Introduction
A number of patients experience unilateral or
bilateral hydronephroses due to advanced pelvic tumor compression,
ureteral malignant invasion or retroperitoneal fibrosis caused by
radiation therapy. Subcutaneous nephrovesical bypass (SNVB) is a
minimally invasive, effective and safe procedure for patients with
ureteral obstruction resulting from advanced malignant disease. As
an alternative procedure to percutaneous nephrostomy, SNVB offers
patients a better quality of life (QoL) (1). Retrograde insertion of a double J
stent is the first-line therapy; however, stent failure occurs in
nearly half of all treated patients (2). An alternative option is to perform a
permanent percutaneous nephrostomy (PCN), which has become a safe
and widely used technique in the last 20 years (3,4).
However, PCN diminishes patient QoL due to a number of possible
complications, including obstruction or dislocation of the
nephrostomy tube and urinary tract infection (UTI) (5). The aim of the present study was to
maintain an acceptable patient QoL and restore kidney function by
performing SNVB in 24 patients.
Materials and methods
Patients
SNVB stents (N=30) were implanted in 24 patients
diagnosed with unilateral or bilateral obstructed ureters due to
progressive metastatic, end-stage disease at the Department of
Urology, Huai’an First People’s Hospital, Nanjing Medical
University (Huai’an, China) between January 2008 and December 2012.
Patients included 14 males and 10 females with a mean age of 56.6
years (range 42–73 years). The cases either displayed bilateral
ureteral obstruction (n=6), a left ureteral obstruction (n=11) or a
right ureteral obstruction (n=7). Among the 24 patients, six had
colorectal carcinoma, five had esophageal cancer, five had uterine
cervical cancers, four had gastric carcinoma, two had ovarian
cancers and two had prostatic adenocarcinoma. Patients with
radiation cystitis were excluded. All of the selected patients
provided written informed consent prior to entering this study, and
the study was approved by the ethics committee of Huai’an First
People’s Hospital, Nanjing Medical University, (Huai’an,
China).
All patients had advanced pelvic malignancy
compression or invasion resulting in unilateral or bilateral
ureteral obstruction as observed by magnetic resonance imaging
(MRI) and retrograde insertion. Double J stenting was impossible
for all patients studied, and 10 patients had been treated
previously with PCN. Urinalysis, serum creatinine (SCr), glomerular
filtration rate (GFR) and ultrasonography were measured in all
patients preoperatively, 3 days postoperatively, and every 3 months
after that at follow-up appointments, and patient QoL scores were
evaluated. All patients were monitored at follow-up appointments
until they succumbed to malignant disease.
Operative technique
An SNVB set consists of a 9F/54-cm special double J
stent as a nephrovesical bypass, an 18-G renal puncture needle, a
guide wire, an 8–12 F fascia dilator, a 12 F/35-cm malleable
tunneler and a 12F half-trough bladder puncture needle (C.R. Bard,
Inc., Murray Hill, NJ, USA). Each tip of the J stent is open and
the side holes only appear within the curved part.
All patient procedures were performed under general
anesthesia by the same surgeon. The first eight patients were
initially placed in a prone position for kidney access, and then
rotated to an anterior oblique elevation (45°) position to access
the bladder and place the distal part of the bypass. As the
procedures were completed, it was determined that a more effective
approach would be to place subsequent patients in an anterior
oblique elevation (45°) position immediately to permit access to
the kidney and bladder simultaneously without having to change the
patient position.
Under ultrasound guidance, needle puncture to the
inferior calyceal system was performed from the posterior axillary
line. The distance was measured from the skin to the target renal
calyx by ultrasound (Pro Focus 2202; BK Medical Ultrasound Systems,
Denmark). The guide wire was placed into the target renal calyx
when urine was detected in the needle. Tracts were dilated to 12F
using sequential Amplatz dilators (C.R. Bard, Inc., Murray Hill,
NJ, USA). The pelvic component of the bypass tube was placed into
the renal pelvis along the guide wire. The depth of the inserted
stent was in accordance with the distance from the skin to the
target renal calyx, excluding the curved part, so that the curved
part was located in the target renal calyx. The stent was fixed
within subcutaneous tissue with a 3-0 nylon suture to prevent stent
dislocation. The skin entry site was widened by ~1 cm and the
subcutaneous tunnel was extended from the nephrostomy incision to
the point 2–3 cm above the pubic symphysis using the 12F malleable
tunneler. A 1-cm incision was created where the tunneler punctured
the suprapubic skin. The pendulous section of the stent was
inserted into the hollow tunneler from the nephrostomy incision to
the suprapubic incision. The stent was fixed by hand and the
tunneler was extracted from the suprapubic incision. Via the small
skin incision, a cystostomy was established using a 12F half-trough
bladder puncture needle under direct vision of flexible cystoscopy.
