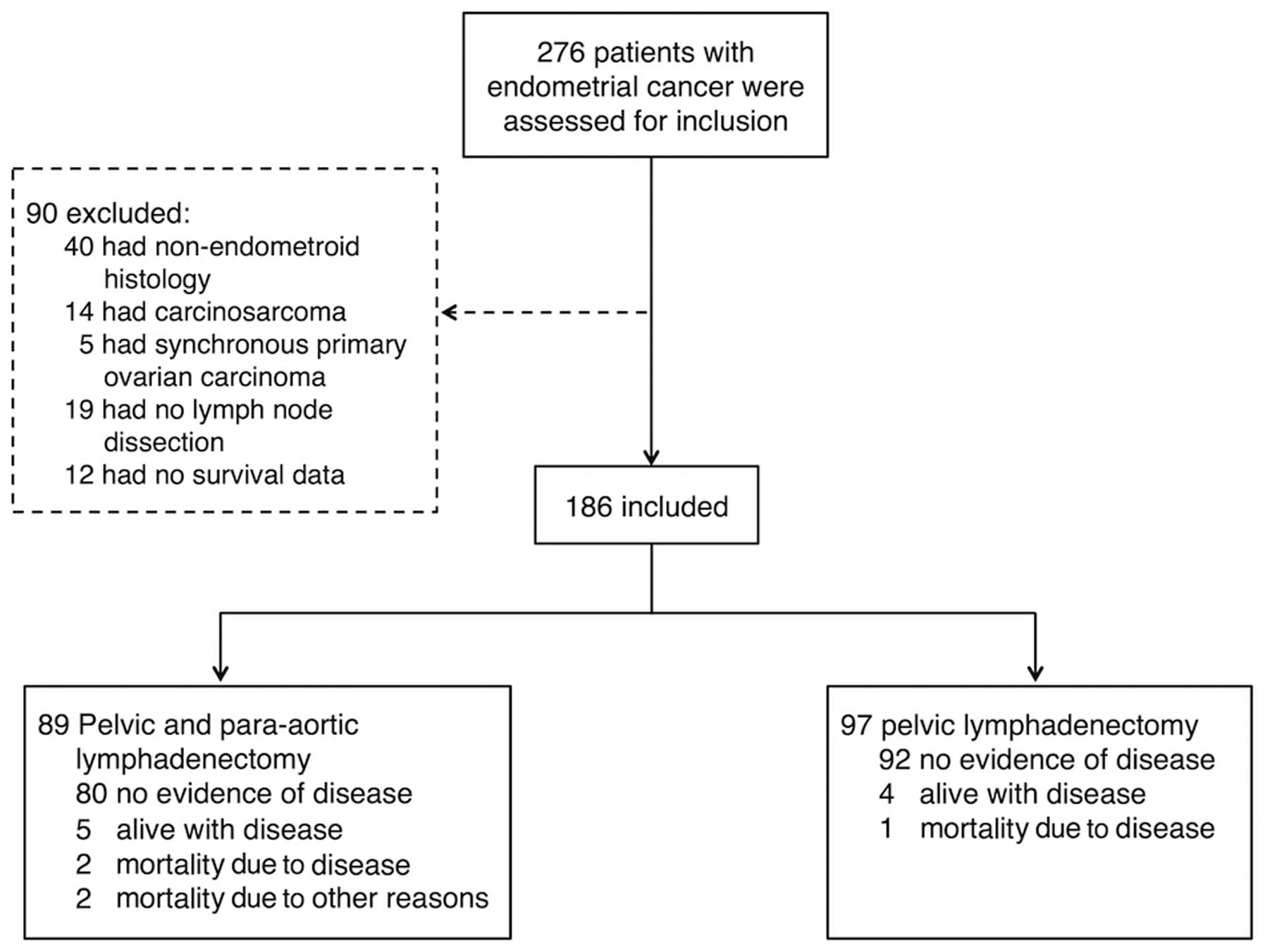
A total of 276 patients were assessed for inclusion
in the current study. Of these patients, 90 were excluded from the
analysis: 40 had a non-endometrioid histology, 14 presented with
carcinosarcoma, five had synchronous ovarian carcinoma, 19 had not
undergone LN dissection and 12 had no survival data. Thus, analysis
of a total of 186 patients, comprised of 97 patients in the PLND
group and 89 in the PPaLND group (Fig.
