Introduction
Salivary duct carcinomas (SDC) are aggressive,
high-grade salivary malignancies first described by Kleinsasser
et al (1). The tumors are
characterized by a histological resemblance to ductal carcinoma of
the breast. The reported incidence of SDC is 1–3% among all
salivary tumors(2). This tumor
exhibits aggressive clinical behavior with a tendency for early
cervical lymphadenopathies and distant metastases to the lungs and
bones and thus, the prognosis of SDC is highly unfavourable
(3). Surgical resection followed by
radiation is the treatment of choice, however, locoregional
recurrences and distant metastases have frequently been reported
(4). The disease is rarely found in
the parotid gland. The present case study reviews the clinical data
of a patient with SDC in the deep lobe of the parotid gland and
discusses the relevant literature. Written informed consent was
obtained from the patient.
Case report
A 54-year-old male presented with a moderate,
painless swelling of the right parotid region that had been
apparent for two weeks. The patient had no history of fever or
other constitutional symptoms. A physical examination revealed a
firm, but mobile, lump that was not fixed to the overlying skin.
The functioning of the facial nerve was within normal limits. Upon
clinical examination, palpation identified no enlarged or
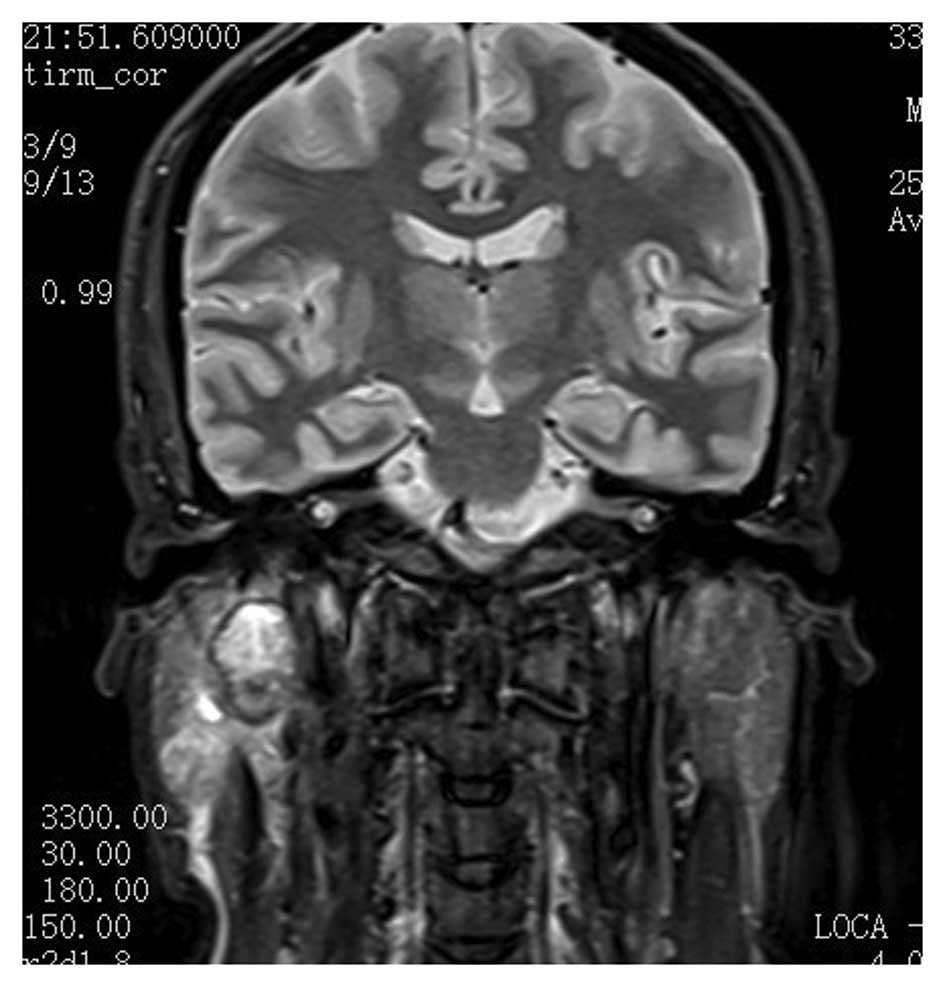
pathological lymph nodes. Magnetic resonance imaging (Fig. 1) identified a neoplasm of ~2 cm in
diameter located in the deep lobe of the parotid gland and
involving the exofacial parotid gland. This lesion was clinically
and radiologically classified as cT3cNxcM0 according to the World
Health Organization International Classification of Tumors
(5). Computed tomography of the
chest appeared negative for distant metastatic lesions.