The bladder component of the bypass stent was inserted into the
bladder under wire guidance. The stent was fixed to the
subcutaneous tissue. The two small skin incisions were closed with
3-0 absorbable sutures. A urethral catheter was inserted for 1
week. Antibiotic prophylactics were administered 24 h
preoperatively and 5 days postoperatively.
Statistics
To compare numerical variables, a two-paired
Student’s t-test was used, and P<0.05 was considered to indicate
a statistically significant difference (Statistical Analysis
Software, V8.0; SAS Institute Inc., Cary, NC, USA).
Results
In total, 30 SNVB stents were successfully implanted
in 24 patients. No operative or immediate postoperative mortalities
occurred. The mean operation time was 78 min (range, 52–118 min)
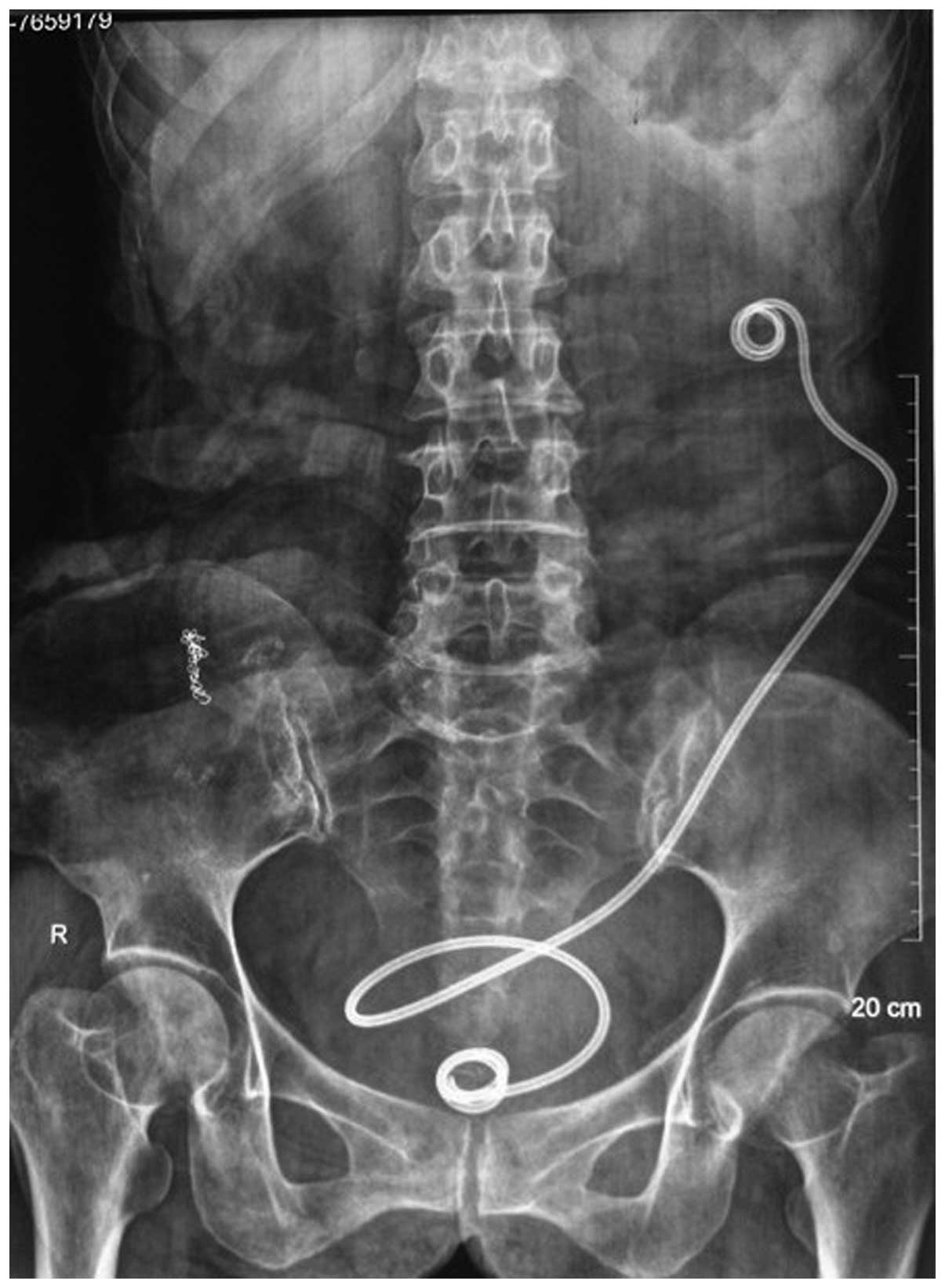
for SNVB. A kidney, ureter and bladder radiology film captured 3
days post-surgery confirmed that the SNVB stents were properly
placed (Fig. 1).
