Introduction
Papillary thyroid carcinoma (PTC) accounts for
>90% of newly diagnosed thyroid cancers. The incidence of PTC
has been increasing in previous decades, without significantly
modifying the characteristic low aggressive behavior, in spite of
frequent lymph node involvement (1). Overall, 20–50% of patients with PTC
experience cervical lymph node metastasis (2). Usually, metastases from PTC occur in a
stepwise fashion from the central to lateral neck compartments.
Therefore, the central compartment lymph nodes are at the greatest
risk of metastases from PTC. While the effectiveness of therapeutic
central neck dissection (CND) is undisputed, there is no consensus
on the role of CND in clinically node-negative patients with PTC.
Proponents of CND propose that CND offers more accurate staging and
may decrease the probability of locoregional recurrence (2–4).
Opponents of elective CND highlight the potential for increased
morbidity secondary to the risk of recurrent laryngeal nerve injury
and hypoparathyroidism (5,6). The overall risk and benefits of the
CND procedure must be determined on a patient-by-patient basis.
Therefore, the purpose of the present study was to analyze the
safety of CND and the risk factors of central nodal metastases in
PTC without clinical cervical lymph node metastasis
(cN0). Written informed consent was obtained from all
patients.
Patients and methods
Patients
The present study was a three-year single
institutional retrospective study, approved by the Medical Ethics
Committee at The Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, Zhejiang, China). The inclusion
criteria of the study were as follows: i) Pathologically confirmed
PTC; ii) pre-operative ultrasonography, with or without computed
tomography (CT) imaging of the thyroid and cervical lymph node
basins; iii) no evidence of nodal disease based on negative
physical examination, negative findings on pre-operative neck
ultrasonography or CT, or the presence of lyphadenopathy <5 mm
in diameter (cN0) (7);
iv) normal vocal cords, confirmed by flexible fiberoptic
laryngoscopy, and a normal parathyroid hormone (PTH) level (range,
15–65 pg/ml); v) CND (ipsilateral or bilateral) during planned
total thyroidectomy (primary or completion); and vi) medical
records and histological data available for review. A total of 389
patients who underwent CND for PTC between January 1, 2012 and
March 31, 2013 met the inclusion criteria for participation. The
exclusion criteria for the study were: i) Previous thyroid or
parathyroid surgery; ii) previous neck surgery; iii) previous neck
irradiation; iv) concomitant surgery for hyperparathyroidism; and
v) surgery for locoregional recurrence.
Surgery
Total thyroidectomy (TT) and CND were conducted by
one of three surgeons during the study period, all using a similar
technique. The study period was selected to maximize homogeneity of
the surgical technique such that 70% of CNDs were performed by a
single surgeon. An ultrasonic scalpel (Harmonic Focus; Johnson
& Johnson, New Brunswick, NJ, USA) was used in cases of
hemostasis. Routinely, recurrent laryngeal nerves were identified
and exposed until their insertion in the larynx and parathyroid
glands were identified and preserved. When devascularization or
incidental removal of the parathyroid glands was suspected, a
muscular autoimplantation procedure followed. Boundaries of the CND
(level VI) were as decided according to the American Thyroid
Association classification (8,9). Lymph
nodes in this compartment included the pretracheal, paratracheal,
prelaryngeal (Delphian) and perithyroidal nodes, including the
lymph nodes medial and lateral to the recurrent laryngeal nerves.
The superior boundary was defined as the cricoid cartilage, the
inferior boundary was the innominate artery and the lateral
boundaries were the common carotid arteries. Central neck
dissection was performed as either an ipsilateral dissection, on
the same side as the primary tumor, or as a bilateral dissection.
The pretracheal lymph nodes were included with the ipsilateral CND.
In general, bilateral CND was considered for patients with
pre-operative or intra-operative evidence of ipsilateral central
compartment adenopathy, contralateral central neck adenopathy or
bilateral disease in the thyroid.
Following surgery, frequencies and patterns of CND
metastases were analyzed with respect to patient characteristics,
age and gender, and pathological variables, consisting of tumor
size, histological type and primary tumor location (extra-thyroid
invasion or not, multifocal or unifocal). For multiple primary
lesions, the diameter of the largest dominant tumor was used in the
analyses.
