Introduction
Follicular dendritic cells (FDCs), which exhibit a
dendritic configuration and occasional multinucleation, can be
found in primary and secondary lymphoid follicles at nodal or
extranodal sites. FDCs are characterized by indistinct borders,
oval nuclei, delicate nuclear membranes, faintly eosinophilic
cytoplasm, small but distinct nucleoli, and their reaction to
cluster of differentiation (CD)21, CD23, CD35, fascin and clusterin
(1). The cells play a major role in
antigen presentation and antigen-dependent maturation of the B-cell
immune response (2). Based on the
normal distribution of FDCs, FDC sarcoma (FDCS) presents within the
lymph nodes and at extranodal sites. Since FDCS has been fairly
well-characterized morphologically and possesses a distinct
immunophenotype, the possibility of diagnosis is currently more
readily established for spindle cell lesions in the lymph nodes.
However, extranodal FDCS is less well recognized, although its
occurrence has been noted since 1994 (3). In the present study, the literature
was reviewed in order to obtain a deeper recognition of FDCS
arising from extranodal sites. Prior to 2013, there were only 142
cases of extranodal FDCS reported in the English language
literature, with up to one-fourth of those cases being initially
misdiagnosed. In the current study, four cases of extranodal FDCS
are presented, and the clinicopathological features of extranodal
FDCS reported in the literature are reviewed.
Materials and methods
Samples
The four cases were retrieved following consultation
at the Xin Hua Hospital Affiliated to Shanghai Jiaotong University
School of Medicine (Shanghai, China). One case has already been
reported as FDCS (4), but
additional tests have since been performed. The clinical
information was obtained from the hospital and outpatient records,
and from the contributing pathologists and clinicians. Histological
materials were processed in a routine manner, with formalin
fixation and paraffin embedding for hematoxylin and eosin staining.
Ethical approval for this study was obtained from the Ethics
Committee of Xin Hua Hospital Affiliated to Shanghai Jiaotong
University School of Medicine.
Immunohistochemistry
Immunohistochemical staining was performed on the
formalin-fixed, paraffin-embedded tissue sections using the
EnVision method (Leica BOND-MAX, Leica Biosystems, Newcastle Upon
Tyne, UK). The antibodies used in the immunohistochemistry were for
CD21, CD23, CD35, vimentin, CD20, CD3, desmin, CD68, cytokeratin
(CK), CD117, CD34, thyroid transcription factor-1, S100 protein,
human melanoma black 45 (HMB45), smooth muscle actin (SMA),
epithelial membrane antigen (EMA) and Ki-67. Appropriate positive
and negative controls were simultaneously evaluated.
Literature review
Previous studies were obtained from the MEDLINE
database, a major index literature source, using the term
‘follicular dendritic cell sarcoma/tumor’. Nodal FDCS was not
included. An effort was made to identify cases that had been
reported more than once and only the case with the most recently