Mean patient follow-up time was 10.6 months (range,
6–36 months). The SNVB stents were well tolerated, but during
follow-up, all but six patients succumbed to progressive metastatic
disease. Following the surgical procedure, hydronephrosis was
completely resolved in 16 of 30 affected kidneys (53.3%) and was
reduced in the remaining kidneys. Patient data for preoperative and
postoperative outcomes are provided in Table I. There were no major perioperative
complications. Certain patients experienced mild hematuria, which
disappeared after 1–2 days. Common procedural complications are
presented in Table II.
 | Table IGFR, serum creatinine, QoL mean value
before and after operation (range). |
Table I
GFR, serum creatinine, QoL mean value
before and after operation (range).
| Glomerular filtration
rate (ml/min) | Serum Creatinine
(μmol/l) | QoL |
|---|
| Pre operation | 25±4.8 (18–26) | 256±46 (85–662) | 3.4±1.4 (0–5) |
| Post operation | 45±5.3 (28–58) | 124±23 (88–176) | 7.6±1.0 (5–9) |
| P-value | <0.01 | <0.001 | <0.001 |
 | Table IIComplications related to the
procedure. |
Table II
Complications related to the
procedure.
| Complication | n (%) |
|---|
| Mild hematuria | 10 (33.3) |
| Urinary tract
infection | 6 (23.1) |
| Urinary urgency | 5 (16.7) |
| Subcutaneous
infection | 2 (6.7) |
Discussion
Malignant pelvic tumors or advanced metastasis often
results in unilateral or bilateral ureteral obstruction (6), and this can lead to nephrosis, renal
insufficiency and uremia. These problems may be solved with
retrograde ureteric stenting, which requires periodic stent changes
(7). Gradually, retrograde
insertion of a double J stent in the ureter may fail in the
presence of advanced pelvic malignancies, or it may be complicated
by infection or obstruction (8).
Alternatively, a PCN may be used; however, this requires an
external urine collection device (9). As the malignancy progresses, patients
not only suffer pain due to the advanced tumors, but also
experience nephrostomy complications, including infection,
obstruction and slippage of the nephrostomy tube (10). Additionally, patients experience a
number of inconveniences, including nephrostomy tube and urine bag
changes, bathing with the nephrostomy tube and social issues, all
of which compromise QoL (11,12).
SNVB is not affected by the ipsilateral ureter and
it offers minimal invasion, low risk and easy manipulation
(13). Research shows that no
severe complications occur during this operation, except for the
occasional urinary extravasation and local infection (14). In the current study, all patients
underwent successful surgery and experienced no severe
complications. In the postoperative follow-up period, no bypass
stent displacement or stone formation occurred. Ipsilateral
hydronephrosis and GFR improved markedly. SCr levels were close to
normal and remained stable, and the uremic patient no longer
required dialysis. Patients expressed no typical complaints, such
as back pain and urinary symptoms. QoL scores increased
postoperatively and these data are in accordance with previous
findings (15). Compared with PCN
patients, the patients of the current study no longer required
external urine collection bags or associated equipment, and SNVB
offered greater comfort for sleeping and improved mobility compared
with conventional PCN (16).
SNVB is suitable for patients with ureteral
obstruction due to advanced abdominal pelvic malignancy without
radical surgery. Patients receiving the SNVB procedure should have
a functional bladder and their disease must exclude lower urinary
tract symptoms. SNVB can replace permanent PCN when a double J
stent is not able to be inserted into the ureter endoscopically
(17,18). In the present study, 14 patients
were offered SNVB as end-stage malignancies had reduced their life
expectancy to <12 months.
The results of the current study indicate that the
single double J stent used in the procedure was superior to two
J-tubes connected at the midpoint by a metal connector, which may
result in potential urinary extravasation (16,17).
SNVB stents made of different materials have been reported to
withstand implantation for 6–84 months (19). Regular changing of the SNVB is not
necessary, and long-term complications were rare even in diabetic
patients. SNVBs may be replaced in the face of complications if
patient survival times permit this. Changing the SNVB is not
difficult: The existing subcutaneous channel for the long-term
indwelling bypass stent aids in the replacement (20). Additionally, PCN may be performed
even if the SNVB could not be successfully replaced.
The surgery requires attention to be paid to
perioperative events. Primarily, UTI is common in patients with
ureteral obstructions, particularly in end-stage patients with
malignant diseases. Infection must be treated with antibiotics to
prevent severe complications and procedure failure. Additionally,
the puncture point for renal puncture should be selected in the
posterior axillary line to prevent puncturing the peritoneal cavity
and damaging the bowel. In certain PCN procedures, a new
nephrostomy would be established and isolated from the existing
nephrostomy to reduce infection. The lower calyx should be chosen
as the target renal calyx to facilitate urine drainage. Finally,
the depth of bypass placement in the renal pelvis should be
measured by ultrasound and be between the puncture point and the
target renal calyx. The curved tips with side holes of the bypass
should be placed within the renal pelvis and bladder, preventing
postoperative urinary extravasation. Infection and bypass
obstruction should be prevented to prolong the bypass stent
retention time. For instance, patients should drink water and
urinate as needed, specifically voiding again following an initial
urination to eliminate urine reflux by the bypass stent.