Post-operative follow up
All clinical and pathological reports were reviewed.
Routine follow-up at one, three and six months post-surgery, and
every six months later, included neck ultrasonography and
estimation of the serum thyroid stimulate hormone (TSH) level
(reference range, 0.55–4.78 mU/l). Indirect laryngoscopy was
performed prior to surgery and on the first day after the
procedure. Vocal cord paresis that lasted for less than six months
post-surgery was regarded as transient, but paresis that persisted
for more than six months was regarded as permanent. The total serum
calcium level (reference range, 2.08–2.60 mmol/l) was measured 24 h
after surgery and medical treatment was initiated if the
concentration was <2.08 mmol/l. Medication was started
prophylactically in order to ensure that no patient developed
hypocalcaemia symptoms. When the total serum calcium level was in
the range of 1.8–2.08 mmol/l, calcium salts (1.5–3.0 g daily) were
administered, and when the level was <1.8 mmol/l, calcium plus
calcitriol (0.25–1.0 μg/day) was administered. The levels of serum
calcium, serum phosphate (normal range, 0.81–1.45 mmol/l) and
intact PTH (iPTH; chemiluminescence assay; normal range, 15–65
ng/l; detection limit, 6.0 ng/l) were determined prior to surgery,
on the first day post-surgery and at every follow-up subsequent to
the procedure. A serum calcium level of <2.08 mmol/l together
with a subnormal serum iPTH level (<15 ng/l) was defined as
transient hypoparathyroidism if the level was restored to a normal
value within six months after the withdrawal of calcium therapy.
Permanent hypoparathyroidism was regarded as persistent
hypocalcaemia with a serum iPTH level <15 ng/l for more than six
months after surgery, which required substitution with calcium,
with or without calcitriol.
Statistical analysis
Categorical data were compared using χ2
analysis (univariate analysis) and logistic regression analysis
(multivariate analysis) using SPSS Statistics version 18.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Between January 2012 and March 2013, 389 PTC
patients met the inclusion criteria for the present study, 287
females and 102 males (female:male ratio, 2.82:1) with a mean age
of 42.58±10.87 years. The patients were submitted for TT + CND
(Table I). All the patients were
successfully followed up until March 2014 (median follow-up, 18.5
months; range, 12.0–25.5 months). In 14/389 cases (3.60%),
parathyroid tissue was identified in the final histopathological
analysis. The incidence of surgical complications is reported in
Table II. In three patients
(0.77%), a neck hematoma that required surgical re-exploration was
observed. During the follow-up, only one patient (1/389; 0.26%), a
22-year-old female, experienced disease recurrence in the residual
thyroid gland and received an additional surgery 18 months after
the initial procedure. None of the patients experienced further
metastasis or tumor-associated mortality.
 | Table IDemographic and pathological data of
389 papilliary thyroid carcinoma patients. |
Table I
Demographic and pathological data of
389 papilliary thyroid carcinoma patients.
| Feature | Value | Percentage |
|---|
| Patients |
| Male | 102 | 26.2 |
| Female | 287 | 73.8 |
| Mean age, years | 42.58±10.87a | |
| History |
| Papillary
cancer | 389 | 100.0 |
| Tumor |
| Mean size | 0.71±0.35a | |
| ≤1 cm | 332 | 85.4 |
| >1 cm | 57 | 14.7 |
| Unique | 299 | 76.9 |
| Multifocal | 90 | 23.1 |
| Extrathyroid
invasion | 107 | 27.5 |
| Positive LN | 129 | 33.2 |
 | Table IIComplications in 389 papillary thyroid
carcinoma patients. |
Table II
Complications in 389 papillary thyroid
carcinoma patients.
| Complication | No. of patients | % |
|---|
| Transient
hypothyroidism | 48 | 12.34 |
| Permanent
hypothyroidim | 0 | 0.00 |
| Parathyroid tissue in
the specimen | 14 | 3.60 |
| Transient unilateral
vocal cord paralysis | 16 | 4.11 |
| Permanent unilateral
vocal cord paralysis | 0 | 0.00 |
| Bilateral vocal cord
paralysis | 0 | 0.00 |
| Neck hematoma | 3 | 0.77 |
The mean tumor size was 0.71±0.35 cm (range, 0.1–2
cm) and microcarcinoma (tumor size, ≤1 cm) was diagnosed in 332
patients (85.35%). The fact that all the tumors included were ≤2.0
cm was incidental. The histotype was papillary carcinoma in all
patients. A total of 90 patients (21.14%) possessed multifocal
tumors, 107 patients (27.51%) were found with tumor extrathyroid
invasion and lymph node involvement was identified in 129 patients
(33.16%) (Table I).