1), was conducted. Table I
compares the clinical and pathological characteristics of the
lymphadenectomy groups. The PPaLND group was significantly older
(median age, 59 vs. 55 years; P=0.0034), exhibited significantly
larger tumor sizes (3.5 vs. 2.8 cm; P=0.0023), less laparoscopic
surgery (6.7 vs. 32.0%; P<0.0001), more pelvic LNs removed (26
vs. 22; P=0.018), a greater number of para-aortic LNs removed (14
vs. 0; P<0.0001), an increased number of patients with stage II
or more advanced disease (42.8% vs. 4.1%; P<0.0001), a greater
number of HR patients (25.8 vs. 4.1%; P<0.0001) and more
patients who received adjuvant treatment (75.6 vs. 34.4%;
P<0.0001). The median follow-up time for all patients was 38
months (95% CI, 36.47–42.90).
 | Table IClinical and pathological
characteristics of patients exhibiting endometrioid type
endometrial cancer. |
Table I
Clinical and pathological
characteristics of patients exhibiting endometrioid type
endometrial cancer.
| Lymphadenectomy | |
|---|
|
| |
|---|
| Variable | Pelvic, n=97 | Pelvic and
para-aortic, n=89 | P-value |
|---|
| Median age at
surgery, years (IQR) | 55 (49–61) | 59 (53–65) | 0.003 |
| Histologic subtype, n
(%) |
| Endometrioid,
pure | 67 (69.1) | 45 (50.6) |
0.023a |
| Endometrioid, with
squamous differentiation | 28 (28.9) | 44 (49.4) | |
| Endometrioid,
villoglandular variant | 1 (1.0) | 0 (0.0) | |
| Endometrioid,
ciliated cell variant | 1 (1.0) | 0 (0.0) | |
| FIGO stage, n
(%) |
| IA | 70 (72.2) | 28 (31.5) |
<0.001b |
| IB | 23 (23.7) | 23 (25.8) | |
| II | 0 (0.0) | 14 (15.7) | |
| IIIA | 0 (0.0) | 3 (3.4) | |
| IIIC | 4 (4.1) | 19 (21.3) | |
| IV | 0 (0.0) | 2 (2.2) | |
| Risk of recurrence, n
(%) |
| Low | 57 (58.8) | 21 (23.6) |
<0.001a |
|
Low-intermediate | 21 (21.6) | 20 (22.5) | |
|
High-intermediate | 15 (15.5) | 25 (28.1) | |
| High | 4 (4.1) | 23 (25.8) | |
| Median tumor size, cm
(IQR) | 2.8 (1.5–4.0) | 3.5 (2.3–5.0) | 0.002 |
| Peritoneal cytology
positive, n (%) | 2 (2.1) | 3 (3.4) | 0.670 |
| Surgery, n (%) |
| Laparoscopy | 31 (32.0) | 6 (6.7) | <0.001 |
| Laparotomy | 66 (68.0) | 83 (93.3) | |
| Median lymph nodes
removed, n (IQR) |
| Pelvic lymph
nodes | 22 (18–29) | 26 (21–32) | 0.018 |
| Para-aortic lymph
nodes | 0 (0.0) | 14 (9–19) | <0.001 |
| Adjuvant treatment, n
(%) |
| None | 63 (64.9) | 20 (22.5) |
<0.001a |
| Radiotherapy
alone | 31 (32.0) | 45 (50.6) | |
| Chemotherapy
alone | 0 (0.0) | 2 (2.1) | |
| Chemotherapy and
radiotherapy | 2 (2.1) | 15 (16.8) | |
| Unknown | 1 (1.0) | 7 (7.9) | |
| Median follow-up
time, months (95% CI) | 39 (38.14–47.13) | 37 (31.88–41.06) | 0.079 |
Logistic regression analysis was performed to
determine whether the differences between the groups influenced PFS
(Table II). The risk of recurrence
stratification was the only independent factor of PFS. Therefore,
the lymphadenectomy groups were stratified according to GOG risk of
recurrence stratification. Since there were few HR patients in the
PLND group, the HIR and HR groups (HIR/HR), and the LIR and LR
groups (LIR/LR) were combined.
 | Table IIUnivariate and multivariate logistic
regression analysis of factors predicting progression or mortality
in patients exhibiting endometrioid type endometrial cancer. |
Table II
Univariate and multivariate logistic
regression analysis of factors predicting progression or mortality
in patients exhibiting endometrioid type endometrial cancer.