In conclusion, SNVB is a minimally invasive, safe
and effective procedure that can improve renal pelvic drainage for
ureteral obstruction patients with end-stage malignancies. SNVB
offers patients a better QoL and should be considered an
alternative procedure to PCN, which is documented to reduce QoL due
to the need for cumbersome external urine collection devices.
References
|
1
|
Kouba E, Wallen EM and Pruthi RS:
Management of ureteral obstruction due to advanced malignancy:
optimizing therapeutic and palliative outcomes. J Urol.
180:444–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung SY, Stein RJ, Landsittel D, et al:
15-year experience with the management of extrinsic ureteral
obstruction with indwelling ureteral stents. J Urol. 172:592–595.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ekici S, Şahin A and Özen H: Percutaneous
nephrostomy in the management of malignant ureteral obstruction
secondary to bladder cancer. J Endourol. 15:827–829. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watkinson AF, A’Hern RP, Jones A, King DM
and Moskovic EC: The role of percutaneous nephrostomy in malignant
urinary tract obstruction. Clin Radiol. 47:32–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radecka E and Magnusson A: Complications
associated with percutaneous nephrostomies. A retrospective study.
Acta Radiol. 45:184–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chitale SV, Scott-Barrett S, Ho ET and
Burgess NA: The management of ureteric obstruction secondary to
malignant pelvic disease. Clin Radiol. 57:1118–1121. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganatra AM and Loughling K: The management
of malignant ureteral obstruction treated with ureteral stents. J
Urol. 174:2125–2128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg BH, Bianco FJ Jr, Wood DP Jr and
Triest JA: Stent-change therapy in advanced malignancies with
ureteral obstruction. J Endourol. 19:63–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wah TM, Weston MJ and Irving HC:
Percutaneous nephrostomy insertion: outcome data from a prospective
multi-operator study at a UK training centre. Clin Radiol.
59:255–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong LM, Cleeve LK, Milner AD and Pitman
AG: Malignant ureteral obstruction: outcomes after intervention.
Have things changed? J Urol. 178:178–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emmert C, Rassler J and Kohler U: Survival
and quality of life after percutaneous nephrostomy for malignant
ureteric obstruction in patients with terminal cervical cancer.
Arch Gynecol Obstet. 259:147–151. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aravantinos E, Anagnostou T, Karatzas AD,
Papakonstantinou W, Samarinas M and Melekos MD: Percutaneous
nephrostomy in patients with tumors of advanced stage: treatment
dilemmas and impact on clinical course and quality of life. J
Endourol. 21:1297–1302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jurczok A, Loertzer H, Wangner S and
Fornara P: Subcutaneous nephrovesical and nephrocutaneous bypass.
Gynecol Obstet Invest. 59:144–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Desgrandchamps F, Leroux S, Ravery V,
Bochereau G, Menut P and Meria P: Subcutaneous pyelovesical bypass
as replacement for standard percutaneous nephrostomy for palliative
urinary diversion: prospective evaluation of patient’s quality of
life. J Endourol. 21:173–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J and Wang XF: Clinical application of
subcutaneous nephrovesical bypass system. Beijing Da Xue Bao.
42:473–475. 2010.(In Chinese).
|
|
16
|
Schmidbauer J, Kratzik C, Klingler HC,
Remzi M, Lackner J and Marberger M: Nephrovesical subcutaneous
ureteric bypass: long-term results in patients with advanced
metastatic disease-improvement of renal function and quality of
life. Eur Urol. 50:1073–1078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nissenkorn I and Gdor Y: Nephrovesical
subcutaneous stent: an alternative to permanent nephrostomy. J
Urol. 163:528–530. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerullis H, Ecke TH, Schwartmann K, Heuck
CJ, Eimer C, Bagner JW, et al: Nephrocutaneous bypass in ureteral
obstruction. Urology. 76:480–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jabbour ME, Desgrandchamps F, Angelescu E,
Teillac P and Le Duc A: Percutaneous implantation of subcutaneous
prosthetic ureters: long-term outcome. J Endourol. 15:611–614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cockburn JF and Nisbet P: Percutaneous
radiologic replacement of blocked nephrovesical stent. AJR Am J
Roentgenol. 170:1109–1110. 1998. View Article : Google Scholar : PubMed/NCBI
|