Introduction
The prognosis of patients with T1aN0M0 renal cell
carcinoma (RCC) is favorable, and recurrence is rare. Risk factors
for recurrence in clinical T1a (cT1a) RCC have been previously
evaluated (1–4). Takayama et al reported that
symptomatic cancer, sarcomatoid component, and C-reactive protein
(CRP) levels ≥0.4 mg/dl were risk factors for recurrence in cT1a
RCC (1). In addition, Kume et
al reported that microvascular invasion (MVI) was an
independent predictor for distant metastasis of RCC with a diameter
of ≤3 cm (2). Since patients with
cT1a RCC include those with pathological T3a (pT3a) RCC, cT1a
tumors theoretically, frequently include more aggressive tumors
compared with patients with pT1a tumors. Although pT1a RCC tumors
generally recur less frequently than cT1a, there are a small number
of patients with pT1a disease recurrence.
Two studies have evaluated the predictors for
recurrence in patients with pT1a RCC (5,6). Kim
et al (5) revealed that MVI
and tumor necrosis were independent predictors for recurrence.
Nishikimi et al (6)
evaluated RCC patients with clear cell RCC using multivariate
analysis and found that Fuhrman grade, growth pattern and tumor
necrosis were significantly associated with disease-free survival.
As the majority of pT1a RCCs are less aggressive and recurrence is
rare, longer follow-up intervals are generally accepted compared
with RCCs at higher pathological stages (7). Kim et al (5) reported that 9 out of 93 pT1aN0M0
patients exhibited distant metastasis (mean follow-up duration,
63.6 months). Furthermore, Nishikimi et al (6) reported that 25 of 293 pT1aN0M0
patients exhibited distant metastasis (median follow-up duration,
62 months). If patients with pT1a RCC with a high risk of
recurrence are identified, clinicians can monitor these patients
closely and counsel them regarding the risk for recurrence.
The aim of the current study was to identify the
risk factors for predicting recurrence in patients with pT1aN0M0
RCC. We evaluated the clinical characteristics of patients with
pT1aN0M0 RCC in whom the disease recurred. In addition, we assessed
the clinical characteristics of patients with pT1bN0M0 RCC ≤5 cm,
who had a recurrence.
Patients and methods
We reviewed the medical records of patients with RCC
undergoing radical nephrectomy (RN) or partial nephrectomy (PN) at
the Department of Urology, National Defense Medical College
(Saitama, Japan) between 1990 and 2011. The study cohort consisted
of 133 patients in whom neither preoperative radiological or
pathological examination of surgical specimens indicated distant or
lymph node metastasis (N0M0 patients), and whose tumors were
pathologically confirmed as pT1a. Of these patients, 101 underwent
RN and 32 underwent PN. Their ages ranged from 32 to 89 years
(mean, 60.8±12.2). Local recurrence and metastasis were monitored
by examining each patient postoperatively at 3–6 month intervals
for the first 5 years, and every 6–12 months thereafter. Follow-up
included physical examination, laboratory tests, chest radiography,
abdominal and chest computed tomography and, if necessary,
radionuclide bone scanning. The total follow-up time ranged from 1
to 261 months (median, 57.8). Recurrence-free survival (RFS) was
evaluated using the date at which local recurrence or metastatic
disease was identified, and overall survival (OS) was determined
using either the date of death or the date of the last follow-up
examination.
The clinicopathological factors evaluated are listed
in Table I, and included age, gender, tumor size, histological
subtype, histological tumor grade, MVI, histological tumor
necrosis, CRP levels, and Eastern Cooperative Oncology Group
performance status (ECOG PS) (8).
These factors were compared between patients with recurrence
postoperatively (n=5, 3.8%), and those without (n=128). Tumors were
staged according to the 2002 TNM classification system (9), and nucleolar grading in a three-grade
system was determined (10). Tumor
necrosis was defined as microscopic coagulative necrosis (6,11); the
presence of necrosis that was apparent on gross examination was
excluded. Preoperative elevation of CRP was defined as CRP ≥0.3
mg/dl, as previously described (12,13).
