Introduction
Renal cell carcinoma (RCC) is a kidney cancer that
derives from the lining of the proximal convoluted tubule and the
small tubes in the kidney that filter the blood to remove waste
products. RCC is the most common type of kidney cancer in adults,
responsible for ~80% of cases, and is also known to be the most
lethal of all the genitourinary tumors (1).
Although an increasing number of individuals succumb
to RCC and studies have begun to focus on the differentially
expressed genes and microRNAs (miRNAs) in RCC, the signals and
mechanisms that govern miRNA transcriptional regulation remain
unclear. Experimental data indicates that differentially expressed
genes and differentially expressed miRNAs play key roles in the
development, metastasis and therapy of RCC. For example,
polymorphisms in vascular endothelial growth factor are associated
with an increased risk of developing RCC (2). The tumor suppressor gene DKK1 induces
apoptosis and inhibits proliferation in human RCC cells (3). The majority of regulatory genes encode
transcription factors (TFs), which modulate gene expression by
binding the regulatory sequences of their target genes. TFs and
miRNAs are prominent regulators of gene expression (4). TFs are proteins that can activate or
repress transcription by binding cis-regulatory elements,
located in the upstream regions of genes and regulate gene
expression at the transcriptional level, either individually or
joint with other proteins.
MiRNAs can be located within numerous genes and are
named for their host genes. Rodriguez et al indicated that
miRNAs were transcribed in parallel with their host transcripts and
two different transcription classes of miRNAs, exonic and intronic,
were identified that may require slightly different mechanisms of
biogenesis (5). Baskerville and
Bartel indicated that intronic miRNAs and their host genes
exhibited a close association (6).
Intronic miRNAs and their host genes are usually coordinately
expressed in biological progression. They usually act as potential
partners to achieve biological function and also affect the
alteration of pathways (7). All
results suggest that miRNAs can work together with their host genes
or separately to contribute to the progression of cancer. In the
present study, when the miRNA is differently expressed the host
genes are considered to also be differently expressed and involved
in the progression of cancer.
The present study aimed to extract the associations
between genes, miRNAs and their host genes, and these
transcriptional associations were considered to be a point of
penetration to build the regulatory network of the genes and miRNAs
involved in RCC. Three levels of networks were obtained, the
differentially expressed, associated and global networks. The
global network contained all the experimentally validated pathways
for the genes and miRNA. However, this network was so complex that
the pathways associated with RCC could not be easily identified.
Therefore, the other two networks were used for further research.
Pathways for the differentially expressed elements were extracted
separately and the differentially expressed network partially
uncovered how RCC forms. The topology network was found in the
progression of RCC. The similarities and differences between the
three networks were compared and analyzed to distinguish the key
nodes and pathways. The network of differentially expressed
elements partially uncovered the mechanism of RCC.
Materials and methods
Material collection and data
processing
Target miRNA interactions were extracted from two
databases, TarBase 5.0 (http://diana.cslab.ece.ntua.gr/tarbase/) and
miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/). TarBase 5.0 is a
comprehensive database with experimentally supported animal miRNA
targets and miRTarBase is a database of experimentally validated
miRNA-targets interactions. Different databases use different
symbols to represent miRNAs and genes. In order to unify the method
of symbolic representation, the official symbols from the National
Center for Biotechnology Information (NCBI) database, which can be
accessed online (www.ncbi.nlm.nih.gov/gene/), were used. These
experimentally validated data strongly support the present study.
This dataset was considered to be set U1.
The TF-miRNA interactions were extracted from
TransmiR (http://cmbi.bjmu.edu.cn/transmir) (8). The data on TransmiR were extracted
from the public literature and biological experiments. This dataset
was considered to be set U2.
Information on the miRNA and host genes was
extracted from miRBase (http://www.mirbase.org/) and NCBI. MiRBase provides a
collection of all the confirmed human miRNAs. The official symbols
and official IDs from NCBI were used to indicate host genes and
their miRNAs. This dataset was considered to be set
U3.