updated information was included in the present report. A total of
142 cases of extranodal FDCS were retrieved from the English
literature (5–86). A total of 11 cases were excluded
from the analysis due to omitted clinical data.
Statistical analysis
The association between the various
clinicopathological features and a higher event rate (recurrence,
metastases, and mortality) were assessed using the χ2
test. Data analyses were generated using SPSS for Windows, version
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was used to indicate
a statistically significant difference.
Results
Report of four cases
Clinical data
The case summaries of the present four cases of
extranodal FDCS are shown in Table
I. The tumors were located in the tonsils, stomach, liver and
lungs, respectively.
 | Table IClinical data of four novel cases of
extranodal follicular dendritic cell sarcoma. |
Table I
Clinical data of four novel cases of
extranodal follicular dendritic cell sarcoma.
| Case | Gender | Age | Site | Maximal size,
cm | Manifestation | Initial
diagnosis | Treatment | Outcome at
follow-up (months) |
|---|
| 1 | M | 65 | Tonsil | No data | Tonsil pain | SCC | Tonsillectomy | NED (25) |
| 2 | F | 53 | Stomach | 14 | Debilitation,
abdominal distention, anepithymia | GIST | Surgery
Radiotherapy
Hemotherapy | AWD (10) |
| 3 | M | 49 | Liver | 9 | Right upper
quadrant pain | Inflammatory
pseudotumor | Surgery | Intra-abdominal
recurrence (8) |
| 4 | F | 76 | Lung | 3 | Dyspnea | Malignant
melanoma | Surgery | Succumbed to
disease |
Histopathological findings
Macroscopically, the four tumors were
well-circumscribed, exhibiting a solid, nodular, grey-yellow or
dust-colored cut surface, similar to sarcoma or lymphoma.
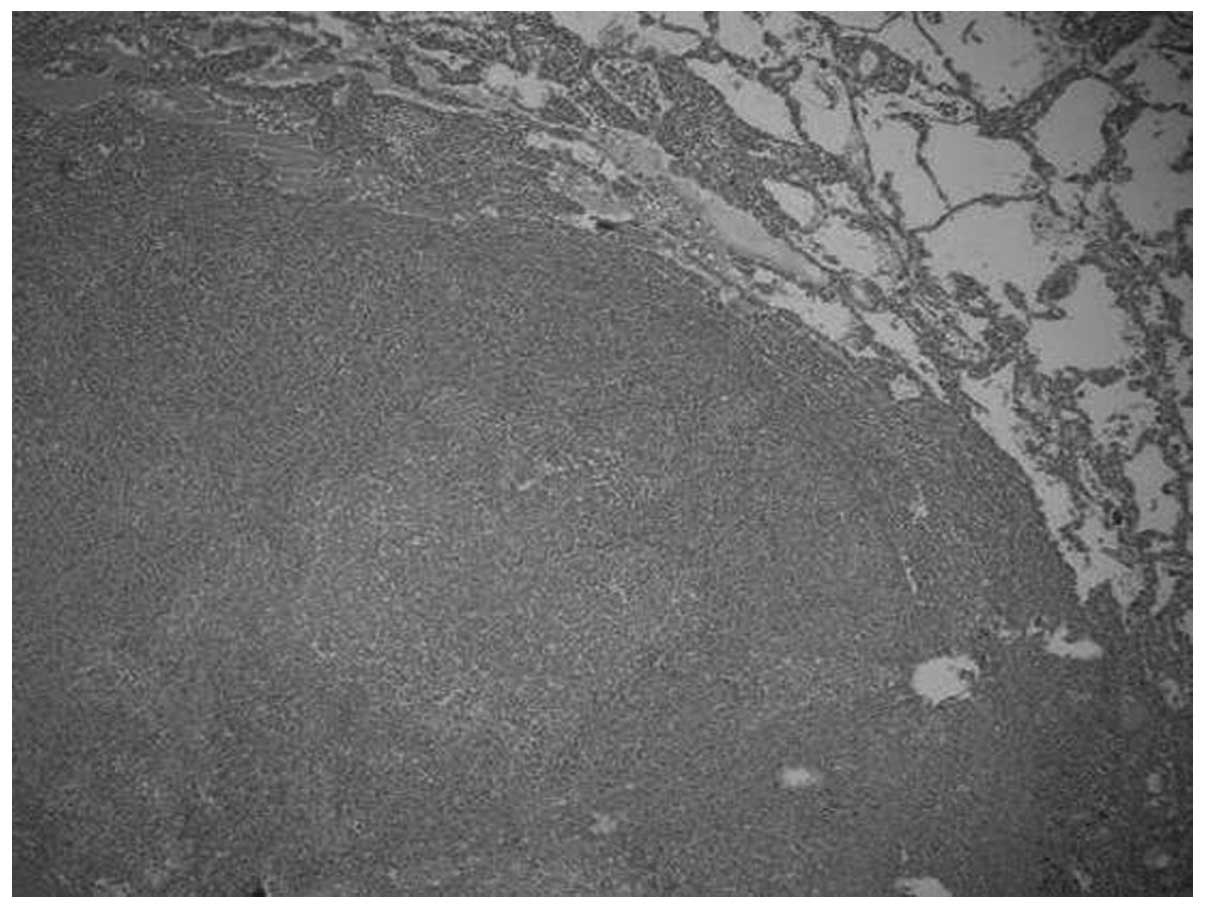
Microscopically, the tumors were relatively well-circumscribed
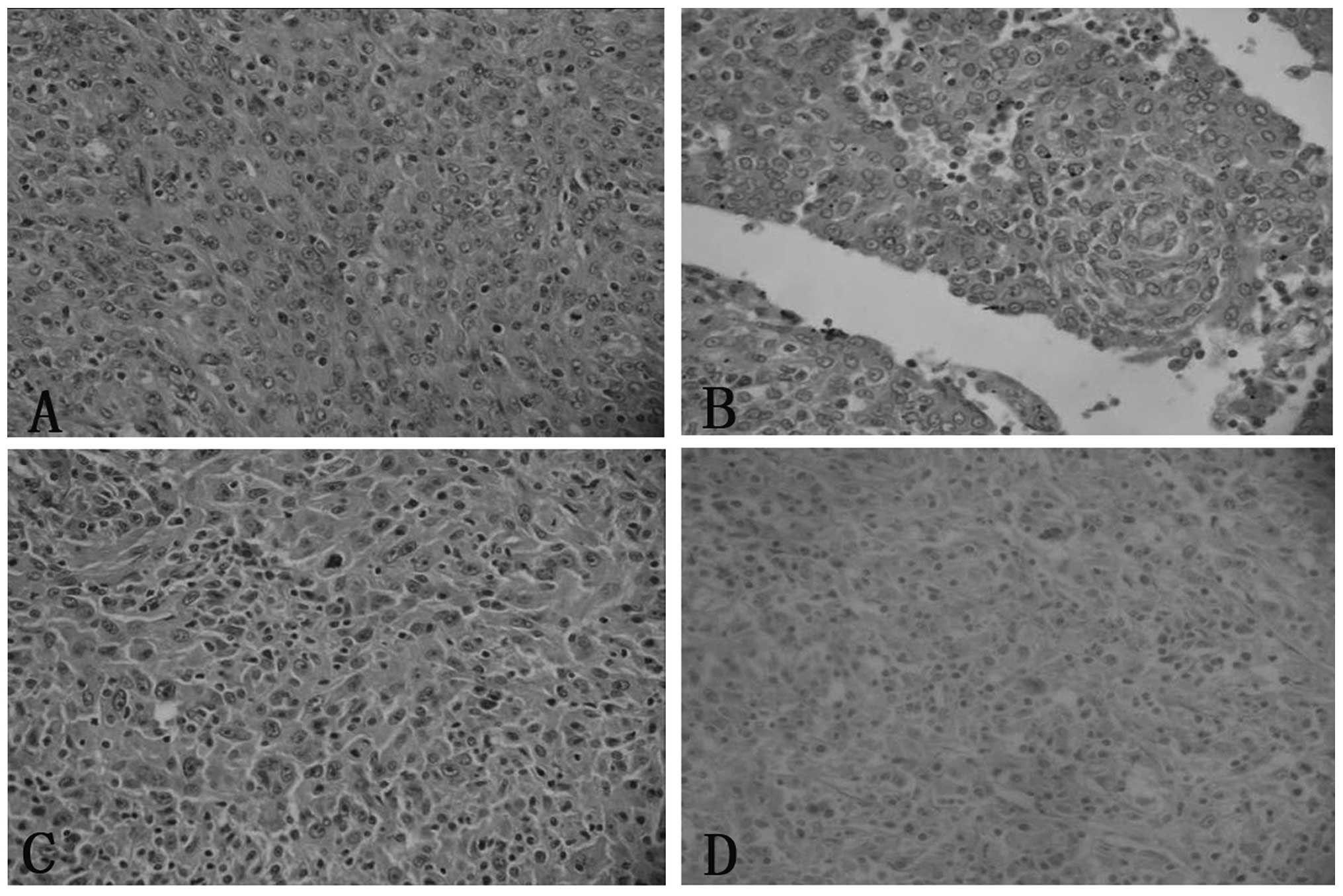
(Fig. 1). Cases one and four
revealed an epithelioid cell morphology, with a whorled, diffuse or
trabecular growth pattern, while cases two and three exhibited an
increased proportion of spindled cells. The growth pattern in these
cases was fascicular, storiform and whorled (Fig. 2). In all cases, the tumor cells
possessed a moderate amount of faintly eosinophilic cytoplasm and
indistinct cell borders. Binucleated or multinucleated tumor cells
were occasionally observed. The nuclei were oval to elongated in
shape, with thin and lightly-stained purplish nuclear membranes,
vesicular or stippled chromatin and distinct nucleoli. Certain
nuclei contained round intranuclear pseudoinclusions (Fig. 2). Mitoses were not prominent and
were absent in three cases and in 6–8/10 high-power fields (HPFs)
in case two. Necrosis was observed in three cases. There were small
lymphocytes scattered throughout the tumors and clustered around
vessels. In case two, certain irregular pseudolacuna-like blood
vessels were found, the insides of which exhibited plump
eosinophilic protein-like liquids that resembled the perivascular
spaces observed in thymoma.