We also reviewed the clinicopathological factors of
patients with pT1b tumors ≤5 cm, and of those with and without
recurrence.
Statistical analysis
Results are presented as the mean ± standard
deviation, and differences in variables between groups were
compared using the Mann-Whitney U test. The independence of fit of
categorical data was analyzed using the χ2 test.
Survival curves were constructed using the Kaplan-Meier method, and
differences between groups were assessed using the log-rank test.
To determine independent factors predicting recurrence in patients
with pT1aN0M0 RCC, univariate and multivariate analyses were
performed using the Cox proportional-hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
patients with disease recurrence (Table
I)
Five out of 133 patients with pT1a (3.8%) exhibited
disease recurrence (median follow-up, 57.8 months). The mean age of
these five patients (three males and two females) was 60.8 years
(56–75). Four patients underwent right nephrectomy and one
underwent left nephrectomy. The mean diameter of the five tumors
was 3.5 cm, and all were ≥3 cm. The ECOG PS in three patients was
0, in one patient was 1 and in the remaining patient was 3.
Metastases were detected in the lungs of three patients, the
mediastinal lymph node in one and the contralateral kidney of one
patient. The time from nephrectomy to recurrence was <1 year in
two patients (3.5 and 5.9 months), and >4 years in three
patients (48.2, 61.2 and 77.5 months). All five patients had clear
cell-type RCC; four tumors were histological grade 2 and one was
grade 3. Two of the five tumors (40%) had microvascular invasion
and three (60%) had histological tumor necrosis. Two patients (40%)
had preoperative CRP levels ≥0.3 mg/dl. No patients had
thrombocytosis.
Comparison of clinicopathological factors
between patients with and without recurrence (Table I)
Tumor size and the percentage of tumor necrosis were
significantly higher in patients with recurrence than in those
without. Age (P=0.2636), gender (P=0.5930), size of the tumor
(P=0.1769), ECOG PS (P=0.0778), RCC subtype (P=0.6203), the
presence of grade 3 component (P=0.4325), the presence of MVI
(P=0.1114) and CRP (P=0.0515) were not significantly different
between the two groups.
Impact of clinicopathological factors on
recurrence in patients with pT1aN0M0 RCC
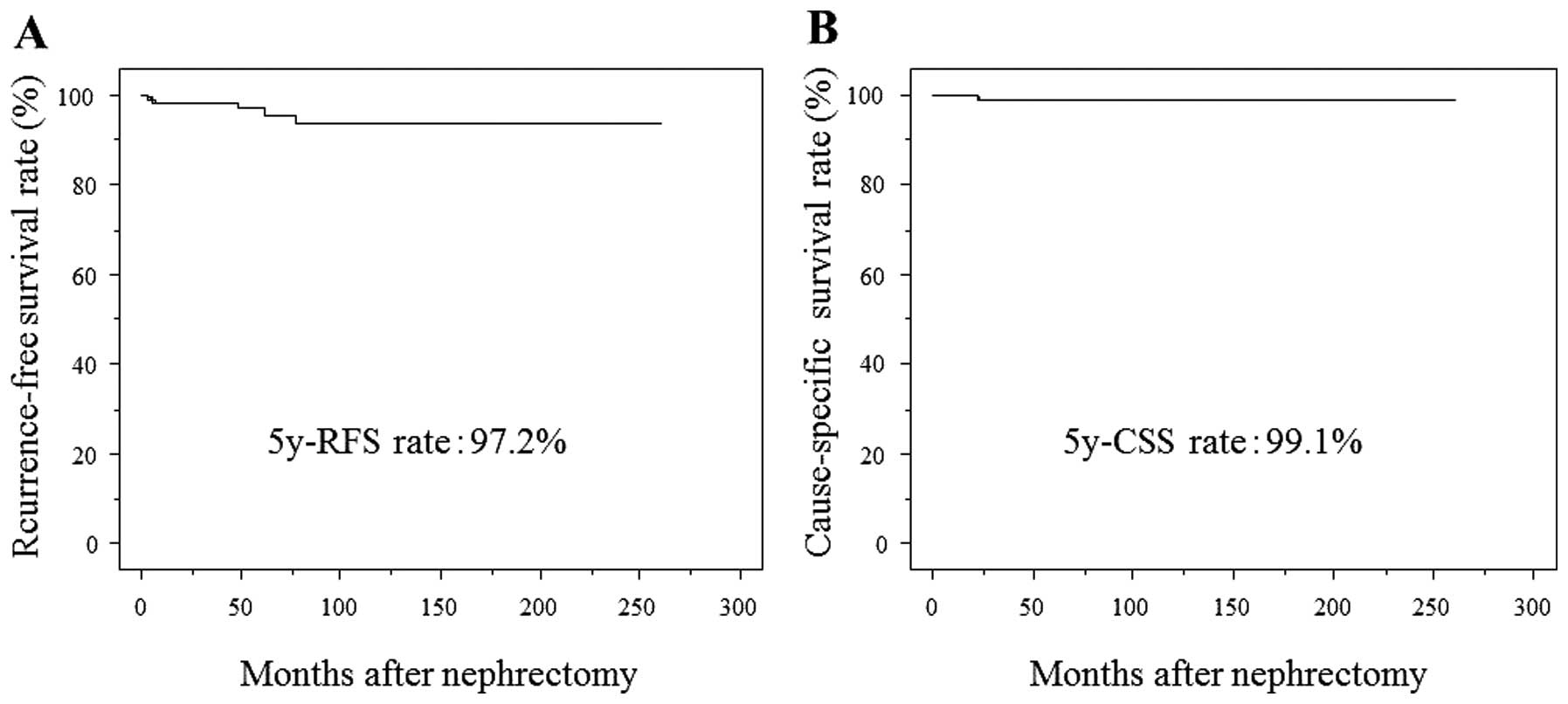
In all patients with pT1aN0M0 RCC, the 5-year RFS
and CSS rates were 97.2 and 99.1%, respectively (Fig. 1). Kaplan-Meier analysis revealed