| Unadjusted | Adjusted |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age at surgery,
years |
| <62 | 1 | | | 1 | | |
| ≥62 | 5.55 | 1.75–17.63 | 0.004 | 1.57 | 0.47–5.26 | 0.460 |
| Risk of
recurrence |
|
Low/Low-intermediate | 1 | | | 1 | | |
|
High/High-intermediate | 10.26 | 3.38–31.12 | <0.001 | 9.42 | 1.21–73.62 | 0.032 |
| Adjuvant
therapy |
| No | 1 | | | 1 | | |
| Yes | 3.74 | 1.20–11.61 | 0.023 | 0.96 | 0.13–6.97 | 0.970 |
| Histologic
subtype |
| Endometrioid,
pure | 1 | | | | | |
| Endometrioid,
other | 1.67 | 0.57–4.94 | 0.350 | - | - | - |
| Tumor size, cm |
| <4 | 1 | | | | | |
| ≥4 | 2.05 | 0.68–6.22 | 0.200 | - | - | - |
| Peritoneal
cytology |
| Negative | 1 | | | | | |
| Positive | 3.32 | 0.43–25.92 | 0.220 | - | - | - |
| Surgery |
| Laparoscopy | 1 | | | | | |
| Laparotomy | 2.50 | 0.72–8.66 | 0.150 | - | - | - |
| Lymph node
dissection |
| Pelvic lymph node
dissection | 1 | | | | | |
| Pelvic and
para-aortic lymph node dissection | 2.21 | 0.73–6.73 | 0.150 | - | - | - |
| Pelvic lymph nodes
removed, n |
| <25 | 1 | | | | | |
| ≥25 | 1.36 | 0.47–3.87 | 0.570 | - | - | - |
Of the 119 LIR/LR patients, 78 underwent PLND and 41
underwent PPaLND. The PPaLND group included significantly more
patients with stage II disease (24.4 vs. 0%; P=0.0167), fewer
patients receiving laparoscopic surgery (4.9 vs. 33.3%;
P<0.0001), a greater number of para-aortic LNs removed (15 vs.
0; P<0.0001) and more patients receiving adjuvant treatment when
compared with the PLND group (55.0 vs. 20.5%; P<0.0001; Table III). Multivariate logistic
regression analysis revealed no significant association between
these covariates and PFS (Table
IV). Of the 67 HIR/HR patients, 19 underwent PLND and 48
underwent PPaLND. The lymphadenectomy groups were comparable with
regard to their baseline characteristics (Table V).
 | Table IIIClinical and pathological
characteristics of the patients with low/low-intermediate risk of
endometrioid type endometrial cancer. |
Table III
Clinical and pathological
characteristics of the patients with low/low-intermediate risk of
endometrioid type endometrial cancer.
|
Lymphadenectomy | |
|---|
|
| |
|---|
| Variable | Pelvic, n=78 | Pelvic and
para-aortic, n=41 | P-value |
|---|
| Median age at
surgery, years (IQR) | 54 (48–59) | 50 (50–62) | 0.061 |
| Histologic subtype,
N (%) |
| Endometrioid,
pure | 57 (73.1) | 25 (61.0) |
0.180a |
| Endometrioid, with
squamous differentiation | 19 (24.3) | 16 (39.0) | |
| Endometrioid,
villoglandular variant | 1 (1.3) | 0 (0.0) | |
| Endometrioid,
ciliated cell variant | 1 (1.3) | 0 (0.0) | |
| FIGO stage, n
(%) |
| IA | 67 (85.9) | 26 (63.4) |
<0.001b |
| IB | 11 (14.1) | 5 (12.2) | |
| II | 0 (0.0) | 10 (24.4) | |
| Grade, n (%) |
| I | 69 (88.5) | 34 (82.9) |
0.330b |
| II | 9 (11.5) | 6 (14.6) | |
| III | 0 (0.0) | 1 (2.5) | |
| Myometrial
invasion, n (%) |
| <1/2 | 67 (85.9) | 31 (75.6) | 0.160 |
| ≥1/2 | 11 (14.1) | 10 (24.4) | |
| Lymphovascular
invasion, n (%) | 2 (2.6) | 1 (2.4) | 0.970 |
| Median tumor size,
cm (IQR) | 2.5 (1.0–3.5) | 3.0 (2.0–4.0) | 0.054 |
| Peritoneal cytology
positive, n (%) | 0 (0.0) | 1 (2.4) | 0.350 |
| Surgery, n (%) |
| Laparoscopy | 26 (33.3) | 2 (4.9) | <0.001 |
| Laparotomy | 52 (66.7) | 39 (95.1) | |
| Median lymph nodes
removed, n (IQR) |
| Pelvic lymph
nodes | 22 (18–27) | 26 (21–31) | 0.080 |
| Para-aortic lymph
nodes | 0 (0.0) | 15 (9–19) | <0.001 |
| Adjuvant treatment,
n (%) |
| None | 62 (79.5) | 18 (43.9) |
<0.001a |
| Radiotherapy
alone | 16 (20.5) | 21 (51.1) | |
| Chemotherapy
alone | 0 (0.0) | 0 (0.0) | |
| Chemotherapy and
radiotherapy | 0 (0.0) | 1 (2.5) | |
| Unknown | 0 (0.0) | 1 (2.5) | |
| Median follow up
time, months (95% CI) | 38
(36.91–46.81) | 36
(31.37–46.14) | 0.440 |
 | Table IVUnivariate and multivariate logistic
regression analysis of factors predicting progression or fatality
in patients with low/low-intermediate risk endometrioid type
endometrial cancer. |
Table IV
Univariate and multivariate logistic
regression analysis of factors predicting progression or fatality
in patients with low/low-intermediate risk endometrioid type
endometrial cancer.