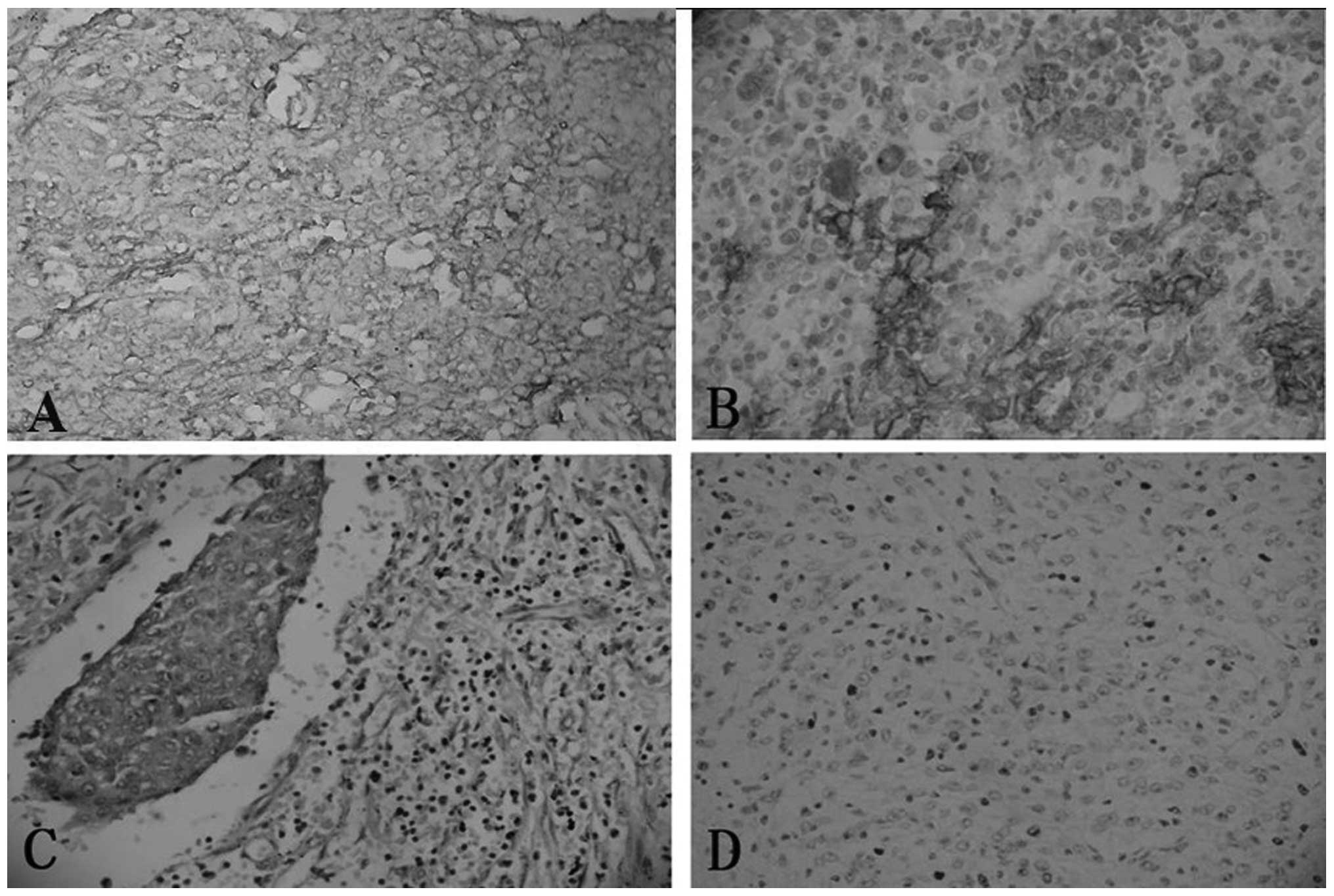
Immunohistochemical findings
The staining of CD21, CD23, CD35 and vimentin was
performed for all four cases, while the staining of CD68, S-100 and
EMA was performed for only three cases. Immunohistochemically, all
four cases were diffusely positive for CD21, CD23 and vimentin
expression, and focally positive for CD35 expression. Two of three
cases were focally positive for CD68 expression, and one of three
cases was focally positive for S-100 and EMA expression (Fig. 3). Case two exhibited a negative
result for the expression of GIST markers CD34 and CD117, while
case four exhibited a negative result for thyroid transcription
factor and the melanoma marker HMB45. Staining for these markers
was not performed in the other cases. All the present cases were
negative for CK and SMA. For the associated lymphocytes, T cells
(CD3+) outnumbered B cells (CD20+). The Ki-67
labeling index of case one was >1%, while the indexes of cases
two and four were 15 and 40%, respectively.
Literature review
Clinical features
A total of 142 cases of extranodal FDCS were
retrieved from the literature. The patients, 77 females and 65
males (female to male ratio, 1.2:1), ranged in age from 9–82 years
old. The tumor affected various anatomical sites. The more
predominant extranodal sites of tumor involvement were the tonsils
(28), pharynx (19), liver (18), spleen (17), intra-abdominal but external viscera
(14), soft tissue of the neck
(11), mediastinum (6), lungs (4), gastrointestinal tract (three in the
stomach, four in the small bowel, two in the large intestine and
one in the anal canal), thyroid, breast, palate, parotid gland
(3) and pancreas (2). Other organ sites consisted of the
intracalvarium, pleura, dura mate spinalis, pelvis and soft tissue
of the thigh, with one case occurring in each location. In total,
12 cases exhibited lymph node and extranodal FDCS, and 9 cases
exhibited distant metastases at the time of presentation.
Pathological findings
The average size of the tumors in all sites was 7.0
cm, with a range of 1–22 cm. Intra-abdominal tumors (n=55)
exhibited an average tumor size of 10.2 cm (range, 3–22 cm),
whereas the average tumor size of extra-abdominal sites (n=72) was
only 5.4 cm (range, 1–16 cm) (P<0.05). Data regarding tumor size
were unavailable for 15 cases.
The macroscopic and microscopic findings of the
cases from the literature were similar to those described in the
present four cases. Macroscopically, the tumors were
well-circumscribed with a solid, nodular, grey-yellow or
dust-colored cut surface, similar to sarcoma or lymphoma.
Microscopically, the tumors were circumscribed from the surrounding
parenchyma, with or without a fibrous capsule. The tumor cells
varied from plump and spindled to epithelioid, with a moderate
amount of faintly eosinophilic cytoplasm and indistinct cell
borders. Binucleated or multinucleated tumor cells were
occasionally observed. The nuclei were oval to elongated in shape,
with a thin and lightly stained purplish nuclear membrane,
vesicular or stippled chromatin, and distinct nucleoli.