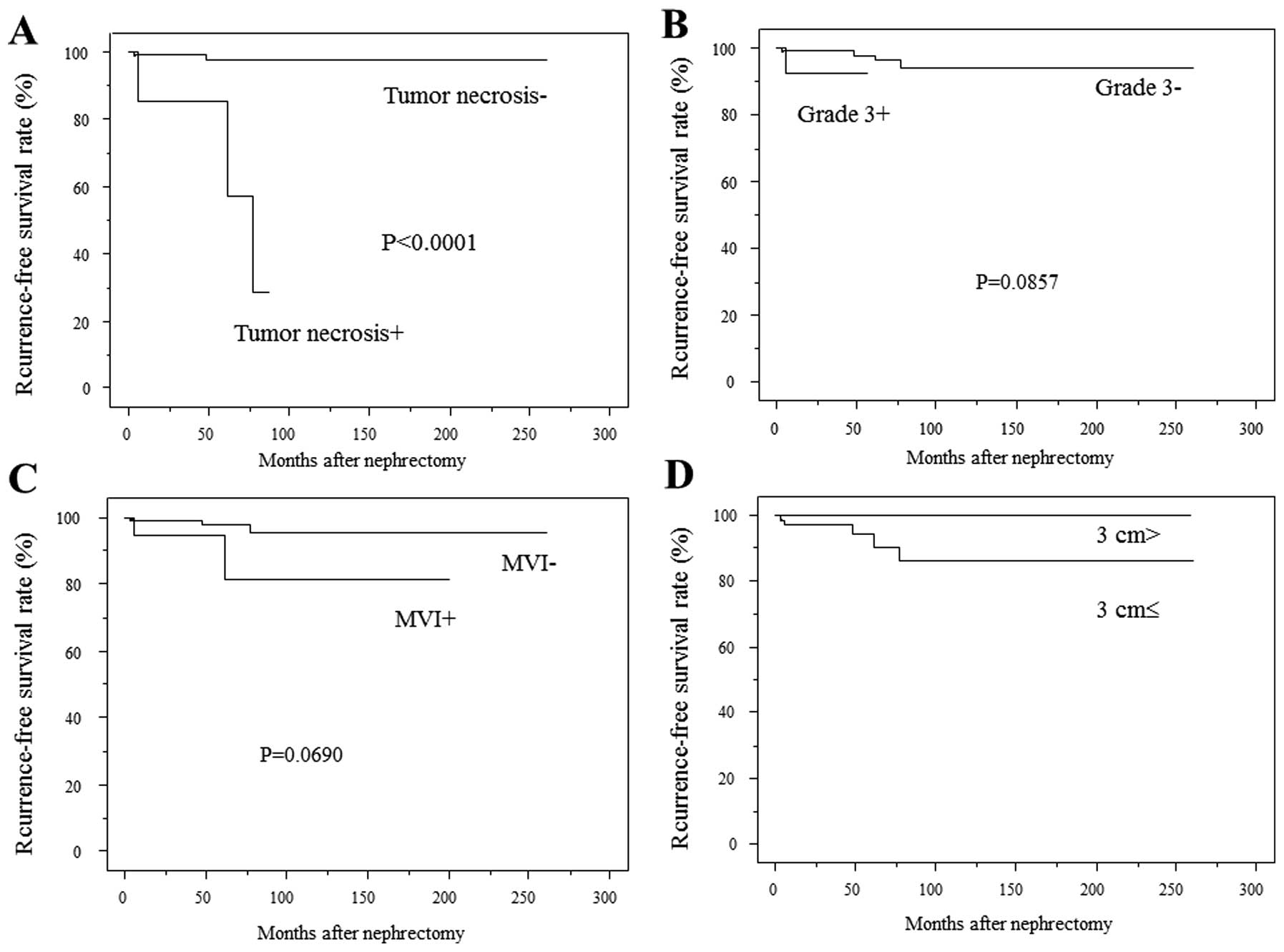
that the recurrence rate was significantly higher in patients with
histological tumor necrosis than in those without (P<0.0001)
(Fig. 2A). The 5- and 7-year RFS
rates were 85.7 and 28.6% in patients with tumor necrosis, and 97.9
and 97.9% in patients without tumor necrosis, respectively. The
recurrence rates were not significantly different between patients
with a grade 3 component and those without (Fig. 2B), or between patients with and
without MVI (Fig. 2C). Patients
with a tumor size <3 cm had no recurrence (Fig. 2D).
Factors predicting recurrence in patients
with pT1aN0M0 RCC
The Cox proportional-hazards regression model was
used to evaluate factors predicting recurrence. Univariate analysis
showed that tumor size (P=0.0379) and presence of tumor necrosis
(P=0.0003) were significantly associated with RFS. Multivariate Cox
proportional-hazards regression model analysis revealed that the
presence of tumor necrosis was the only significant predictor of
RFS (P=0.0143) (Table II).
 | Table IIMultivariate analysis for predicting
recurrence in patients with pT1aN0M0 RCC (n=133). |
Table II
Multivariate analysis for predicting
recurrence in patients with pT1aN0M0 RCC (n=133).