| Unadjusted | Adjusted |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Adjuvant
therapy |
| No | 1 | | | | | |
| Yes | 2.87 | 0.13–62.0 | 0.500 | - | - | - |
| Surgery |
| Laparoscopy | 1 | | | | | |
| Laparotomy | 3.88 | 0.17–90.93 | 0.400 | - | - | - |
| FIGO stage |
| I | 1 | | | | | |
| II | 0.33 | 0.01–39.95 | 0.650 | - | - | - |
| Age at surgery,
years |
| <54 | 1 | | | | | |
| ≥54 | 5.58 | 0.33–93.37 | 0.230 | - | - | - |
| Tumor size, cm |
| <2.5 | 1 | | | | | |
| ≥2.5 | 0.18 | 0.01–3.01 | 0.240 | - | - | - |
| Pelvic lymph nodes
removed |
| <25 | 1 | | | | | |
| ≥25 | 1.20 | 0.07–19.39 | 0.900 | - | - | - |
 | Table VClinical and pathological
characteristics of the patients with high/high-intermediate risk
endometrioid histological subtype of endometrial cancer. |
Table V
Clinical and pathological
characteristics of the patients with high/high-intermediate risk
endometrioid histological subtype of endometrial cancer.
|
Lymphadenectomy | |
|---|
|
| |
|---|
| Variable | Pelvic, n=19 | Pelvic and
para-aortic, n=48 | P-value |
|---|
| Median age at
surgery, years (IQR) | 62 (52–70) | 62 (54–67) | 0.600 |
| Histologic subtype,
n (%) |
| Endometrioid,
pure | 10 (52.6) | 20 (41.7) |
0.420a |
| Endometrioid, with
squamous differentiation | 9 (47.4) | 28 (58.3) | |
| Endometrioid,
villoglandular variant | 0 (0.0) | 0 (0.0) | |
| Endometrioid,
ciliated cell variant | 0 (0.0) | 0 (0.0) | |
| FIGO stage, n
(%) |
| IA | 3 (15.8) | 2 (4.2) |
0.100b |
| IB | 12 (63.2) | 18 (37.5) | |
| II | 0 (0.0) | 4 (8.3) | |
| IIIA | 0 (0.0) | 3 (6.2) | |
| IIIC | 4 (21.0) | 19 (39.6) | |
| IVB | 0 (0.0) | 2 (4.2) | |
| Grade, n (%) |
| I | 3 (15.8) | 9 (18.8) |
0.170c |
| II | 14 (73.7) | 24 (50.0) | |
| III | 2 (10.5) | 15 (31.2) | |
