Introduction
Primary hepatic lymphoma (PHL) is an extremely rare
malignancy. PHL is defined as an extranodal lymphoma of the liver
without involvement of any other organs. PHL constitutes 0.4% of
cases of extranodal non-Hodgkin’s lymphoma (NHL), and comprises
~0.01% of all NHL (1). PHL occurs
in males twice as often as in females, and the usual age at
presentation is >50 years (2).
The symptoms are usually non-specific, however, the most common
symptom is abdominal pain. Additionally, laboratory tests and
cancer markers are non-specific. Liver biopsies remain the most
valuable tool for the diagnosis of PHL (3). The CHOP (cyclophosphamide,
doxorubicin, vincristine, prednisone) chemotherapy regimen is the
standard treatment and the prognosis of patients with PHL is
associated with PHL subtypes (4).
The current study presents an unusual case of primary NHL with
rectal cancer. A review of the literature with regard to the
clinical features, diagnosis and management of PHL is also
provided. Written informed consent was obtained from the patient
for publication of this case study.
Case report
In November 2012, a 56-year-old male presented to
the Affiliated Tumor Hospital and Oncology School of Guangxi
Medical University (Nanning, Guangxi, China) with a 3-month history
of bloody stools. The patient exhibited no symptoms of a fever,
night sweats, nausea, vomiting, chest pain, abdominal pain,
diarrhea, loss of appetite, changes in bowel habits or weight loss.
A physical examination showed no notable results. No superficial
lymphadenopathy was present.
The laboratory results included a hemoglobin level
of 146.00 g/l and a white cell count of 4.79×109/l, with