Intranuclear pseudoinclusions were found in certain cases. A
mitotic count was available in 80 cases, with a median of 4/10 HPFs
ranging between 0 and 50/10 HPFs. In total, 51 cases possessed a
mitotic count of <5/10 HPFs. Information on necrosis was
available in 70 cases, of which 34 cases (48.6%) exhibited
coagulative necrosis. There were small lymphocytes scattered
throughout the tumors and clustered around vessels.
Immunohistochemically, all the cases were positive
for at least one of the FDC markers, including CD21, CD23, CD35,
fascin and clusterin, and were negative for CK. Staining for CD117,
CD34, desmin, HMB45 and CD1α, CD68, S100, and EMA revealed variable
results among the different cases. Desmoplakin, a
desmosome-associated protein, was detected in the majority of the
cases. The associated lymphocytes were more commonly T cells
(CD3+) rather than B cells (CD20+). Prominent
cell processes that were focally joined by well-formed cell
junctions and desmosome-like cytoplasm were exhibited
ultrastructurally by the tumor cells.
Discordant diagnoses at the initial
evaluation
Overall, 40 cases were misdiagnosed at the time of
the initial evaluation. In each of these cases, the diagnosis of
FDCS was not considered at the initial evaluation. The disease
entities that were considered consisted of interdigitating
reticulum cell sarcoma, minor salivary gland tumors, malignant
peripheral nerve sheath tumors, reactive response, inflammatory
pseudotumors, malignant fibrous histocytomas, meningioma,
mesenchymal tumors with neural differentiation, schwannoma,
Hodgkin’s lymphoma, angiosarcoma, malignant melanoma, large-cell
lymphoma, spindle cell carcinoma, primitive neuroectodermal tumors,
malignant myoepithelial carcinoma and gastrointestinal stromal
tumors.
Correlation between
clinicopathological findings and patient outcome
Follow-up information was available in 130 cases,
with a follow-up duration period of 0.5 to 324 months (mean, 34.5
months). The overall recurrence, metastasis and mortality rates
were 49.2% (64 cases), 21.5% (28 cases), and 13.8% (18 cases),
respectively. Local recurrence occurred in 25 patients (20.5%)
following resection (range, 6–180 months). Distant metastases
occurred in 28 patients and the metastatic sites included the
liver, lung, bones, ovaries, thyroid gland, omentum, lymph nodes
and soft tissue. At follow-up, it was determined that 18 (13.8%)
patients had succumbed to the disease, 38 (29.2%) were alive with
the disease, and 74 (56.9%) patients were alive with no evidence of
the disease. Both clinicopathological features and follow-up
information were available for 109 patients; the association
between the various clinicopathological features of early-stage
disease and a higher event rate (recurrence and mortality) was
analyzed for these patients, the results of which are summarized in
Table II. A large tumor size (≥7.5
cm) and high mitotic rate (≥5/10 HPF) were associated with
mortality, but not recurrence.
 | Table IIAnalysis of factors associated with a
higher risk of recurrence or mortality in localized disease. |
Table II
Analysis of factors associated with a
higher risk of recurrence or mortality in localized disease.
| Parameter | Disease-free,
n | Recurrence, n | Mortality, n | P-valuea | P-valueb |
|---|
| Age, years | | | | 0.017 | 0.752 |
| ≥50 | 38 | 10 | 7 | | |
| <50 | 25 | 21 | 8 | | |
| Gender | | | | 0.310 | 0.765 |
| Female | 34 | 20 | 8 | | |
| Male | 29 | 11 | 7 | | |
| Tumor size, cm | | | | 0.391 | 0.006 |
| ≥7.5 | 18 | 7 | 9 | | |
| <7.5 | 41 | 18 | 4 | | |
| Location | | | | 0.128 | 0.095 |
|
Intra-abdominal | 28 | 7 | 9 | | |
|
Extra-abdominal | 36 | 23 | 6 | | |
| Mitotic count | | | | 0.304 | 0.004 |
| ≥5/10 HPFs | 7 | 8 | 6 | | |
| <5/10 HPFs | 31 | 11 | 1 | | |
| Necrosis | | | | 0.012 | 0.367 |
| Present | 24 | 5 | 4 | | |
| Absent | 14 | 12 | 1 | | |
Discussion
The majority of FDCS occurs in adults, with a mild
female predilection. The clinical characteristics of FDCS include a
painless mass. It is important that doctors are aware of FDCS and
are able to recognize this tumor, as it is extremely similar to a
wide range of other tumors and tumor-like lesions. Usually, FDCS is
not considered in routine diagnoses, particularly when it occurs in
extranodal sites. FDCS can be missed even after immunohistochemical
studies, as the markers for FDC are not included among the routine
antibody panel that is used for the investigation of
poorly-differentiated neoplasms. Thus, tumors arising from
extranodal sites are often misdiagnosed or excluded during the
initial diagnosis, and this is corrected only when there is tumor
recurrence or metastasis (78). In
the present four cases, three were misdiagnosed, which could lead
to unnecessary treatment and associated morbidity. This prompted
the investigation of potential diagnostic pitfalls and a detailed
analysis of extranodal FDCS.
The diagnosis of FDCS is established based on
morphological and immunohistochemical findings. Histologically, the
tumors in the present study were composed of spindle to oval-shaped
cells, which were arranged in sheets, interlacing fascicles or
whorls. The tumor cells exhibited plump eosinophilic cytoplasm,
with ill-defined cell borders, forming a less diffuse growth
pattern. The nuclei of the tumor cells were small, oval or round in
shape, and exhibited a vesicular chromatin pattern. Certain
multinucleate tumor cells were also present. Nuclear pleomorphisms
and scattered mitotic figures, often <3 mitoses/10 HPFs, were
observed. The neoplastic cells were intermixed with small mature
lymphocytes and plasma cells, which surrounded the blood vessel and
formed a cuff-like structure.
The correct diagnosis of FDCS must also be made from
immunohistochemistry studies. Ultrastructural studies are desirable
when making a diagnosis of FDCS, but are not essential. FDCS cells
demonstrate a similar immunophenotype to normal FDCs. CD21, CD23,
CD35, fascin, and clusterin are more specific markers for FDCS.