Clinicopathological factors affecting central nodal
metastases in patients were assessed by univariate analysis and are
summarized in Table III for
patients with conventional PTC and in Table IV for patients with a tumor size of
≤1.0 cm. There was no significant difference between patients with
central nodal metastases with respect to age [P=0.067; P-value for
microcarcinoma (P1)=0.202] or tumor focality (P=0.289;
P1=0.221). However, there were significant differences
between patients with central nodal metastases with respect to
gender (P=0.006; P1=0.007), tumor size (P=0.014;
P1=0.001) and extrathyroid invasion (P=0.04;
P1=0.039).
 | Table IIIClinicopathological factors affecting
central nodal metastases. |
Table III
Clinicopathological factors affecting
central nodal metastases.
| Characteristic | No. of patients | No. of LN-positive
patients (%) | P-value |
|---|
| Age, years |
| <45 | 228 | 84 (36.8) | |
| ≥45 | 161 | 45 (28.0) | 0.067 |
| Gender |
| Male | 102 | 45 (44.1) | |
| Female | 287 | 84 (29.3) | 0.006 |
| Tumor size, cm |
| ≤1 | 332 | 102 (30.7) | |
| >1 | 57 | 27 (47.4) | 0.014 |
| Extrathyroid
invasion |
| Yes | 107 | 44 (41.1) | |
| No | 282 | 85 (30.1) | 0.040 |
| Tumor focality |
| Unique | 299 | 95 (31.8) | |
| Multifocal | 90 | 34 (37.8) | 0.289 |
 | Table IVClinicopathological factors affecting
central nodal metastases in microcarcinoma patients (n=332). |
Table IV
Clinicopathological factors affecting
central nodal metastases in microcarcinoma patients (n=332).
| Characteristic | No. of patients | No. of LN-positive
patients, % |
P1-value |
|---|
| Age, years |
| <45 | 201 | 67 (33.3) | |
| ≥45 | 131 | 35 (26.7) | 0.202 |
| Gender |
| Male | 85 | 36 (42.4) | |
| Female | 247 | 66 (26.7) | 0.007 |
| Tumor size, cm |
| ≤0.5 | 144 | 31 (21.5) | |
| >0.5 | 188 | 71 (37.8) | 0.001 |
| Extrathyroid
invasion |
| Yes | 80 | 32 (40.0) | |
| No | 252 | 70 (27.8) | 0.039 |
| Tumor focality |
| Unique | 255 | 74 (29.0) | |
| Multifocal | 77 | 28 (36.4) | 0.221 |
In the multivariable analysis, the male gender and
tumor size were found to be significantly associated with central
lymph node metastasis for all the patients in the study (Tables V and VI).
 | Table VMultivariate logistic regression
analysis of central lymph node involvement. |
Table V
Multivariate logistic regression
analysis of central lymph node involvement.
| Variables in the
equation |
|---|
|
|
|---|
| Risk factors | B | S.E. | Wald | df | Sig. | Exp(B) |
|---|
| Gender | 0.650 | 0.246 | 7.016 | 1 | 0.008 | 1.916 |
| Tumor size | 1.319 | 0.337 | 15.288 | 1 | 0.000 | 3.738 |
| Extrathyroid
invasion | 0.182 | 0.255 | 0.510 | 1 | 0.475 | 1.200 |
| Constant | −1.907 | 0.279 | 46.848 | 1 | 0.000 | 0.149 |
 | Table VIMultivariate logistic regression
analysis of central lymph node involvement in microcarcinoma. |
Table VI
Multivariate logistic regression
analysis of central lymph node involvement in microcarcinoma.