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variables | P-value | P-value | Odds ratio | Relative risk ratio
95% CI |
|---|
| Age | 0.1591 | | | |
| Gender | 0.7189 | | | |
| ECOG PS | 0.1151 | | | |
| Tumor side | 0.2449 | | | |
| Tumor size | 0.0379 | 0.3622 | 2.355a | 0.0373–14.866 |
| Grade 3 component
(+) | 0.1353 | | | |
| MVI (+) | 0.0975 | | | |
| Tumor necrosis
(+) | 0.0003 | 0.0143 | 14.286 | 1.701–125 |
| CRP (≥0.3 mg/dl) | 0.1061 | | | |
Comparison of clinicopathological factors
in patients with pT1b ≤5 cm with and without recurrence
We reviewed the clinicopathological factors of
patients with tumors larger than pT1a tumors (pT1b tumors
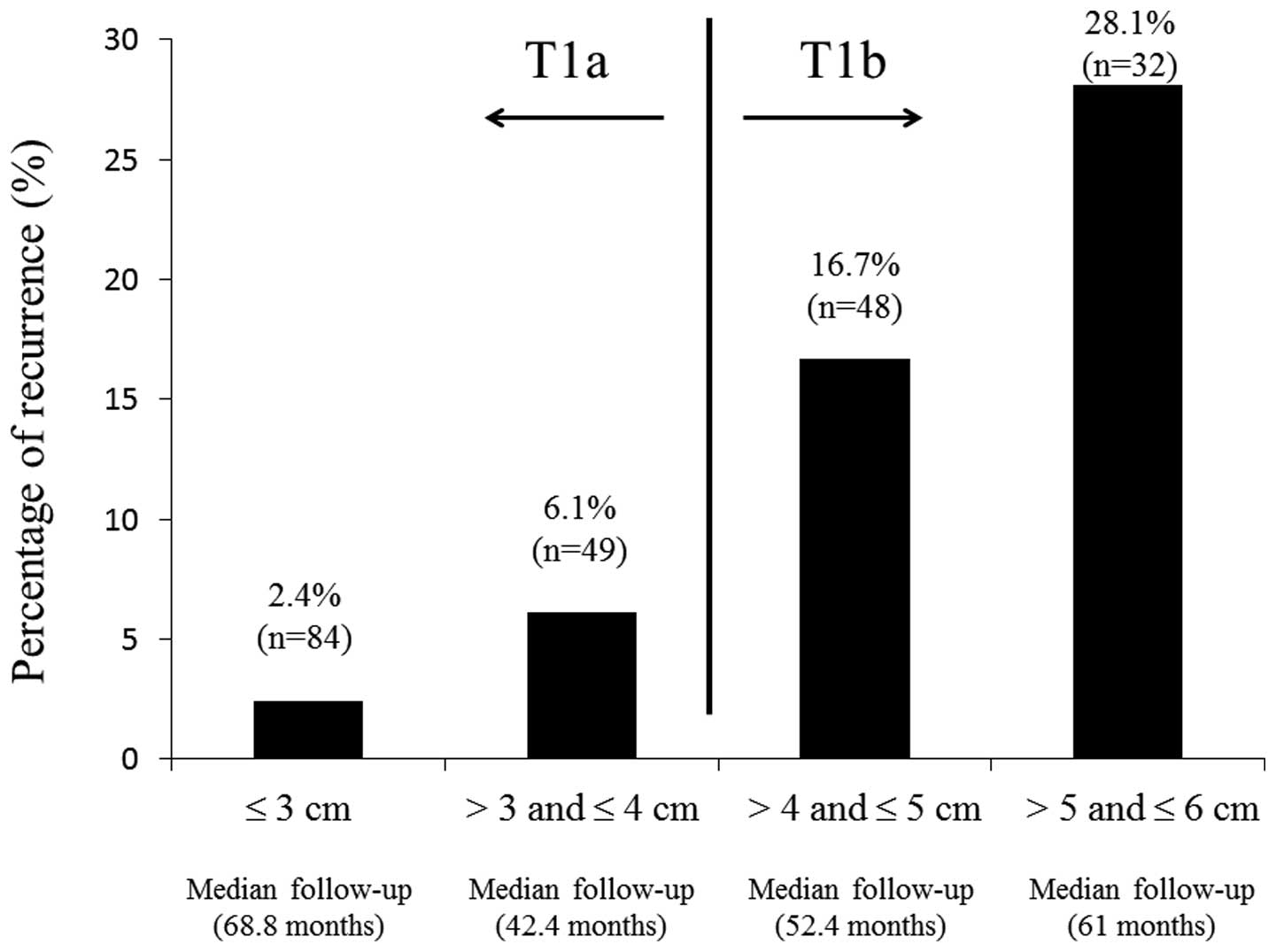
>4cm-≤5 cm) with and without recurrence. As shown in Fig. 3, the percentage of RCC patients with
recurrence gradually increased according to tumor size. The
percentage of patients with pT1aN0M0 >3 cm (n=49; median
follow-up time, 42.4 months) was 6.1%, whereas the percentage of
patients with pT1b ≤5 cm (n=48; median follow-up time, 52.4 months)
was 16.7%. When the clinicopathological factors of patients with
pT1b tumors ≤5 cm with and without recurrence were compared, the
percentage of tumor necrosis (P=0.0261) and gender (P=0.0367) were
significantly different (Table
III), suggesting that tumor necrosis may be an important
predictor for the recurrence of small RCCs.