| Myometrial
invasion, n (%) |
| <1/2 | 4 (21.0) | 6 (12.5) | 0.450 |
| ≥1/2 | 15 (78.9) | 42 (87.5) | |
| Lymphovascular
invasion, n (%) | 6 (31.6) | 19 (39.6) | 0.540 |
| Median tumor size,
cm (IQR) | 3.5 (3.0–4.0) | 3.5 (2.5–5.3) | 0.600 |
| Peritoneal cytology
positive, n (%) | 2 (10.5) | 2 (4.2) | 0.320 |
| Surgery, n (%) |
| Laparoscopy | 5 (26.3) | 4 (8.3) | 0.110 |
| Laparotomy | 14 (73.7) | 44 (91.7) | |
| Median lymph nodes
removed, n (IQR) |
| Pelvic lymph
nodes | 27 (17–32) | 26 (22–32) | 0.600 |
| Paraaortic lymph
nodes | 0 (0.0) | 13 (8–20) | <0.001 |
| Adjuvant treatment,
n (%) |
| None | 1 (5.3) | 2 (4.2) |
0.210a |
| Radiotherapy
alone | 15 (78.9) | 24 (50.0) | |
| Chemotherapy
alone | 0 (0.0) | 2 (4.2) | |
| Chemotherapy and
radiotherapy | 2 (10.5) | 14 (29.1) | |
| Unknown | 1 (5.3) | 6 (12.5) | |
| Median follow-up
time, months (95% CI) | 39
(34.29–57.40) | 39
(28.60–40.44) | 0.140 |
Survival analysis
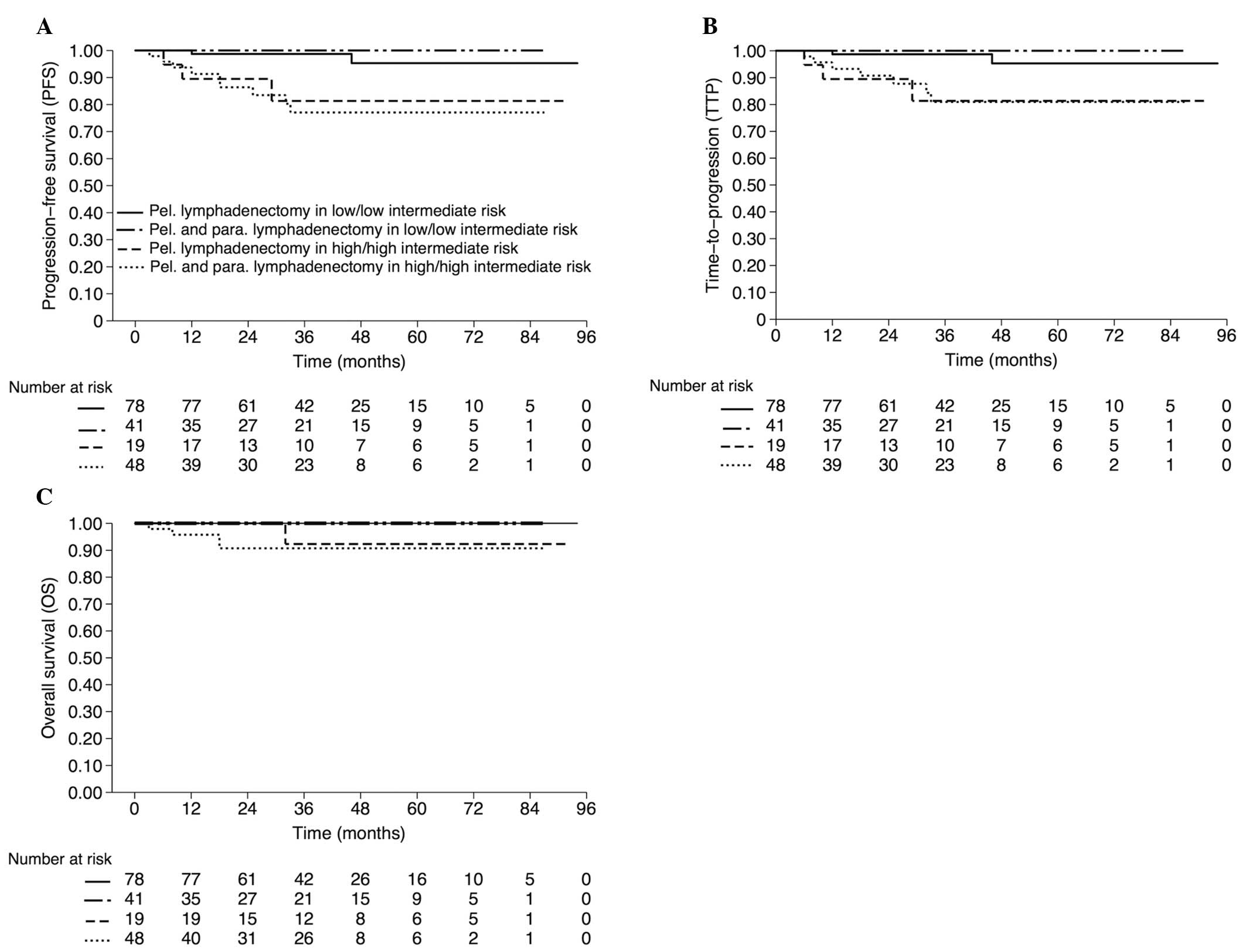
The estimated three-year OS, PFS and TTP rates for
patients with LIR/LR stratified by lymphadenectomy groups were as
follows: PLND, 100, 98.7 and 98.7%, respectively; and PPaLND, all
100%. The estimated three-year OS, PFS and TTP rates for patients
with HIR/HR were as follows: PLND, 92.3, 81.3 and 81.3%; and
PPaLND, 90.7, 77.1 and 80.9%, respectively (Fig. 2). No statistically significant
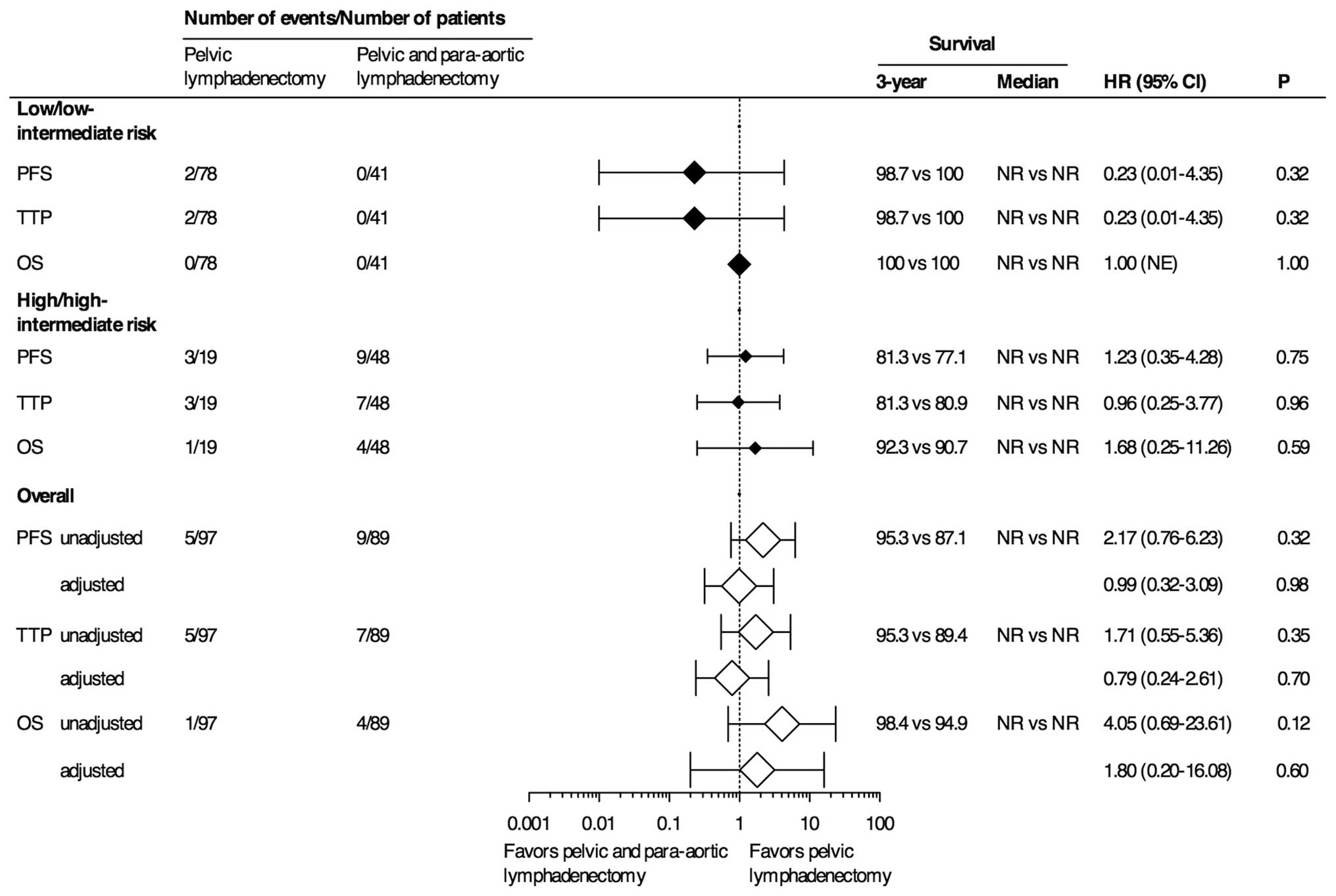
differences were identified between three-year OS, PFS and TTP
rates, regardless of the risk of recurrence stratification, between
the lymphadenectomy groups (98.4, 95.3 and 95.3%, respectively for
PLND; and 94.9, 87.1% and 89.4, respectively for PPaLND; Fig. 3).
Discussion
A survival comparison of PLND and PPaLND was
conducted in the present study in patients with endometrioid type