CD68, S-100, EMA, and LCA are variably immunoreactive, while
results for CK, CD1α, CD31, CD34, and HMB-45 are negative (84). A previous study demonstrated that
EGFR and clusterin exhibit high specificity for diagnosis (1). Fascin has been revealed to be
extremely non-specific among spindle cell tumors, indicating that
they do not have a follicular dendritic lineage (1). High D2–40 expression has been revealed
in FDCS, while weak or no expression has been found in other
dendritic cell tumors (41,87). Electron microscopy reveals that the
tumor cells have long, slender cytoplasmic processes, which were
connected by desmosomes, few cellular organs and no Birbeck
granules (78).
Although FDCS possesses certain morphological and
immunophenotypical features, those that occur in extranodal sites
are particularly rare and extremely similar to other soft-tissue
tumors, even poorly-differentiated cancers. For example, in the
cases from the literature review, FDCS of the parapharyngeal region
was misdiagnosed as ectopic meningioma (24), pars palatalis was misdiagnosed as
acinic cell carcinoma (88) and
primary FDCS of the alimentary tract was misdiagnosed as
mesenchymal neoplasm of the abdominal cavity (43), diffuse large-cell lymphoma of the
liver (8) and low-degree malignant
tumor of the mesentery (57).
Therefore, an awareness of the morphological
features of FDCS and the appropriate application of FDC markers for
any tumors exhibiting an unusual appearance, should aid a correct
diagnosis (74). In case two of the
present study, stomach FDCS was misdiagnosed as a GIST, in which
the tumor cells usually exhibit less eosinophilic cytoplasm
compared with the cells of spindle cell tumors, which exhibit
greater specific differentiation, similar to smooth muscle and
neurogenic tumors. Immunohistochemically, the tumor cells of GISTs
are positive for CD117 and CD34, but negative for FDC markers.
Another type of neoplasm that has a dendritic cell origin is
interdigitating dendritic cell sarcoma, which also shares certain
immunophenotypical, histological and ultrastructural features with
FDCS. However, interdigitating dendritic cell sarcoma is
S-100-positive, but negative for FDC markers. When FDC markers are
present and there is no CD45 expression, the diagnosis of
interdigitating dendritic cell sarcoma can be excluded. Sparse
intracytoplasmic organelles and interdigitating cytoplasmic
processes are typical ultrastructural features, but interdigitating
dendritic cell sarcoma does not exhibit desmosomes. In addition, a
differential diagnosis should include malignant melanoma, ectopic
meningioma, malignant fibrohistiocytoma, ectopic thymoma, malignant
peripheral nerve sheath tumor, lymphepithelioma and malignant
lymphoma. Among these possibilities, malignant melanoma is positive
for HMB-45 and ectopic meningioma is positive for vimentin and EMA.
Malignant fibrohistiocytoma is characterized by spindle cells in a
storiform pattern, multinucleate tumor giant cells, marked
cytological atypia and positive histiocyte marker expression.
Ectopic thymoma is positive for CK and terminal deoxynucleotidyl
transferase expression in lymphocytes. However, all the
aforementioned lesions are negative for FDC markers (77).
The etiopathogenesis of FDCS remains unclear. Novel
investigations have revealed that FDCS was associated with
hyaline-vascular Castleman’s disease, in which FDC hyperplasia can
develop into FDCS, similar to certain nodal counterparts (20). Cheuk et al (45) reported a series of inflammatory
pseudotumor (IPT)-like FDCS, which were positive for Epstein-Barr
virus-encoded RNA (EBER) in all cases. Shia et al (14) suggested that the IPT-like FDCS may
represent an earlier stage of FDCS, which may exert an inciting
factor associated with EBV. Upon review of studies of cases that
had previously been tested for EBV, it was discovered that 10 out
of 11 cases of hepatic FDCS exhibited positive EBER expression by
in situ hybridization (47),
whereas 11 out of 17 splenic cases were positive for the virus. EBV
was not detected in any of the cases located in other sites.
Recently, it was found that tumor cells were uniformly positive for
EBV in all six studied cases of IPT-like FDC sarcoma of the spleen,
and there were predominant immunoglobulin (Ig)G4+ plasma
cells in the tumor nodules, suggesting that IPT-like FDC sarcoma of
the spleen may be closely associated with EBV and the
IgG4-associated disease (86).
Therefore, there is a pathogenetic difference between liver and
splenic FDCS and other FDCS. However, EBV was not detected in case
three, an FDCS of the liver, in the present study. In addition,
certain studies revealed that FDCS coexisting with myasthenia
gravis was also associated with immature T cells. These cases
suggested that myasthenia gravis may be a paraneoplastic
manifestation of FDCS and that FDCS may be capable of mediating
aberrant immune activation (89–91).
Other data suggested that the p53-mediated pathway may possibly be
involved in the development or progression of FDCS (75). However, the association was not
evident in any lesion in the present cases.
The biological behavior of FDCS is difficult to
predict, as it is generally considered to be a low-grade
soft-tissue sarcoma. Although originally known as an FDC tumor, the
term FDCS was proposed by Chan et al (6) in order to emphasize the clinical
behavior of the neoplasm as a sarcoma rather than a lymphoma. Until
recently, the tumor was believed to be indolent with a tendency to
recur, but with a low risk of metastasis. Studies of larger cohorts
and for longer follow-up periods have since concluded that FDCS is
more aggressive than previously hypothesized, and that the tumor
should be considered as an intermediate-grade malignancy at least
(6,92,93).
In the present study, a large tumor size and a high mitotic rate
were found to be associated with mortality, but an intra-abdominal
location and the presence of necrosis were not associated with a
poor prognosis. However, other studies have found that poor
prognostic factors included an intra-abdominal location, a tumor
size of >6 cm, a high mitotic rate, necrosis and nuclear
pleomorphism (92,93,14).
In case two of the present study, cell atypia and 6–8 mitoses/10
HPFs were observed, which indicated a poor prognosis. There were
multiple metastases in the liver at the time of surgery. In
addition, the marked intratumoral immature T-cell burden may also
perform a role as an adverse prognostic factor in FDCS (89). Radical resection of the tumor is the
primary therapy, but the value of radiotherapy and chemotherapy in
the treatment of this neoplasm remains uncertain.
In conclusion, extranodal FDCS is a rare, often
misdiagnosed, malignant tumor. An increased awareness of the
morphological spectrum of FDCS and appropriate immunostains for FDC
differentiation should aid in the recognition of FDCS. It is
imperative for the biological behavior of this tumor to be clearly
recognized. Increased awareness of the existence of FDCS may aid a
reduction in the potential for diagnostic error.