| Variables in the
equation |
|---|
|
|
|---|
| Risk factors | B | S.E. | Wald | df | Sig1. | Exp(B) |
|---|
| Gender | 0.760 | 0.271 | 7.832 | 1 | 0.005 | 2.137 |
| Tumor size, cm | 1.958 | 0.572 | 11.708 | 1 | 0.001 | 7.086 |
| Extrathyroid | 0.235 | 0.292 | 0.648 | 1 | 0.421 | 1.265 |
| Constant | −2.312 | 0.388 | 35.532 | 1 | 0.000 | 0.099 |
From the statistics, it was indicated that compared
with females, males were more vulnerable to developing positive
lymph node involvement in the present study. The incidence of
central lymph node metastasis appeared to be higher with a larger
tumor size. The correlation coefficient between tumor size and
central lymph node metastasis was 0.228, which was calculated using
a two-tailed Pearson product-moment correlation (P<0.01).
Discussion
Differentiated PTC exhibits a high propensity to
spread to regional lymph nodes. As aforementioned, the reported
incidence of clinically positive lymph nodes ranges between 20 and
50% (2), and was 33.2% in the
present study. A higher proportion (80–90%) of patients exhibit
subclinical lymph node metastases (micrometastases) at the time of
surgical intervention (10–12). Despite the high incidence, lymph
node metastases are not considered prognostic for poor survival in
patients with well-differentiated PTC (13). Therefore, treatment of the cervical
lymph nodes in well-differentiated PTC remains controversial. The
primary argument for performing CND in the treatment of
well-differentiated PTC is to more accurately stage the tumor.
Accurate staging allows for improved risk stratification and the
more rational application of levothyroxine suppression and adjuvant
therapy, such as iodine 131I ablation (2–4). The
presence or absence of pathological lymph nodes in neck dissection
specimens has been reported to correlate with the incidence of
disease recurrence. Elective CND may aid in the prevention of local
recurrences in the central compartment where re-operation can be
challenging (2–4). Therefore, there are proponents and
opponents of elective CND. Certain PTC lesions (~25%), particularly
in the older patient population (≥45 years old), concentrate
radioactive iodine poorly (14–16).
In these cases, radioactive iodine treatment may not adequately
treat residual nodal micrometastasis. Thus, it is likely that
elective CND is most beneficial at the time of initial surgery for
selected high-risk patients.
Determining the patients that are high risk prior to