EC according to the GOG risk of recurrence stratification. The
results revealed no evidence of a survival advantage for PPaLND
when compared with PLND in either of the LIR/LR or the HIR/HR
patients. The aim of the present study was to histologically
examine the outcomes in patients exhibiting endometrioid type EC.
Histology is important, as the non-endometrioid EC subtypes have
different immunophenotypes, natural histories and outcomes, which
are determined by the tumor cell type (11,12).
Various studies have assessed the survival effect of
lymphadenectomy in EC. The majority of studies have compared
outcomes in patients who received a lymphadenectomy and those who
did not. Trimble et al (13)
noted a survival benefit among patients with stage I and grade 3
disease, but not those with grade 1 or grade 2 disease. Cragun
et al (14) reported that
patients who had >11 pelvic LNs removed exhibited significantly
improved OS. However, aortic lymphadenectomy was not found to be
beneficial in terms of improved survival in patients with apparent
early-stage EC. In a large population-based analysis involving
42,184 patients, Smith et al (15) reported that lymphadenectomy
conferred a disease-specific survival advantage. The improved
disease-specific survival was most pronounced for patients with
>11 LNs removed, or those with a disease stage II or higher.
However, the survival benefits observed in the
above-mentioned studies, particularly in the patients presenting
with high-grade tumors or those who had a greater number of LNs
removed, frequently result from the misinterpretation of the
disease stage. A certain proportion of patients who do not receive
lymphadenectomy may be expected to develop LN metastases.