a normal differential. The levels of alanine aminotransferase,
aspartate aminotransferase, alkaline phosphatase and lactate
dehydrogenase were also within normal limits. The serum tumor
marker results included a cancer antigen 125 level of 22.49 U/ml,
and a cancer antigen 15-3 level of 8.92 U/ml, as well as normal
levels of serum α-fetoprotein and carcinoembryonic antigen.
Serology was positive for the hepatitis B virus (HBV), and negative
for the hepatitis C virus (HCV) and the human immunodeficiency
virus.
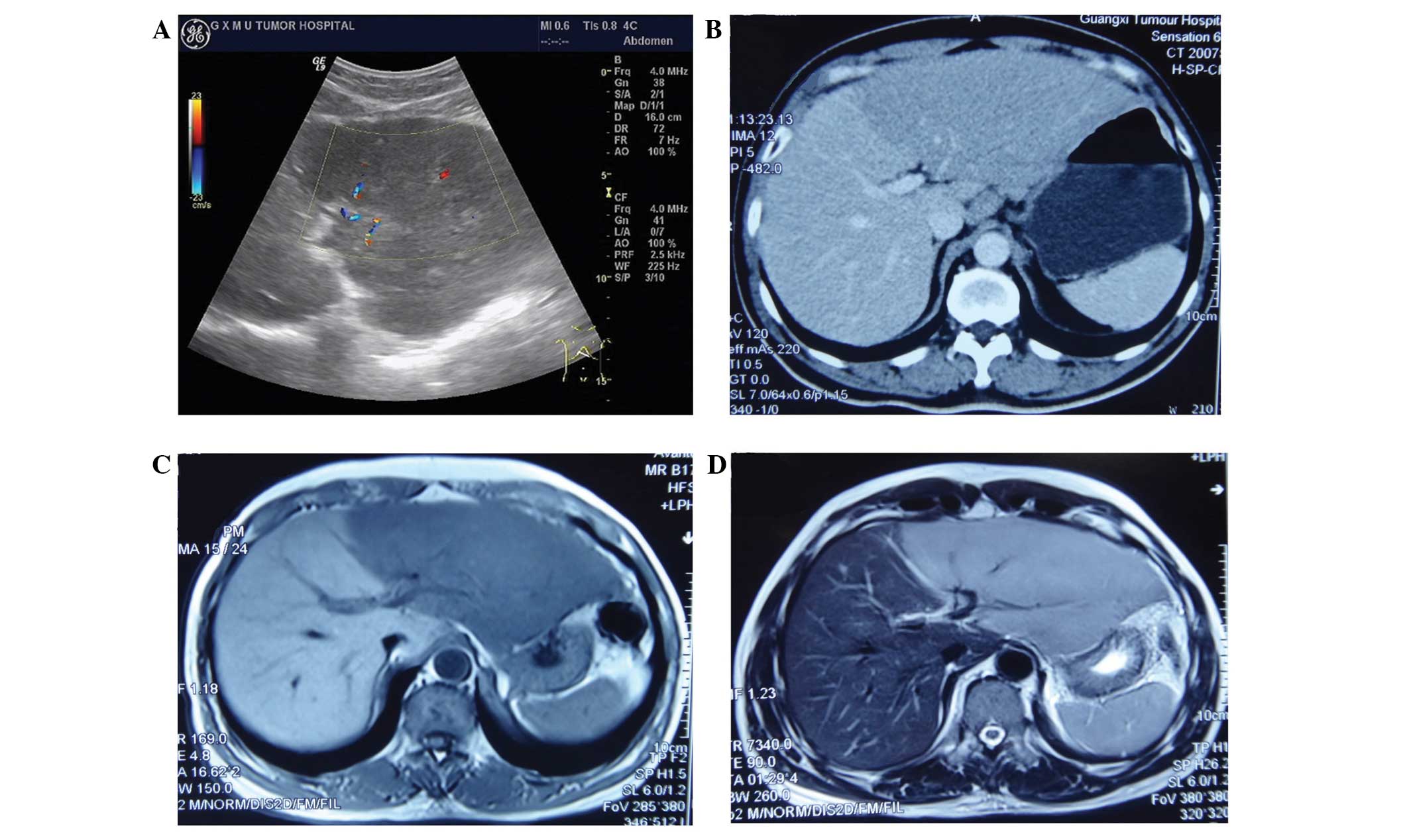
Imaging studies of the left lobe of the liver showed
abnormal increases with smooth edges, and mixed iso- and
hypoechogenicity in the ultrasound examination. Hypodensity in the
pre-contrast phase and no enhancement in the post-contrast phase
was observed on computed tomography (CT). Hypointensity was
observed on T1-weighted imaging (WI) and hyper-intensity on T2WI by
magnetic resonance imaging (MRI) (Fig.
1). Radiography and CT did not reveal any mediastinal and
abdominal lymphadenopathy. The pancreas, spleen, and biliary tract
were normal.
Colonoscopy showed a tumor in the bowel wall growing
out from the anus by 3–5 cm, with surface erosion, and pathological
analysis of a biopsy specimen that was obtained revealed a
moderately-differentiated adenocarcinoma. Histological analysis
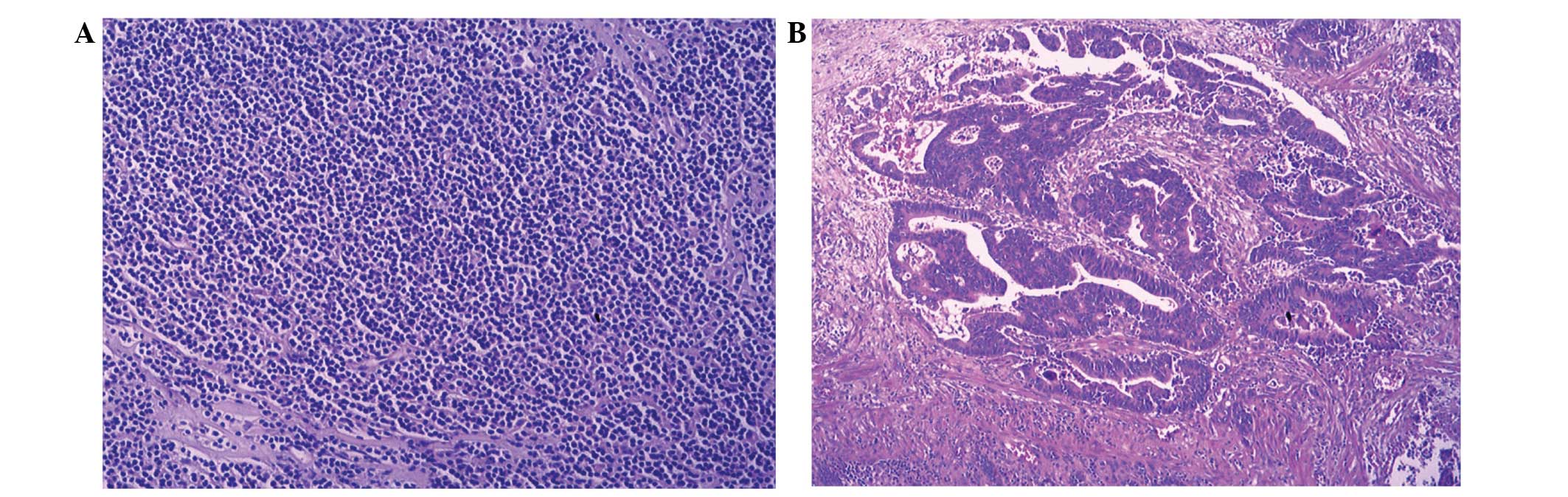
following ultrasound-guided biopsy of the lesion in the left lobe
of the liver showed diffuse infiltrates and small to intermediate
atypical cells consistent with lymphoma (Fig. 2), Immunostaining of the tumor cells
showed reactivity for cluster of differentiation 79a, pax-5, light
chain λ and B-cell lymphoma-2.