The differentially expressed genes of RCC were
mainly extracted from the KEGG pathway database and Cancer Genetics
Web, which can be accessed online (www.cancerindex.org). The KEGG pathway database
(www.kegg.com/kegg/pathway.html) consists
of graphical diagrams of biochemical pathways and certain known
regulatory pathways. The RCC pathway map was obtained from this
database. The map demonstrates all the validated mutated RCC genes.
Similar methods were used to extract the mutated RCC genes from
Cancer Genetics Web (accessed online at www.cancer-genetics.org). To complete the data
collection, studies on the mutated genes of RCC were manually
searched for using the Science Citation Index (SCI; Thomson-Reuters
Corporation, New York, NY, USA). The RCC associated genes include
the differentially expressed genes of RCC and other associated
genes for which the pertinent literature was manually searched. In
the present study, differentially expressed genes were considered
to be a part of the associated genes in the RCC-associated network.
Additionally, TFs that may be involved in RCC were extracted using
the P-match method. P-Match is a novel tool to identify TF binding
sites in DNA sequences. The tool combines pattern matching with
weight matrix approaches, and thus provides higher accuracy of
recognition than each of the methods alone. TFs were considered to
be RCC-associated genes and only the TFs in set
U2 were focused on. Promoter region sequences,
1,000 and 5,000 nt in length, of targets targeted by mutated miRNAs
were downloaded from the University of California Santa Cruz
database (http://genome.ucsc.edu/). The P-match
method was used to identify the TF binding sites in the 1000 and
5000 nt promoter region sequences and mapped TFBSs onto the
promoter region of targets. The matrix library of P-Match is
contains sets of known TF-binding sites collected in TRANSFAC, and
therefore provides the possibility of searching for a large variety
of TF binding sites. The complete data of differentially expressed
genes and associated genes were considered to be data set
U4.
Differentially expressed miRNAs were extracted from
mir2Disease (9). Mir2Disease is a
manually curated database that aims to provide a comprehensive
resource of miRNA deregulation in various human diseases. To
complete the data collection, studies on RCC were searched for
using SCI. The complete differentially expressed miRNAs and
associated miRNAs of RCC are considered as set
U5.
Three level networks construction
The transcriptional network of RCC is an extremely
complex regulatory network. Differentially expressed genes and
miRNAs play key roles in this network. They participate in cancer
progression, including carcinogenesis, metastasis and therapy.
Therefore, the core network in RCC was extracted using the
following method: Differentially expressed data from
U4 and U5 was mapped onto
U1, U2 and
U3, and then the regulatory associations of
TF-miRNA, miRNA-targets and host gene-miRNA were extracted.
Following the combination of all the associations, the core network
was obtained.
In addition to differentially expressed genes and
miRNAs, the RCC-associated genes and miRNAs also influence the key
cellular processes of RCC. Therefore, the network of RCC-associated
elements was used to further illuminate the regulatory network of
RCC. Naturally, this network includes the core network and it has
more complex regulatory associations compared with the core
network. The regulatory network in RCC was extracted using the
aforementioned method.
The former two networks present extremely important
regulatory associations in RCC. In addition to the experimentally
validated genes and miRNAs that are included in the former two
networks, certain genes and miRNAs that are not experimentally
validated may be involved in the progression of RCC. In the third
network, the complete TF and miRNA interaction that were present in
the associated network were mapped onto U1,
U2 and U3, and then the
regulatory associations of TF-miRNA, miRNA-targets and host
gene-miRNA were extracted. Following the combination of all the
associations, the expanded global network was obtained.
Results and Discussion
Core transcriptional network of RCC
Through statistical analysis, a core transcriptional
network that attempts to describe the mechanism of human RCC was
obtained. There were two TFs, phosphatase and tensin homolog (PTEN)
and tumor protein p53 (TP53) in this network, which were regarded
as the essential regulatory elements.