Therefore, the outcomes of those studies reflect the difference in
survival between the patients that are surgically identified to be
true early-stage without LN involvement and those who are presumed
to be early-stage with an unknown LN status.
Recently, the results of two large RCTs revealed
that there was no significant survival benefit of a lymphadenectomy
in patients with presumed early-stage EC (6,7).
However, certain concerns regarding these trials have been raised,
including the number of LNs removed and the inclusion of patients
with LR of LN involvement. In particular, in the ASTEC trial
(7), despite the lymphadenectomy
group consisting of more patients with high-risk and advanced
disease, radiotherapy was administered to an equal number of
patients in each group. This factor resulted in the overtreatment
of the patients in the no lymphadenectomy group.
Studies that examined patients who had the pelvic
nodes removed as well as the nodes in the para-aortic region have
indicated a survival benefit of lymphadenectomy, particularly in
patients with node-positive disease (3–5).
However, these studies had certain limitations, including small
sample sizes, non-standardized adjuvant treatment strategies,
heterogeneous tumor histology and uncertain inclusion criteria. One
retrospective study (16) observed
a significantly longer OS period in a PPaLND group as compared with
a PLND group (hazard ratio, 0.53; 95% CI, 0.38–0.76; P=0.0005).
Furthermore, this association was observed in the subgroup of
patients with intermediate risk or HR (P=0.0009). However, OS was
not associated with lymphadenectomy type in the LR patient
subgroup. One major concern regarding the results of the study by
Todo et al (16) was the
lack of uniformity between the adjuvant treatment procedures for
the intermediate and HR patients. In the PPaLND cohort, adjuvant
treatment was limited to chemotherapy. In the PLND cohort, the
patients received radiotherapy or chemotherapy depending on the
preference of the patient and the discretion of the physician.
Therefore, whether the improved survival, particularly in the HR
patients, was associated with the para-aortic lymphadenectomy
itself or with the therapeutic effect of chemotherapy on occult
metastases is unclear. Conversely, another retrospective cohort
that compared PLND with PPaLND in intermediate or HR patients
indicated an improved disease-free survival rate in the patients
who underwent PLND (80 vs. 62%; P=0.02) (17). However, the OS values were not
significantly different between the two groups (P=0.93); in
addition, the PLND group was more likely than the PPaLND group to
have received multimodal adjuvant treatment.
The strengths of the present study include the
analysis of a single histological type, the administration of
adjuvant treatment as determined by the risk of recurrence analysis
proposed by the GOG, the employment of relatively uniform surgical
procedures/techniques and the adequacy of staging performed by
subspecialized gynecological oncologists. The limitations include
potential entry bias with case-selection, the lack of uniformity in
the adjuvant treatment of HR-stage III patients, the relatively
short median follow-up time and the small number of HR patients,
particularly in the PLND group.
Recommendations regarding the use of adjuvant
treatment in HIR/HR patients with any histological subtype or in
patients with any stage of non-endometrioid histology vary widely.
The lack of standardization is apparent in previous studies
(3–5,16,17).
The current GOG-258 trial compares the combination of chemotherapy
and radiotherapy with chemotherapy alone (18). The PORTEC-3 trial compares the
combination of chemotherapy and radiation with radiation alone
(19). These trials may aid with
determining the most appropriate adjuvant treatment modality for
patients with optimally resected HR disease.
Following clarification of these issues, the
therapeutic effect of lymphadenectomy may be assessed more
thoroughly. However, concerns regarding bias and the use of
adjuvant treatment in the PLND alone arm of the trial remain; for
example, selection of the adjuvant treatment to be administered in
patients with positive pelvic nodes but unknown para-aortic node
status. Prior studies have shown that over half of patients with
positive pelvic nodes exhibit positive para-aortic LN metastases
(20,21). Thus, the subjects would experience a
50% chance of concurrent para-aortic metastases or the other 50%
may be overtreated. Notably, skip metastases, the occurence of
isolated para-aortic LN metastases in individuals with negative
pelvic nodes, has been reported in ~1% surgically staged EC
patients (22,23). In the current study, this rate was
3.3%. Determining which adjuvant therapy is administered to
patients with negative pelvic nodes and whether the 1–3% likelihood
of skip metastases is negligible requires further analysis.
In conclusion, investigating the therapeutic effect
of lymphadenectomy, particularly in HR patients, is not considered
to be possible based on the findings of the present study. Although
performing an extended lymphadenectomy may provide valuable
prognostic data, the procedure is solely acceptable as an
experimental determinant of therapeutic intent.
References
|
1
|
FIGO. Classification and staging of
malignant tumours in the female pelvis. Int J Gynaecol Obstet.
28:1901989.