Bone marrow biopsy showed normal cellularity with
maturing trilineage hematopoiesis in normal proportions. No
histological or immunophenotypical evidence of B-cell lymphoma was
present.
The patient was diagnosed with rectal adenocarcinoma
with primary liver lymphoma, tending towards a large B-cell
lymphoma of the mucosa-associated lymphoid tissue-type, given that
no additional foci of lymphoma were found anywhere else in the
body. Laparoscopic resection of the rectal cancer and liver biopsy
specimens was performed simultaneously. The pathology of the
hepatic tumor confirmed the previous diagnosis. The patient
received six cycles of
cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP)
chemotherapy (750 mg/m2 cyclophosphamide on day 1; 50
mg/m2 doxorubicin on day 1; 1.4 mg/m2
vincristine on day 1; and 100 mg/m2 prednisone on days
1–5) for six months, subsequent to the surgery. CT showed no
metastasis of the rectal cancer and no change to the PHL at six
months post-chemotherapy. To date, the patient’s condition remains
stable.
Discussion
Although secondary liver involvement of lymphoma in
the advanced stage is common, PHL, which is defined as lymphoma
either confined to the liver or having major liver involvement, is
extremely rare (1,5) and represents <1% of all extranodal
lymphomas (6).
The exact cause of PHL remains unclear. A number of
recent studies have shown a higher prevalence of HCV or HBV
infection in PHL patients (4,7,8).
Hepatitis C is found in 40–60% of patients with PHL (2). Other studies have hypothesized that PH
may be associated with cirrhosis and immunosuppressive drugs
(9,10). However, the patient in the present
study was positive for HBV and also diagnosed with rectal cancer,
but the association between the PHL and rectal cancer was not
clear. No cases exhibiting this combination have been reported in
the past.
PHL occurs in males twice as often as in females,
and the usual age at presentation is 50 years (2). The symptoms are usually non-specific,
however, the most common symptom is abdominal pain. Hepatomegaly
also occurs frequently, while jaundice is an occasional finding
upon physical examination (1,11).
Additionally, laboratory tests and cancer markers are
non-specific.
Upon the imaging of PHL, certain characteristics are
shown. Upon ultrasound examination, the lesions are of mixed iso-
and hypoechogenicity, with a hypoechoic rim. On CT scans, the
lesions appear as hypodense in the pre-contrast phase and as rim
enhancement in the post-contrast phase (12,13).
On MRI, the lesions show hypointensity on T1WI and hyperintensity
on T2WI. These imaging features differ from focal nodular
hyperplasia, hepatocellular carcinoma, cholangiocarcinoma or
metastases (14). The imaging
examinations in the present study revealed the majority of the
usual PHL features, such as hepatomegaly of the left lobe of the
liver.
The final diagnosis of PHL relies on histological
examination. Liver biopsies remain the most valuable tool for the
diagnosis of PHL (3). The majority
of cases of PHL are diffuse large B-cell lymphoma. Other
histological subtypes of PHL include high-grade tumors
(lymphoblastic and Burkett lymphoma; 17%), follicular lymphoma
(4%), diffuse histiocytic lymphoma (5%), lymphoma of the
mucosa-associated lymphoid tissue-type, anaplastic large-cell
lymphoma, mantle cell lymphoma and T-cell-rich B-cell lymphoma
(3). The present patient underwent
a liver biopsy twice and was diagnosed with primary B-cell
lymphoma, tending towards the B-cell lymphoma of the
mucosa-associated lymphoid tissue-type, given that no additional
foci of lymphoma were found anywhere else in the body.