The primary surgical treatment for the patient
consisted of a total parotidectomy conserving the facial nerve and
a modified ipsilateral radical neck dissection, as the
intraoperative histology suggested a malignant tumor. The excision
site was covered by a sternocleidomastoid muscle flap.
Intraoperatively, surgeons identified eight hard and enlarged lymph
nodes at cervical levels III and IV on the right side of the neck.
The largest lymph node, which measured 1.5×1.5 cm, was situated at
level III and showed early infiltration of the sternocleidomastoid
muscle. Frozen sections of the lymph nodes confirmed that six out
of eight nodes contained malignant cells, highly suggestive of
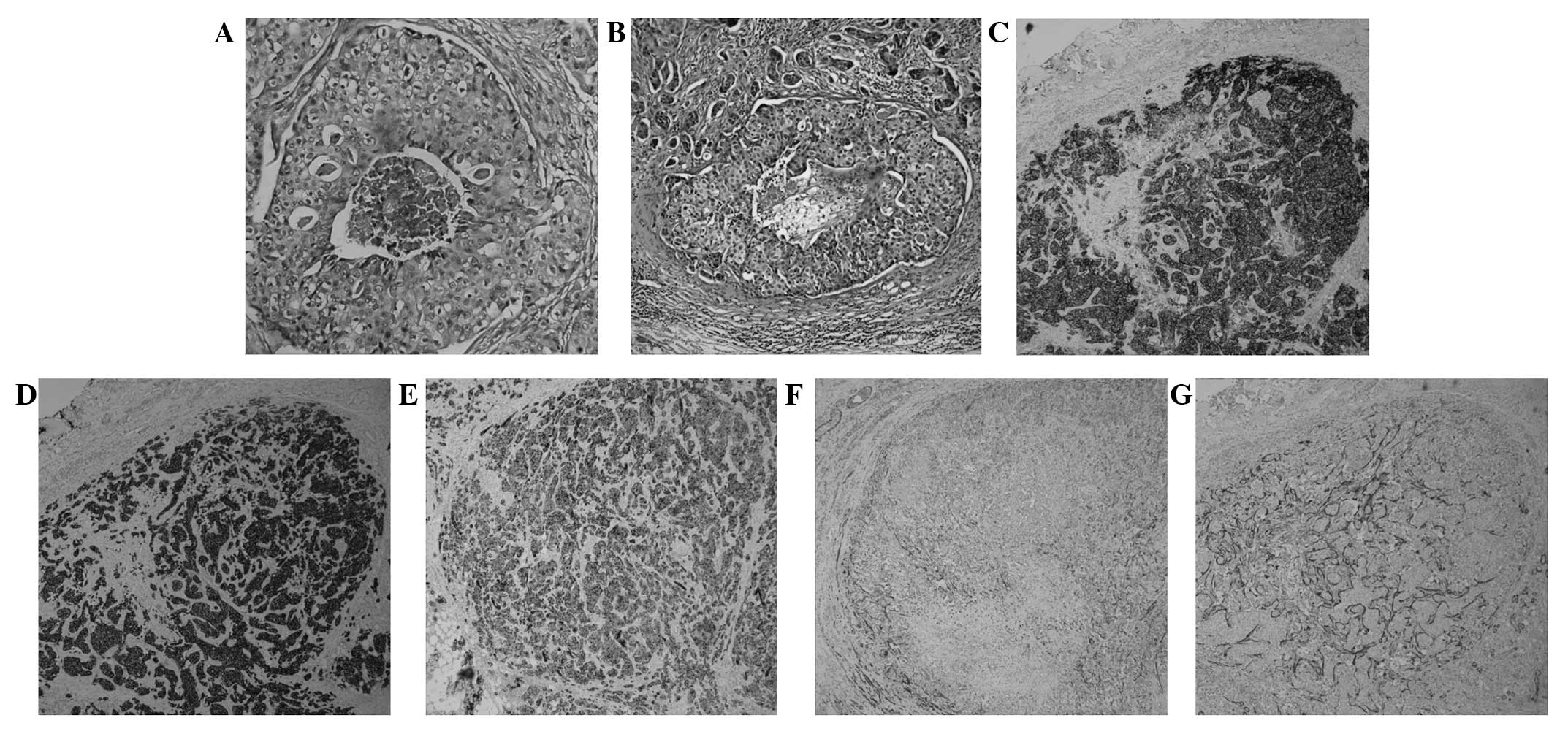
salivary gland carcinoma (Fig. 2B).
Upon final histopathological examination, a diagnosis of SDC was
confirmed. All the resection margins were free from tumor and the
tumor-free margin was <1.0 cm. In order to further investigate
the tissue samples, several immunohistochemical markers were
analyzed, including human epidermal growth factor 2 (HER-2), high
molecular weight cytokeratin (CK-H), CK8/CK18, p63, calponin, the
estrogen receptor (ER) and the progesterone receptor (PR).
The patient experienced no complications during
post-operative healing. Upon examination, the surgical margins were
clear, therefore, no further surgery was required. Fifteen days
after surgery, post-operative radiation therapy (60 Gy; 2 Gy, twice
a day, five days a week) was applied to the surgical bed and the
right neck area due to the aggressive nature of the tumor. The
final pathological staging was pT4pN2pM0, and three years after
treatment, the patient remains free from tumor recurrence.
Discussion
SDCs generally affect males in the fifth or sixth
decades of life, with the average age of occurrence at 60 years.
Valeri et al (2) declared
SDC to be a rare form of parotid tumor originating from the major
or minor salivary glands and accounting for 0.2–2% of all salivary
gland tumors (3). The majority of
cases of SDC present as a rapidly enlarging firm mass accompanied
by facial paralysis or pain. Cervical adenopathy and lymph node
invasion are identified in 35% (4)
and 40–80% (6) of SDC patients,
respectively. In the present case study, eight hard lymph nodes
were observed along the sternocleidomastoid, with six of them
demonstrating histopathological involvement.