References
|
1
|
Grogg KL, Macon WR, Kurtin PJ and
Nascimento AG: A survey of clusterin and fascin expression in
sarcomas and spindle cell neoplasms: strong clusterin
immunostaining is highly specific for follicular dendritic cell
tumor. Mod Pathol. 18:260–266. 2005. View Article : Google Scholar
|
|
2
|
Wu J, Qin D, Burton GF, Szakal AK and Tew
JG: Follicular dendritic cell-derived antigen and accessory
activity in initiation of memory IgG responses in vitro. J Immunol.
157:3404–3411. 1996.PubMed/NCBI
|
|
3
|
Chan JK, Tsang WY and Ng CS: Follicular
dendritic cell tumor and vascular neoplasm complicating
hyaline-vascular Castleman’s disease. Am J Surg Pathol. 18:517–525.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang LF, Wang RF, Wang ZC, et al:
Follicular dendritic cell sarcoma of stomach: report of a case.
Zhonghua Bing Li Xue Za Zhi. 37:210–211. 2008.(In Chinese).
PubMed/NCBI
|
|
5
|
Perez-Ordonez B, Erlandson RA and Rosai J:
Follicular dendritic cell tumor: report of 13 additional cases of a
distinctive entity. Am J Surg Pathol. 20:944–955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan JK, Fletcher CD, Nayler SJ and Cooper
K: Follicular dendritic cell sarcoma. Clinicopathologic analysis of
17 cases suggesting a malignant potential higher than currently
recognized. Cancer. 79:294–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biddle DA, Ro JY, Yoon GS, et al:
Extranodal follicular dendritic cell sarcoma of the head and neck
region: three new cases, with a review of the literature. Mod
Pathol. 15:50–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vargas H, Mouzakes J, Purdy SS, Cohn AS
and Parnes SM: Follicular dendritic cell tumor: an aggressive head
and neck tumor. Am J Otolaryngol. 23:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoh K, Hibi G, Yamamoto Y, et al:
Follicular dendritic cell tumor in the oro-pharyngeal region:
report of a case and a review of the literature. Oral Oncol.
39:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tisch M, Hengstermann F, Kraft K, von
Hinüber G and Maier H: Follicular dendritic cell sarcoma of the
tonsil: report of a rare case. Ear Nose Throat J. 82:507–509.
2003.PubMed/NCBI
|
|
11
|
Grogg KL, Lae ME, Kurtin PJ and Macon WR:
Clusterin expression distinguishes follicular dendritic cell tumors
from other dendritic cell neoplasms: report of a novel follicular
dendritic cell marker and clinicopathologic data on 12 additional
follicular dendritic cell tumors and 6 additional interdigitating
dendritic cell tumors. Am J Surg Pathol. 28:988–998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Idrees MT, Brandwein-Gensler M, Strauchen
JA, Gil J and Wang BY: Extranodal follicular dendritic cell tumor
of the tonsil: report of a diagnostic pitfall and literature
review. Arch Otolaryngol Head Neck Surg. 130:1109–1113. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Domínguez-Malagón H, Cano-Valdez AM,
Mosqueda-Taylor A and Hes O: Follicular dendritic cell sarcoma of
the pharyngeal region: histologic, cytologic, immunohistochemical,
and ultrastructural study of three cases. Ann Diagn Pathol.
8:325–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shia J, Chen W, Tang LH, et al: Extranodal
follicular dendritic cell sarcoma: clinical, pathologic, and
histogenetic characteristics of an underrecognized disease entity.
Virchows Arch. 449:148–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aydin E, Ozluoglu LN, Demirhan B and
Arikan U: Follicular dendritic cell sarcoma of the tonsil: case
report. Eur Arch Otorhinolaryngol. 263:1155–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clement P, Saint-Blancard P, Minvielle F,
Le Page P and Kossowski M: Follicular dendritic cell sarcoma of the
tonsil: a case report. Am J Otolaryngol. 27:207–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDuffie C, Lian TS and Thibodeaux J:
Follicular dendritic cell sarcoma of the tonsil: a case report and
literature review. Ear Nose Throat J. 86:234–235. 2007.PubMed/NCBI
|
|
18
|
Fan YS, Ng WK, Chan A, et al: Fine needle
aspiration cytology in follicular dendritic cell sarcoma: a report
of two cases. Acta Cytol. 51:642–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vaideeswar P, George SM, Kane SV,
Chaturvedi RA and Pandit SP: Extranodal follicular dendritic cell
sarcoma of the tonsil - case report of an epithelioid cell variant
with osteoclastic giant cells. Pathol Res Pract. 205:149–153. 2009.
View Article : Google Scholar
|
|
20
|
Chan AC, Chan KW, Chan JK, et al:
Development of follicular dendritic cell sarcoma in
hyaline-vascular Castleman’s disease of the nasopharynx: tracing
its evolution by sequential biopsies. Histopathology. 38:510–518.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Georgalas C, Kanagalingam J, Gallimore A
and O’Flynn P: Follicular dendritic cell sarcoma arising from the
hypopharynx. J Laryngol Otol. 118:317–318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Encabo RS, McHugh J, Carrau RL, Kassam A
and Heron D: Follicular dendritic cell sarcoma of the nasopharynx.
Am J Otolaryngol. 29:262–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Araújo VC, Martins MT, Salmen FS and
Araújo NS: Extranodal follicular dendritic cell sarcoma of the
palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
87:209–214. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desai S, Deshpande RB and Jambhekar N:
Follicular dendritic cell tumor of the parapharyngeal region. Head
Neck. 21:164–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galati LT, Barnes EL and Myers EN:
Dendritic cell sarcoma of the thyroid. Head Neck. 21:273–275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chou YY, How SW and Huang CH: Follicular
dendritic cell sarcoma of the soft palate. J Formos Med Assoc.
104:843–847. 2005.
|
|
27
|
Yu L and Yang SJ: Primary follicular
dendritic cell sarcoma of the thyroid gland coexisting with
Hashimoto’s thyroiditis. Int J Surg Pathol. 19:502–505. 2011.
View Article : Google Scholar
|
|
28
|
Fisher C, Magnusson B, Hardarson S and
Smith ME: Myxoid variant of follicular dendritic cell sarcoma
arising in the breast. Ann Diagn Pathol. 3:92–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi PC, To KF, Lai FM, et al: Follicular
dendritic cell sarcoma of the neck: report of two cases complicated
by pulmonary metastases. Cancer. 89:664–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pruneri G, Masullo M, Renne G, et al:
Follicular dendritic cell sarcoma of the breast. Virchows Arch.