The optimal therapy for PHL remains unclear and the
outcomes are uncertain. Certain clinicians use surgery only, while
others prefer chemotherapy alone or combined with radiotherapy
(1). Although chemotherapy is used
to treat the majority of patients, certain physicians undertake a
multimodality approach, which also integrates surgery and
radiotherapy (1). The CHOP regimen
is the standard treatment for patients with diffuse large B-cell
lymphoma. The addition of rituximab to the CHOP regimen, when given
in eight cycles, augments the complete response rate and prolongs
event-free and overall survival times in older patients with
diffuse large B-cell lymphoma, without significantly increasing the
clinical toxicity (15,16). The present patient received one
cycle of CHOP chemotherapy following a laparoscopic resection of
the rectal cancer. CT showed no metastasis of the rectal cancer and
no change of the PHL at six months post-chemotherapy. At present,
the patient’s condition is stable and is attending follow-up
examinations to monitor any long-term effects.
In conclusion, PHL associated with rectal
adenocarcinoma is extremely rare and to the best of our knowledge,
has never reported. The cause of PHL remains unclear. Diagnosis of
this condition is important, and if the clinical conditions are
indicative of PHL, a liver biopsy should be obtained. As the
optimal therapy is unclear, the overall survival rate for patients
with PHL tends to be poor.
Acknowledgements
This study was supported by grants from the Key
Research Project of the Health Department of Guangxi Zhuang
Autonomous Region (grant no. 2012087).
References
|
1
|
Noronha V, Shafi NQ, Obando JA and Kummar
S: Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol
Hematol. 53:199–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haider FS, Smith R and Khan S: Primary
hepatic lymphoma presenting as fulminant hepatic failure with
hyperferritinemia: a case report. J Med Case Rep. 2:2792008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Page RD, Romaguera JE, Osborne B, et al:
Primary hepatic lymphoma: favorable outcome after combination
chemotherapy. Cancer. 92:2023–2029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Q, Liu HP, Gu YJ and Cong WM:
Clinicopathological and survival features of primary hepatic
lymphoma: an analysis of 35 cases. Zhonghua Zhong Liu Za Zhi.
35:689–692. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Masood A, Kairouz S, Hudhud KH, Hegazi AZ,
Banu A and Gupta NC: Primary non-Hodgkin lymphoma of liver. Curr
Oncol. 16:74–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agmon-Levin N, Berger I, Shtalrid M,
Schlanger H and Sthoeger ZM: Primary hepatic lymphoma: a case
report and review of the literature. Age Ageing. 33:637–640. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somaglino C, Pramaggiore P and Polastri R:
Primary hepatic lymphoma in a patient with chronic hepatitis B and
C infection: diagnostic pitfalls and therapeutic challenge. Updates
Surg. 66:89–90. 2014. View Article : Google Scholar
|
|
8
|
Kaneko F, Yokomori H, Sato A, et al: A
case of primary hepatic non-Hodgkin’s lymphoma with chronic
hepatitis C. Med Mol Morphol. 41:171–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakayama S, Yokote T, Kobayashi K, et al:
Primary hepatic MALT lymphoma associated with primary biliary
cirrhosis. Leuk Res. 34:e17–e20. 2010. View Article : Google Scholar
|
|
10
|
Golli L, Taïeb J, Boleslawski E, et al:
Mucosa-associated lyphoid tissue hepatic lymphoma with low-grade
malignancy associated with primary biliary cirrhosis. Gastroenterol
Clin Biol. 27:127–129. 2003.(In French). PubMed/NCBI
|
|
11
|
Salmon JS, Thompson MA, Arildsen RC and
Greer JP: Non-Hodgkin’s lymphoma involving the liver: clinical and
therapeutic considerations. Clin Lymphoma Myeloma. 6:273–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maher MM, McDermott SR, Fenlon HM, et al:
Imaging of primary non-Hodgkin’s lymphoma of the liver. Clin
Radiol. 56:295–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elsayes KM, Menias CO, Willatt JM, Pandya
A, Wiggins M and Platt J: Primary hepatic lymphoma: imaging
findings. J Med Imaging Radiat Oncol. 53:373–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coenegrachts K, Vanbeckevoort D, Deraedt K
and Van Steenbergen W: Mri findings in primary non-Hodgkin’s
lymphoma of the liver. JBR-BTR. 88:17–19. 2005.PubMed/NCBI
|
|
15
|
Winter MC and Hancock BW: Ten years of
rituximab in NHL. Expert Opin Drug Saf. 8:223–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marcus R and Hagenbeek A: The therapeutic
use of rituximab in non-Hodgkin’s lymphoma. Eur J Haematol.
(Suppl): 5–14. 2007. View Article : Google Scholar
|