In 2005, SDC was defined as an independent entity by
the World Health Organization, labeling it as ‘an aggressive
adenocarcinoma, which resembled high-grade breast ductal
carcinoma’. SDC was previously divided into two categories;
low-grade and high-grade SDC. The low-grade SDC was recognized as a
rare, cystic, proliferative carcinoma that resembled the spectrum
of breast lesions, including atypical ductal hyperplasia and
micropapillary and cribriform low-grade ductal carcinoma in
situ (7). Low-grade SDC has
subsequently been defined as a classification termed low-grade
cribriform cystadenocarcinoma. Under the current definition of SDC,
the present case study defines high-grade SDCs as tumors that
consist of solid invasive cancer nests with polygonal cancer cells
surrounding a comedo-like necrosis. In the present case study, it
was observed that the intraductal component of the primary foci and
the malignant lymph nodes exhibited central comedo necrosis
associated with a cribriform, solid or micropapillary architecture
(Fig. 2A and B). SDC is generally a
hematoxylin and eosin stain-based diagnosis, however, specific
immunohistochemical and staining techniques may confirm a diagnosis
in certain cases, and immunomarkers may be beneficial for future
therapeutic approaches. Immunohistochemically, SDC is positive for
the expression of low molecular weight CKs and epithelial membrane
antigen (8). Nikitakis et al
(9) demonstrated that CK7 was
diffusely positive in the majority of malignant salivary gland
tumors and that CK20 was intermittently focally stained. In the
present case study, immunohistochemistry of the tumor sample
identified that CK-H expression was diffusely positive, whilst
CK8/CK18 expression was moderately positive (Fig. 2D and E). SDC lesions are usually
negative when stained for the expression of S-100 protein or
basal-myoepithelial markers, such as CK 5/6 and 14, p63, calponin
and smooth muscle myosin heavy chain (8). However, the present case study
revealed that p63 and calponin were weakly positive in the
myoepithelium surrounding the ducts, which suggested that the
surrounding cells of the in situ lesions were neoplastic
(Fig. 2F and G). The overexpression
of HER2 protein, identified in ~90% of SDC cases (10), was apparent in the present case
study (Fig. 2C). Significant
differences have been identified between the hormone receptor
profiles of SDC and invasive ductal carcinoma of the breast. The
presence of the ER and PR is found in 75% of cases of breast
cancer, however, positivity for these markers is rare in SDC
(9). However, SDC analysis in the
present study found the samples to be ER- and PR-negative. Based on
these data, Simpson proposed that SDCs could be classified into
three main groups: Luminal androgen receptor-positive,
HER2-positive and basal phenotype, which may form the basis for
prognostic information and novel therapeutic possibilities
(8).
Due to the infiltrative nature of SDC, radical
surgery is the primary treatment; this involves the surgical
removal of the tumor by parotidectomy with or without conservation
of the facial nerve, followed by neck dissection to allow for
ipsilateral lymph node excision. However, the rate of locoregional
recurrence is high and the prognosis for survival is poor in the
case of insufficient resection margins, particularly in cases with
lymph node invasion (6). Lymphatic
embolism and perineural, extraparotid and/or lymphatic invasion are
further indicators of a poor prognosis. Post-operative radiation
therapy is mandatory in advanced cases of SDC, whereas
chemoradiotherapy is generally reserved for metastatic forms of the
tumor. The prognosis may be improved in tumors measuring <2 cm
(6,11), however, the five-year
recurrence-free survival rate remains at ~30% (2,12).
Previous studies have demonstrated that HER2 is an
effective therapeutic target for patients with HER2-positive breast
cancers. di Palma et al (1)
suggested that certain individuals with advanced SDC treated with
trastuzumab (an anti-HER2 monoclonal antibody) demonstrated
promising results. Therefore, patients with HER2 subtype SDCs may
benefit from targeted therapies using anti-HER2 monoclonal
antibodies, including trastuzumab and pertuzumab, or HER2 tyrosine
kinase inhibitors, such as lapatinib.
SDC is a rare and aggressive salivary gland
malignancy for which treatment is surgical resection and neck
dissection, with adjuvant radiation therapy reserved for the more
advanced forms. The current report may increase knowledge with
regard to SDCs. The primary clinical symptom presented by the
patient in this case was a painless mass in the right deep parotid.
Therefore, the pathological and immunohistochemical analysis of SDC
is required to diagnose patients with a painless mass in the deep
parotid, in order to avoid misdiagnosis. Furthermore, since SDC
usually develops aggressively with the possibility of early distant
metastasis and local recurrence, this indicates that surgery and
postoperative radiation are beneficial for SDC patients.
HER2-targeted therapies may therefore be a novel and effective
future treatment choice for certain SDC patients. Furthermore,
additional studies focusing on the etiology and mechanism of SDC
are required.