441:194–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bradshaw EJ, Wood KM, Hodgkinson P,
Lucraft H and Windebank KP: Follicular dendritic cell tumour in a
9-year-old child. Pediatr Blood Cancer. 45:725–727. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kapucuoglu N, Percinel S, Ventura T, et
al: Dendritic cell sarcomas/tumours of the breast: report of two
cases. Virchows Arch. 454:333–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fassina A, Marino F, Poletti A, et al:
Follicular dendritic cell tumor of the mediastinum. Ann Diagn
Pathol. 5:361–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kröber SM, Marx A, Aebert H, Dohmen BM and
Kaiserling E: Sarcoma of follicular dendritic cells in the dorsal
mediastinum. Hum Pathol. 35:259–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawasaki T, Watanabe G, Hasegawa G and
Naito M: Multiple extranodal follicular dendritic cell tumors
initially presenting in the soft tissue in the chest wall. Pathol
Int. 56:30–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guettier C, Validire P, Emilie D, et al:
Follicular dendritic cell tumor of the mediastinum: expression of
fractalkine and SDF-1alpha as mast cell chemoattractants. Virchows
Arch. 448:218–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang L, Admirand JH, Moran C, Ford RJ and
Bueso-Ramos CE: Mediastinal follicular dendritic cell sarcoma
involving bone marrow: a case report and review of the literature.
Ann Diagn Pathol. 10:357–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han JH, Kim SH, Noh SH, et al: Follicular
dendritic cell sarcoma presenting as a submucosal tumor of the
stomach. Arch Pathol Lab Med. 124:1693–1696. 2000.PubMed/NCBI
|
|
39
|
Shah RN, Ozden O, Yeldandi A, et al:
Follicular dendritic cell tumor presenting in the lung: a case
report. Hum Pathol. 32:745–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kovács RB, Sattar HA, Krausz T, et al:
Primary follicular dendritic cell sarcoma of the lung.
Histopathology. 49:431–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Denning KL, Olson PR, Maley RH Jr, et al:
Primary pulmonary follicular dendritic cell neoplasm: a case report
and review of the literature. Arch Pathol Lab Med. 133:643–647.
2009.PubMed/NCBI
|
|
42
|
Fonseca R, Tefferi A and Strickler JG:
Follicular dendritic cell sarcoma mimicking diffuse large cell
lymphoma: a case report. Am J Hematol. 55:148–155. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shek TW, Liu CL, Peh WC, Fan ST and Ng IO:
Intra-abdominal follicular dendritic cell tumour: a rare tumour in
need of recognition. Histopathology. 33:465–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang KC, Jin YT, Chen FF and Su IJ:
Follicular dendritic cell sarcoma of the colon mimicking stromal
tumour. Histopathology. 38:25–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheuk W, Chan JK, Shek TW, et al:
Inflammatory pseudotumor-like follicular dendritic cell tumor: a
distinctive low-grade malignant intra-abdominal neoplasm with
consistent Epstein-Barr virus association. Am J Surg Pathol.
25:721–731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Agaimy A and Wünsch PH: Follicular
dendritic cell tumor of the gastrointestinal tract: Report of a
rare neoplasm and literature review. Pathol Res Pract. 202:541–548.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Torres U, Hawkins WG, Antonescu CR and
DeMatteo RP: Hepatic follicular dendritic cell sarcoma without
Epstein-Barr virus expression. Arch Pathol Lab Med. 129:1480–1483.
2005.PubMed/NCBI
|
|
48
|
Khalid T and Folman R: Symptoms in cancer
patients and an unusual tumor: case three. Follicular dendritic
cell sarcoma. J Clin Oncol. 23:9425–9426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Horiguchi H, Matsui-Horiguchi M, Sakata H,
et al: Inflammatory pseudotumor-like follicular dendritic cell
tumor of the spleen. Pathol Int. 54:124–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ren R, Sun X, Staerkel G, Sneige N and
Gong Y: Fine-needle aspiration cytology of a liver metastasis of
follicular dendritic cell sarcoma. Diagn Cytopathol. 32:38–43.
2005. View Article : Google Scholar
|
|
51
|
Granados R, Aramburu JA, Rodríguez JM and
Nieto MA: Cytopathology of a primary follicular dendritic cell
sarcoma of the liver of the inflammatory pseudotumor-like type.
Diagn Cytopathol. 36:42–46. 2008. View Article : Google Scholar
|
|
52
|
Moriki T, Takahashi T, Wada M, et al:
Follicular dendritic cell tumor of the mesentery. Pathol Res Pract.
193:629–639; discussion 640–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamakawa M, Andoh A, Masuda A, et al:
Follicular dendritic cell sarcoma of the omentum. Virchows Arch.
440:660–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sander B, Middel P, Gunawan B, et al:
Follicular dendritic cell sarcoma of the spleen. Hum Pathol.
38:668–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yakushijin Y, Shikata H, Kito K, et al:
Follicular dendritic cell tumor as an unknown primary tumor. Int J
Clin Oncol. 12:56–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chiaramonte MF, Lee D, Abruzzo LV, Heyman
M and Bass BL: Retroperitoneal follicular dendritic cell sarcoma
presenting as secondary amyloidosis. Surgery. 130:109–111. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Kong Y, Lu H and Xu Y: Two cases
of extranodal follicular dendritic cell sarcoma. Chin Med J (Engl).
116:794–797. 2003.
|
|
58
|
Marzano AV, Vezzoli P, Mariotti F, et al:
Paraneoplastic pemphigus associated with follicular dendritic cell
sarcoma and Castleman disease. Br J Dermatol. 153:214–215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Androulaki A, Liapis G, Alexandrou P and
Lazaris AC: Retroperitoneal follicular dendritic cell sarcoma. Int
J Hematol. 84:22006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Padilla-Rodríguez AL, Bembassat M, Lazaro
M and Ortiz-Hidalgo C: Intra-abdominal follicular dendritic cell
sarcoma with marked pleomorphic features and aberrant expression of
neuroendocrine markers: report of a case with immunohistochemical
analysis. Appl Immunohistochem Mol Morphol. 15:346–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Loo CK, Henderson C and Rogan K:
Intraabdominal follicular dendritic cell sarcoma: report of a case
with fine needle aspiration findings. Acta Cytol. 45:999–1004.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hasselblatt M, Sepehrnia A, von FM and
Paulus W: Intracranial follicular dendritic cell sarcoma. Case
report. J Neurosurg. 99:1089–1090. 2003. View Article : Google Scholar
|
|
63
|
Li CF, Chuang SS and Lin CN: A 70-year-old
man with multiple intra-abdominal masses and liver and spleen
metastases. Intra-abdominal follicular dendritic cell sarcoma with
liver and spleen metastases. Arch Pathol Lab Med. 129:e130–e131.