 | Table IIIComparison of clinicopathological
factors between pT1bN0M0 (≤5 cm) patients with recurrence and those
without. |
Table III
Comparison of clinicopathological
factors between pT1bN0M0 (≤5 cm) patients with recurrence and those
without.
| Variables | Patients with pT1b
tumor (≤5 cm) (rec.+) (n=8) | Patients with pT1b
tumor (≤5 cm) (rec. −) (n=40) | P-value |
|---|
| Age (years) | 65±10 | 60±13 | 0.2509 |
| Gender
(male/female) | 8/0 | 25/15 | 0.0367 |
| Side
(right/left) | 3/5 | 21/19 | 0.4386 |
| Tumor size (cm) | 4.6±0.3 | 4.4±0.3 | 0.0563 |
| ECOG PS (0 vs.
1) | 0/8 | 6/33 | 0.2349 |
| Grade 3 (+ vs.
−) | 3/5 | 14/26 | 0.8926 |
| MVI (+ vs. −) | 5/3 | 16/24 | 0.2416 |
| Tumor necrosis (+
vs. −) | 4/4 | 6/34 | 0.0261 |
| CRP (0.3> vs.
0.3) | 5/3 | 11/29 | 0.0552 |
Discussion
In the present study, five of 133 patients with
pT1aN0M0 RCC (3.8%) experienced tumor recurrence (median follow-up
time, 57.8 months). In previous studies, the 5-year RFS rates were
88–93% in patients with pT1aN0M0 RCC (5,6). The
5-year RFS rate in our study was higher than that in the previous
studies. In the current study, patients with recurrence had a
significantly increased tumor size and a higher percentage of tumor
necrosis compared with patients without recurrence. Univariate
analysis for the prediction of recurrence revealed that tumor size
and necrosis were significant factors, but only tumor necrosis was
an independent predictor for recurrence using multivariate
analysis. When patients with pT1bN0M0 RCC with tumors sized ≤5 cm
were evaluated, the percentage of tumor necrosis was higher in
patients with recurrence compared with without recurrence.
Therefore, tumor necrosis appeared to be a strong predictor for
recurrence in small RCCs.
Predictors for recurrence and prognosis in cT1a RCC
have been previously evaluated (1–4).
Takayama et al (1) reported
that symptomatic cancer and the presence of sarcomatoid components
were independent risk factors for metachronous metastasis, and CRP
levels of ≥0.4 mg/dl were an independent prognostic factor for
overall survival. Kume et al (2) reported that MVI was an independent
predictor for metastasis (2).
Furthermore, cT1a RCC patients with tumors ≥3.1 cm exhibited lower
recurrence-free survival rates than those with tumor ≤3.0 cm, and
patients with MVI exhibited lower recurrence-free survival rates
than those without MVI (3). By
contrast, tumor size not identified as an independent progostic
factor in RCC patients with tumors ≤4 cm (4). We hypothesize that tumors in patients
with cT1a RCC are theoretically more aggressive than those with
pT1a RCC. In previous studies, the common site of recurrence in
patients with cT1a RCC was the bone (1,2).
Takayama et al reported that 65% of patients with cT1a with
simultaneous or metachronus metastasis had bone metastasis
(1). The authors also reported that
the presence of a sarcomatoid component was an independent
predictor for prognosis, and four out of the five patients with a
sarcomatoid component exhibited bone metastases. Consistent with
this, Nishikimi et al reported that the bone was a
predominant site of recurrence (10 of 25 recurrent patients, 40%)
in patients with pT1aN0M0 RCC (6).