surgery remains difficult. The present study aimed to identify
factors associated with central neck compartment nodal metastases
as an initial step toward defining the patients that are most
likely to benefit from elective CND. Malignant lymph nodes were
found to occur with high frequency in male patients with a larger
tumor size or extrathyroid invasion. Overall, from the statistical
analysis, tumor size was found to be an extremely significant risk
factor of central lymph node involvement in PTC. There was a
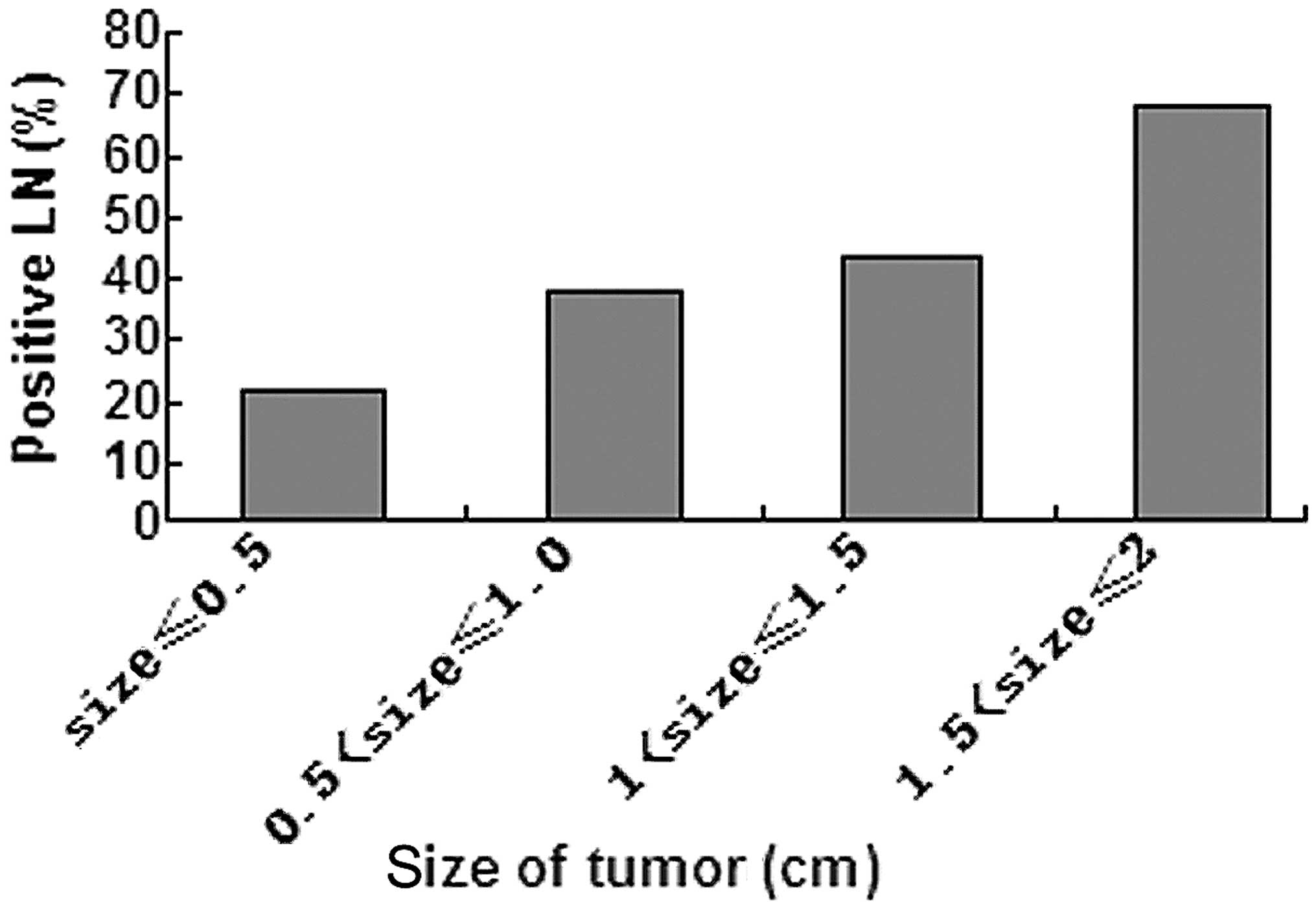
significant difference (P<0.05) in the incidence of central
lymph node metastasis between the four groups (Fig. 1) and the larger the tumor size, the
higher the incidence. Only when the tumor size was ≤0.5 cm, was the
incidence of lymph node involvement <30%.
Post-operative complications in the present study
were comparative to those of other studies and to TT alone without
CND (17–20). None of the patients underwent
permanent hypoparathyroidism or permanent vocal cord paralysis.
During the follow-up period, only one patient experienced
recurrence. It appeared that CND did not increase the chance of the
loco-regional recurrence. The safety of the surgery may partly be
due to the high volume of surgeries and the experience of the
surgeons at the Department of General Surgery at The Second
Affiliated Hospital of Zhejiang University School of Medicine, with
>1,000 cases treated per year. Therefore, the present conclusion
is that it is advisable for male PTC patients (cN0) with
a tumor size of ≥0.5 cm to receive prophylactic CND, particularly
when performed by a surgeon that treats a high volume of PTC
cases.
There are certain limitations to the present study.
First, all the procedures were conducted by three surgeons.
Although they were all surgeons who treat high volumes of PTC cases
and used a similar technique, there are differences between them
with regard to the extent of CND and the safety of the surgery.
Second, the follow-up period was not long enough. Complications,
particularly the recurrence rate or mortality rate due to PTC may
change over time. The third limitation may also have been an
advantage for the present study, as the tumor size was refined to
2.0 cm. If possible, an increased number of patients with larger
tumor sizes should be included in the future. Further observation
and study are required to better define the risk factors of central
lymph node metastasis and the safety of CND in cN0
PTC.