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mariani A, Webb MJ, Galli L and Podratz
KC: Potential therapeutic role of para-aortic lymphadenectomy in
node-positive endometrial cancer. Gynecol Oncol. 76:348–356. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujimoto T, Nanjyo H, Nakamura A, et al:
Para-aortic lymphadenectomy may improve disease-related survival in
patients with multipositive pelvic lymph node stage IIIc
endometrial cancer. Gynecol Oncol. 107:253–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Havrilesky LJ, Cragun JM, Calingaert B, et
al: Resection of lymph node metastases influences survival in stage
IIIC endometrial cancer. Gynecol Oncol. 99:689–695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benedetti Panici P, Basile S, Maneschi F,
et al: Systematic pelvic lymphadenectomy vs. no lymphadenectomy in
early-stage endometrial carcinoma: randomized clinical trial. J
Natl Cancer Inst. 100:1707–1716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitchener H, Swart AM, Qian Q, et al:
Efficacy of systematic pelvic lymphadenectomy in endometrial cancer
(MRC ASTEC trial): a randomised study. Lancet. 373:125–136. 2009.
View Article : Google Scholar
|
|
8
|
Silverberg SG, Kurman RJ, Nogales F,
Mutter GL, Kubik-Huch RA and Tavassoli FA: Tumours of the uterine
corpus Epithelial tumours and related lesions. World Health
Organization Classification of Tumours. Pathology and Genetics of
Tumours of the Breast and Female Genital Organs. Tavassoli FA and
Devilee P: IARC; Lyon, France: pp. 222–223. 2003
|
|
9
|
GOG-249 Protocol. http://www.cancer.gov/clinicaltrials/search/view?cdrid=629591&version=HealthProfessional.
Accessed September 12, 2014
|
|
10
|
FDA. Guidance for Industry. Clinical Trial
Endpoints for the Approval of Cancer Drugs and Biologics. May.
2007, http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071590.pdf%20-%20137k%20-.
Accessed September 12, 2014
|
|
11
|
Soslow RA, Bissonnette JP, Wilton A, et
al: Clinicopathologic analysis of 187 high-grade endometrial
carcinomas of different histologic subtypes: similar outcomes belie
distinctive biologic differences. Am J Surg Pathol. 31:979–987.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Creasman WT, Odicino F, Maisonneuve P, et
al: Carcinoma of the corpus uteri. FIGO 26th Annual Report on the
Results of Treatment in Gynecological Cancer. Int J Gynaecol
Obstet. 95(Suppl 1): S105–S143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trimble EL, Kosary C and Park RC: Lymph
node sampling and survival in endometrial cancer. Gynecol Oncol.
71:340–343. 1998. View Article : Google Scholar
|
|
14
|
Cragun JM, Havrilesky LJ, Calingaert B, et
al: Retrospective analysis of selective lymphadenectomy in apparent
early-stage endometrial cancer. J Clin Oncol. 23:3668–3675. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith DC, Macdonald OK, Lee CM and Gaffney
DK: Survival impact of lymph node dissection in endometrial
adenocarcinoma: a surveillance, epidemiology, and end results
analysis. Int J Gynecol Cancer. 18:255–261. 2008. View Article : Google Scholar
|
|
16
|
Todo Y, Kato H, Kaneuchi M, et al:
Survival effect of para-aortic lymphadenectomy in endometrial
cancer (SEPAL study): a retrospective cohort analysis. Lancet.
375:1165–1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
May T, Shoni M, Vitonis AF, et al: The
role of para-aortic lymphadenectomy in the surgical staging of
women with intermediate and high-risk endometrial adenocarcinomas.
Int J Surg Oncol. 2013:8589162013.PubMed/NCBI
|
|
18
|
Carboplatin and Paclitaxel With or Without
Cisplatin and Radiation Therapy in Treating Patients With Stage I,
Stage II, Stage III, or Stage IVA Endometrial Cancer. http://www.clinicaltrials.gov/show/NCT00942357.
Accessed September 12, 2014
|
|
19
|
PORTEC 3 trial protocol. http://www.clinicalresearch.nl/portec3.
Accessed September 12, 2014
|
|
20
|
Mariani A, Dowdy SC, Cliby WA, et al:
Prospective assessment of lymphatic dissemination in endometrial
cancer: a paradigm shift in surgical staging. Gynecol Oncol.
109:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yokoyama Y, Maruyama H, Sato S and Saito
Y: Indispensability of pelvic and paraaortic lymphadenectomy in
endometrial cancers. Gynecol Oncol. 64:411–417. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mariani A, Keeney GL, Aletti G, et al:
Endometrial carcinoma: paraaortic dissemination. Gynecol Oncol.
92:833–838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abu-Rustum NR, Gomez JD, Alektiar KM, et
al: The incidence of isolated paraaortic nodal metastasis in
surgically staged endometrial cancer patients with negative pelvic
lymph nodes. Gynecol Oncol. 115:236–238. 2009. View Article : Google Scholar : PubMed/NCBI
|