2005.PubMed/NCBI
|
|
64
|
Shen SC, Wu CC, Ng KF, et al: Follicular
dendritic cell sarcoma mimicking giant cell carcinoma of the
pancreas. Pathol Int. 56:466–470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Choi JW, Lee JH, Kim A, et al: Follicular
dendritic cell sarcoma arising in the dura mater of the spine. Arch
Pathol Lab Med. 130:1718–1721. 2006.PubMed/NCBI
|
|
66
|
Karaman E, Saritzali G, Kilic E, Korkut N
and Enver O: Follicular dendritic cell sarcoma of the parotid gland
recurring 6 times within 12 years. J Craniofac Surg. 20:2171–2172.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yamada Y, Haga H, Hernandez M, et al:
Follicular dendritic cell sarcoma of small intestine with aberrant
T-cell marker expression. Pathol Int. 59:809–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hartert M, Ströbel P, Dahm M, et al: A
follicular dendritic cell sarcoma of the mediastinum with immature
T cells and association with myasthenia gravis. Am J Surg Pathol.
34:742–745. 2010.PubMed/NCBI
|
|
69
|
Kim WY, Kim H, Jeon YK and Kim CW:
Follicular dendritic cell sarcoma with immature T-cell
proliferation. Hum Pathol. 41:129–133. 2010. View Article : Google Scholar
|
|
70
|
Suhail Z, Musani MA, Afaq S, Zafar A and
Ahmed Ashrafi SK: Follicular dendritic cell sarcoma of tonsil. J
Coll Physicians Surg Pak. 20:55–56. 2010.PubMed/NCBI
|
|
71
|
Suchitha S, Sheeladevi CS, Sunila R and
Manjunath GV: Extra nodal follicular dendritic cell tumor. Indian J
Pathol Microbiol. 53:175–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Amiri-Kordestani L, Priebat D and Chia SH:
Follicular dendritic cell sarcoma of the neck: case report and
review of current diagnostic and management strategies. Ear Nose
Throat J. 89:E14–E17. 2010.PubMed/NCBI
|
|
73
|
Eun YG, Kim SW and Kwon KH: Follicular
dendritic cell sarcoma of the tonsil. Yonsei Med J. 51:602–604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Duan GJ, Wu F, Zhu J, et al: Extranodal
follicular dendritic cell sarcoma of the pharyngeal region: a
potential diagnostic pitfall, with literature review. Am J Clin
Pathol. 133:49–58. 2010. View Article : Google Scholar
|
|
75
|
Li L, Shi YH, Guo ZJ, et al:
Clinicopathological features and prognosis assessment of extranodal
follicular dendritic cell sarcoma. World J Gastroenterol.
16:2504–2519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Martins PN, Reddy S, Martins AB and
Facciuto M: Follicular dendritic cell sarcoma of the liver: unusual
presentation of a rare tumor and literature review. Hepatobiliary
Pancreat Dis Int. 10:443–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Silver AL, Faquin WC and Deschler DG:
Follicular dendritic cell sarcoma presenting in the submandibular
region in an 11 year-old. International Journal of Pediatric
Otorhinolaryngology Extra. 6:104–106. 2011. View Article : Google Scholar
|
|
78
|
Fareed MM, Memon MA, Rashid A, et al:
Follicular dendritic cell sarcoma of the neck with pulmonary
metastases. J Coll Physicians Surg Pak. 21:561–563. 2011.PubMed/NCBI
|
|
79
|
Li Z, Jin K, Yu X, et al: Extranodal
follicular dendritic cell sarcoma in mesentery: A case report.
Oncol Lett. 2:649–652. 2011.
|
|
80
|
Pyo JS, Kang G, Do SI, et al: Extranodal
follicular dendritic cell sarcoma with rapid growth in parapharynx:
a case report. Korean J Pathol. 46:306–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mondal SK, Bera H, Bhattacharya B and
Dewan K: Follicular dendritic cell sarcoma of the tonsil. Natl J
Maxillofac Surg. 3:62–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Karabulut B, Orhan KS, Guldiken Y and
Dogan O: Follicular dendritic cell sarcoma of the nasopharynx. Int
J Oral Maxillofac Surg. 41:218–220. 2012. View Article : Google Scholar
|
|
83
|
Malik A, Veniyoor A, Fanthome B and Dutta
V: Follicular dendritic cell sarcoma: a diagnostic challenge! J
Cancer Res Ther. 8:306–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hu T, Wang X, Yu C, et al: Follicular
dendritic cell sarcoma of the pharyngeal region. Oncol Lett.
5:1467–1476. 2013.PubMed/NCBI
|
|
85
|
Saygin C, Uzunaslan D, Ozguroglu M,
Senocak M and Tuzuner N: Dendritic cell sarcoma: a pooled analysis
including 462 cases with presentation of our case series. Crit Rev
Oncol Hematol. 88:253–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Choe JY, Go H, Jeon YK, et al:
Inflammatory pseudotumor-like follicular dendritic cell sarcoma of
the spleen: a report of six cases with increased IgG4-positive
plasma cells. Pathol Int. 63:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yu H, Gibson JA, Pinkus GS and Hornick JL:
Podoplanin (D2–40) is a novel marker for follicular dendritic cell
tumors. Am J Clin Pathol. 128:776–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chan JK, Tsang WY, Ng CS, et al:
Follicular dendritic cell tumors of the oral cavity. Am J Surg
Pathol. 18:148–157. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wook YK, Haeryoung K, Yoon KJ, et al:
Follicular dendritic cell sarcoma with immature T-cell
proliferation. Human Pathology. 41:129–133. 2010. View Article : Google Scholar
|
|
90
|
Marc H, Philipp S, Manfred D, et al: A
Follicular Dendritic Cell Sarcoma of the Mediastinum With Immature
T Cells and Association With Myasthenia Gravis. Am J Surg Pathol.
34:742–745. 2010.
|
|
91
|
Hsu C, Vega F, Grimes LM and Hunt KK:
Follicular dendritic cell sarcoma and associated myasthenia gravis:
true, true, related? J Clin Oncol. 29:e369–e371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jaffee ES, Harris NL, Stein H and Vardiman
JW: World Health Organization classification of tumors. Pathology
and genetics of tumors of hematopoietic and lymphoid tissues Lyon:
IARC Press; 2001
|
|
93
|
Chan JKC, Pileri SA, Delsol G, et al:
Follicular dendritic cell sarcoma. WHO Classification of Tumours of
Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris
NL, Jaffe ES, Pileri SA, Stein H, Thiele J and Vardiman JW: 4th
edition. IARC Press; Lyon: pp. 363–364. 2008
|