Acknowledgements
The authors would like to thank Dr. Ping Wang’s
surgery team for their hard work during the procedures and
follow-up period. The authors would also like to thank the patients
who were involved in the study.
References
|
1
|
Pacini F: Changing natural history of
differentiated thyroid cancer. Endocrine. 42:229–230. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper DS, Doherty GM, Haugen BR, et al:
American Thyroid Association Guidelines Taskforce: Management
guidelines for patients with thyroid nodules and differentiated
thyroid cancer. Thyroid. 16:109–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shindo M, Wu JC, Park EE and Tanzella F:
The importance of central compartment elective lymph node excision
in the staging and treatment of papillary thyroid cancer. Arch
Otolaryngol Head Neck Surg. 132:650–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White ML, Gauger PG and Doherty GM:
Central lymph node dissection in differentiated thyroid cancer.
World J Surg. 31:895–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaha AR: Management of the neck in
thyroid cancer. Otolaryngol Clin North Am. 31:823–831. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kowalski LP, Bagietto R, Lara JR, et al:
Prognostic significance of the distribution of neck node metastasis
from oral carcinoma. Head Neck. 22:207–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer. Cooper DS, Doherty GM, Haugen BR, et al: Revised American
Thyroid Association management guidelines for patients with thyroid
nodules and differentiated thyroid cancer. Thyroid. 19:1167–1214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
American Thyroid Association Surgery
Working Group; American Association of Endocrine Surgeons; American
Academy of Otolaryngology-Head and Neck Surgery; American Head and
Neck Society. Carty SE, Cooper DS, Doherty GM, et al: Consensus
statement on the terminology and classification of central neck
dissection for thyroid cancer. Thyroid. 19:1153–1158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chow SM, Law SC, Chan JK, et al: Papillary
microcarcinoma of the thyroid - prognostic significance of lymph
node metastasis and multifocality. Cancer. 98:31–40. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hay ID, Grant CS, van Heerden JA, et al:
Papillary thyroid microcarcinoma: a study of 535 cases observed in
a 50-year period. Surgery. 112:1139–1146. 1992.PubMed/NCBI
|
|
12
|
Qubain SW, Nakano S, Baba M, Takao S and
Aikou T: Distribution of lymph node micrometastasis in pN0
well-differentiated thyroid carcinoma. Surgery. 131:249–256. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaha AR: Implications of prognostic
factors and risk groups in the management of differentiated thyroid
cancer. Laryngoscope. 114:393–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schlumberger M, Challeton C, De Vathaire
F, et al: Radioactive iodine treatment and external radiotherapy
for lung and bone metastasis from thyroid carcinoma. J Nucl Med.
37:598–605. 1996.PubMed/NCBI
|
|
15
|
Sawka AM, Brierley JD, Tsang RW, et al: An
updated systemic review and commentary examining the effectiveness
of radioactive iodine remnant ablation in well-differentiated
thyroid cancer. Endocrinol Metab Clin North Am. 37:457–480. 2008.
View Article : Google Scholar
|
|
16
|
Ganti AK and Cohen EE: Iodine-refractory
thyroid carcinoma. Rev Recent Clin Trials. 1:133–141. 2006.
View Article : Google Scholar
|
|
17
|
Hughes DT, White ML, Miller BS, et al:
Influence of prophylactic central node dissection on postoperative
thyroglobulin levels and radioiodine treatment in papillary thyroid
cancer. Surgery. 148:1100–1106. 2010. View Article : Google Scholar
|
|
18
|
Poppadich A, Levin O, Lee JC, et al: A
multicenter cohort study of total thyroidectomy and routine central
lymph node dissection for cN0 papillary thyroid cancer.
Surgery. 150:1048–1057. 2011. View Article : Google Scholar
|
|
19
|
Giordano D, Valcavi R, Thompson GB, et al:
Complications of central neck dissection in patients with papillary
thyroid carcinoma: results of a study on 1087 patients and review
of the literature. Thyroid. 22:911–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barczyński M, Konturek A, Stopa M and
Nowak W: Prophylactic central neck dissection for papillary thyroid
cancer. Br J Surg. 100:410–418. 2013. View
Article : Google Scholar
|