However, the mechanism for the preference of bone metastasis in
cT1a RCC remains unclear. By contrast, there were no patients with
bone recurrence in the present study. Kim et al reported
that the lungs were the major site of recurrence in patients with
pT1aN0M0RCC (four of nine patients), and only one patient had a
recurrence in the bone (5).
Therefore, it remains controversial whether the bone is a preferred
site of recurrence in pT1aN0M0 RCC.
A small number of studies have set out to identify
predictors for recurrence in pT1aN0M RCC. Nishikimi et al
reported that Fuhrman nucleolar grade, growth pattern and tumor
necrosis were independent predictors for recurrence in pT1aN0M0
clear cell RCC (6). In addition,
Kim et al reported that microvascular invasion and tumor
necrosis were independent predictors for distant metastasis in
pT1aN0M0 RCC (5). Consistent with
these two studies, the current study identified that tumor necrosis
was an independent predictor for recurrence, suggesting that tumor
necrosis may be an important predictor for the recurrence of
pT1aN0M0 RCC.
In the present study, we also evaluated pT1bN0M0 RCC
with a tumor size ≤5 cm. In this population with relatively small
pT1b tumors, patients with a recurrence had a significantly higher
percentage of tumor necrosis than those without recurrence (50 vs.
15%, P=0.0261). This suggests that tumor necrosis may predict the
recurrence of small RCCs, which generally have low recurrence
rates. Moreover, we have previously demonstrated that the
non-normalization of postoperative CRP, pre-CRP elevation,
microvascular invasion, and histological tumor necrosis were
independent predictors for recurrence in N0M0 clear cell RCC
(13). Therefore, tumor necrosis
appears to accurately reflect biological activity, tumor grade and
microvascular invasion; thus, it may predict recurrence of RCC.
MVI is an important predictor for recurrence in low
clinical stage RCC (2,3,14). In
our five patients with recurrence, two (40%) had MVI. In addition,
four of the five patients with recurrence had distant visceral
metastases, and one had LN metastasis. Distant and LN metastasis
theoretically require MVI. Therefore, tumors with a small degree of
MVI may be occasionally diagnosed as lacking MVI. In contrast,
tumor necrosis usually occupies a relatively large area in RCC
specimens compared with MVI. Therefore, the presence of tumor
necrosis is unlikely to be missed during pathological
diagnosis.
Identifying the risk factors for recurrence may be
useful for determining the optimal follow-up period in patients
with pT1a RCC. Antonelli et al defined a follow-up protocol
based on the University of California Los Angelus Integrated
Staging System (15) after surgery
for N0M0 RCC (16). In their study,
pT1 low-risk patients (pT1 and nucleolar grade 1–2, ECOG PS=0)
required thoracic examination every 30 months and abdominal
examination annually for 5 years after surgery. In addition, Hafez
reported that annual follow-up with a medical history, physical
examination, and select laboratory studies were sufficient for
patients with RCC ≤2.5 cm (17). If
we can establish a risk classification system that includes tumor
necrosis as a predictor for recurrence, it may be possible to more
effectively predict recurrence in patients with pT1a RCC.
Therefore, risk classification may be useful for determining
individual-based follow-up periods. Very few patients with pT1aN0M0
RCC have tumor necrosis in RCC specimens, which was demonstrated in
the present study (7/133 patients) and a previous study (8/293
patients) (6). However, if tumor
necrosis is detected, the patients should be followed more closely
than patients without tumor necrosis.
The present study has several limitations. First,
this is a non-randomized, retrospective, single-center study.
Therefore, a prospective study including a large number of patients
is required to confirm these observations. However, the current
study revealed an important finding; tumor necrosis was an
independent predictor for recurrence in pT1aN0M0 RCC.