Acknowledgements
This study was supported by the Department of
Cranio-maxillofacial and Oral Surgery, University Hospital Zürich
(Zurich, Switzerland). The research described in this study was
supported by Guangdong Province Nature Science Foundation (grant
no. S2012010010382) and the Shenzhen Science and Research
Innovation Foundation (grant no. JCY20130402114702120).
Abbreviations:
|
SDC
|
salivary duct carcinoma
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
CK
|
cytokeratin
|
References
|
1
|
Kleinsasser O, Klein HJ and Hübner G:
Salivary duct carcinoma. A group of salivary gland tumors analogous
to mammary duct carcinoma. Arch Klin Exp Ohren Nasen
Kehlkopfheilkd. 192:100–105. 1968.(In German). View Article : Google Scholar
|
|
2
|
Valeri RM, Hadjileontis C, Skordalaki A,
Pandidou A, Vahtsevanos C and Destouni H: Salivary duct carcinoma
of the parotid gland: report of a rare case with a comparative
study of aspiration cytology and histomorphology. Acta Cytol.
49:61–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellis GL and Auclair PL: Tumors of the
Salivary Glands; Salivary Duct Carcinoma. Atlas Tumor of Pathology.
3rd series. Rosai J: Armed Forces Institute of Pathology;
Washington DC: pp. 455–488. 1996
|
|
4
|
Lewis JE, McKinney BC, Weiland LH,
Ferreiro JA and Olsen KD: Salivary duct carcinoma.
Clinicopathologic and immunohistochemical review of 26 cases.
Cancer. 77:223–230. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seifert G, Brocheriou C, Cardesa A and
Eveson JW: WHO International Histological Classification of
Tumours. Tentative Histological Classification of Salivary Gland
Tumours. Pathol Res Pract. 186:555–581. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
BenJelloun H, Maazouzi A, Benchakroun N,
Acharki A, Tawfiq N, Saharoui S and Benider A: Salivary duct
carcinoma of the parotid gland: report of two cases and literature
review. Cancer Radiother. 8:383–386. 2004.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brandwein-Gensler MS, Skálová A and Nagao
T: Salivary duct carcinoma Tumours of the Salivary Glands. World
Health Organization Classification of Tumours Pathology and
Genetics of Head and Neck Tumours. Barnes EL, Eveson JW, Reichart P
and Sidransky D: IARC Press; Lyon, France: pp. 236–237. 2005
|
|
8
|
Simpson RHW: salivary duct carcinoma: new
developments - morphological variants including pure in situ high
grade lesions; proposed molecular classification. Head Neck Pathol.
7(Suppl 1): S48–S58. 2013. View Article : Google Scholar
|
|
9
|
Nikitakis NG, Tosios KI, Papanikolaou VS,
Rivera H, Papanicolaou SI and Ioffe OB: Immunohistochemical
expression of cytokeratins 7 and 20 in malignant salivary gland
tumors. Mod Pathol. 17:407–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson CJ, Barry MB, Vasef MA and Deyoung
BR: Her-2/neu expression in salivary duct carcinoma: an
immunohistochemical and chromogenic in situ hybridization study.
Appl Immunohistochem Mol Morphol. 16:54–58. 2008.
|
|
11
|
Delgado R, Klimstra D and Albores-Saavedra
J: Low grade salivary duct carcinoma. A distinctive variant with a
low grade histology and a predominant intraductal growth pattern.
Cancer. 78:958–967. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jamal AM, Sun ZJ, Chen XM and Zhao YF:
Salivary duct carcinoma of the parotid gland: case report and
review of the literature. J Oral Maxillofac Surg. 66:1708–1713.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
di Palma S, Whitaker S, Potter K and
Pitkin L: Carcinoma ex pleomorphic adenoma successfully treated
with trastuzumab and radiotherapy. Virchows Arch. 461(Suppl 1):
S1442012.
|