Histological tumor necrosis was the only independent
predictor for recurrence in patients with pT1aN0M0 RCC. The
frequency of tumor necrosis was low in patients with pT1aN0M0 RCC.
However, patients with tumor necrosis in RCC specimens had a
significantly higher risk for recurrence compared with those
without tumor necrosis. Therefore, the presence of tumor necrosis
may reflect an aggressive biological activity and be an effective
predictor for recurrence in small RCCs.
Acknowledgements
This abstract was presented at the Annual Meeting of
the International Society of Urology, Oct 1-Oct 3, 2012, Montreal,
QC, Canada, which was published as abstract no. EP. 178 in Urology
80 (Suppl 3A), 2012.
References
|
1
|
Takayama T, Sugiyama T, Kai F, et al:
Characteristics of aggressive variants in T1a renal cell carcinoma.
J Cancer Res Clin Oncol. 137:1653–1659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kume H, Suzuki M, Fujimura T, et al:
Distant metastasis of renal cell carcinoma with a diameter of 3 cm
or less-which is aggressive cancer? J Urol. 184:64–68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitagawa Y, Nakashima K, Shima T, et al:
Clinicopathological outcomes of clinical T1a renal cell carcinoma
by tumor size. Jpn J Clin Oncol. 41:637–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klatte T, Patard JJ, de Martino M, et al:
Tumor size does not predict risk of metastatic disease or prognosis
of small renal cell carcinomas. J Urol. 179:1719–1729. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JM, Song PH, Kim HT and Park TC: The
prognostic factors for patients with pT1a renal cell carcinoma.
Korean J Urol. 51:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishikimi T, Tsuzuki T, Fujita T, et al:
The post-operative pathological prognostic parameters of clear cell
renal cell carcinoma in pT1a cases. Pathol Int. 61:116–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siddiqui SA, Frank I, Cheville JC, Loshe
CM, Leibovich BC and Blute ML: Postoperative surveillance for renal
cell carcinoma: a multifactorial histological subtype specific
protocol. BJU Int. 104:778–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Cancer Institute. Common Toxicity
Criteria, version 2.0. (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf).
Accessed October 19, 2014
|
|
9
|
Greene FL, Page DL, Fleming ID, et al:
Kidney. AJCC Cancer Staging Manual. 6th edition. Springer-Verlag;
New York, NY: pp. 323–328. 2002
|
|
10
|
Ito K, Yoshii H, Asakuma J, et al:
Clinical impact of the presence of the worst nucleolar grade in
renal cell carcinoma specimens. Jpn J Clin Oncol. 39:588–594. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sengupta S, Lohse CM, Leibovich BC, et al:
Histologic coagulative tumor necrosis as a prognostic indicator of
renal cell carcinoma aggressiveness. Cancer. 104:511–520. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito K, Asano T, Yoshii H, et al: Impact of
thrombocytosis and C-reactive protein elevation on the prognosis
for patients with renal cell carcinoma. Int J Urol. 13:1365–1370.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito K, Yoshii H, Sato A, et al: Impact of
postoperative C-reactive protein level on recurrence and prognosis
in patients with N0M0 clear cell renal cell carcinoma. J Urol.
186:430–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gonçalves PD, Srougi M, Dall’lio MF, et
al: Low clinical stage renal cell carcinoma: relevance of
microvascular tumor invasion as a prognostic parameter. J Urol.
172:470–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zisman A, Pantuck AJ, Dorey F, et al:
Improved prognostication of renal cell carcinoma using an
integrated staging system. J Clin Oncol. 19:1649–1657.
2001.PubMed/NCBI
|
|
16
|
Antonelli A, Cozzoli A, Simeone C, et al:
Surgical treatment of adrenal metastasis from renal cell carcinoma:
a single-centre experience of 45 patients. BJU Int. 97:505–508.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hafez KS, Novick AC and Campbell SC:
Patterns of tumor recurrence and guidelines for followup after
nephron sparing surgery for sporadic renal cell carcinoma. J Urol.
157:2067–2070. 1997. View Article : Google Scholar : PubMed/NCBI
|