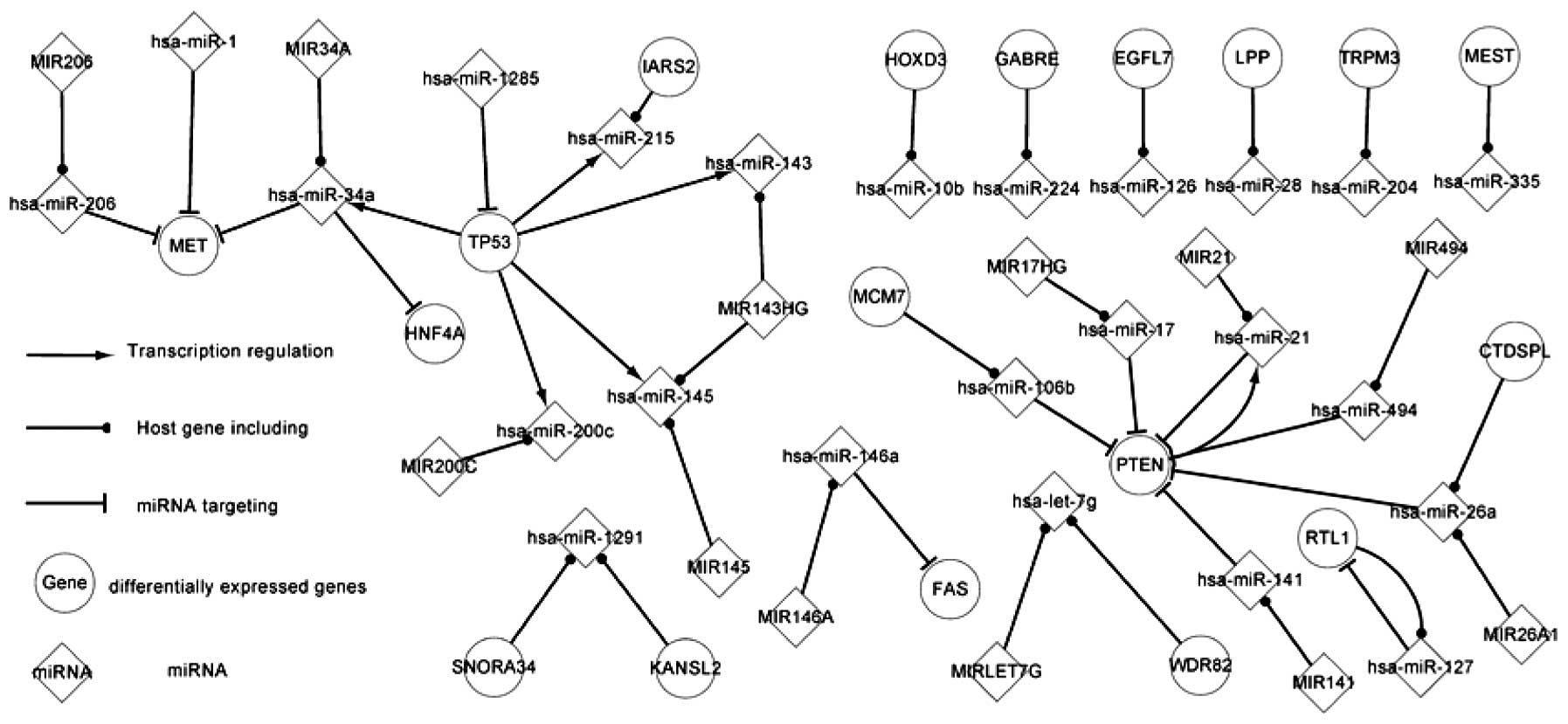
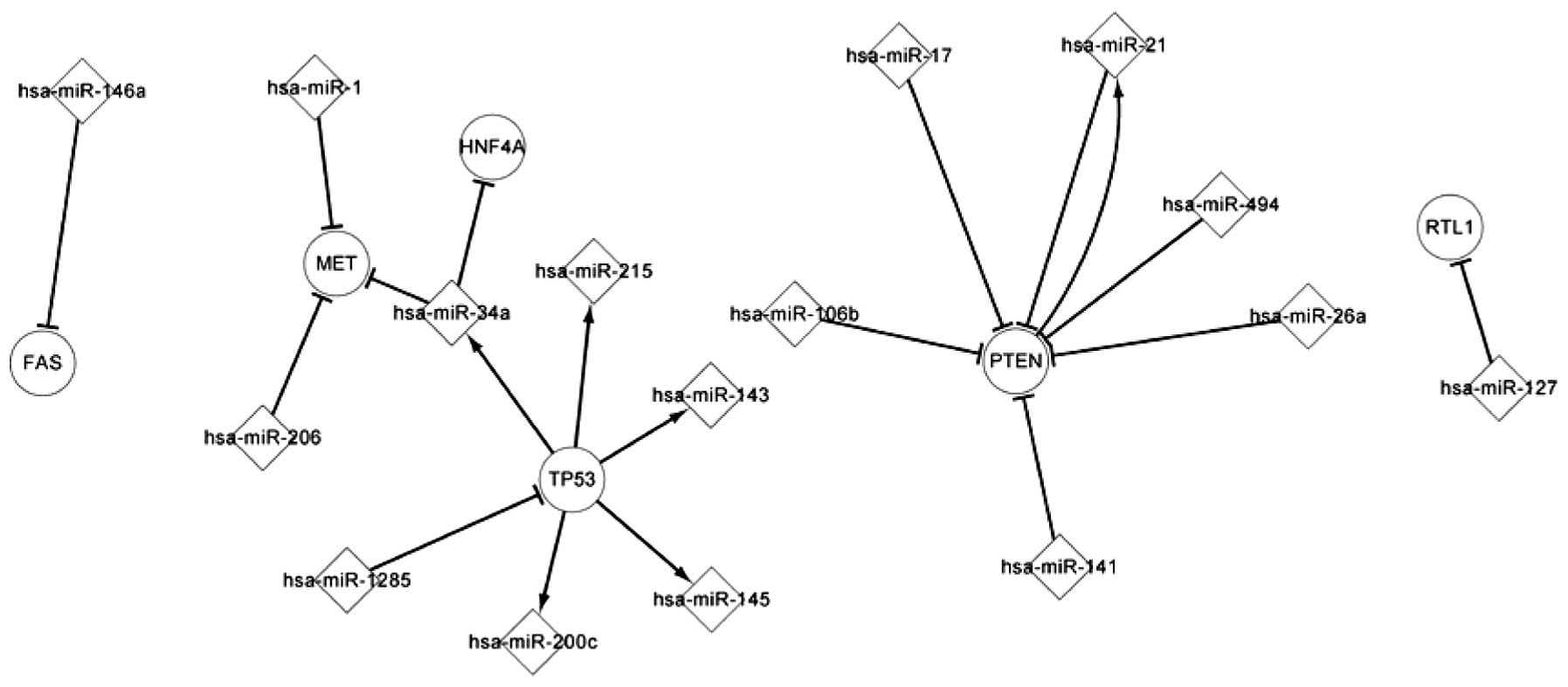
Fig. 1 shows the
core transcriptional network, consisting of few regulatory
pathways. PTEN regulates hsa-miR-21 and TP53 regulates five miRNAs,
consisting of hsa-miR-143, hsa-miR-145, hsa-miR-200, hsa-miR-215
and hsa-miR-34a. These five miRNAs target two genes, HNF4A and MET.
These genes and miRNAs are extremely important in the progression
of RCC. Wirsing et al indicated that miR-34a overexpression
in RCC cooperates with the downregulation of HNF4A mRNA (10).
The product of the TP53 gene is tumor protein p53.
This protein acts as a tumor suppressor and regulates cell division
by preventing cells from growing and dividing too rapidly or in an
uncontrolled way (11). Mutations
in the TP53 gene may aid predictions of whether the RCC will
progress and spread to nearby tissues and whether the disease will
recur following treatment.
The tumor suppressor gene PTEN is mutated or
homozygously deleted in numerous cancers and maps to a region of
10q within the reported region of minimal loss in RCC (12). PTEN participates in two overlaps and
targets hsa-miR-106b, hsa-miR-141, hsa-miR-17, hsa-miR-21,
hsa-miR-26a and hsa-miR-494 whilst simultaneously being targeted by
them.
The present study attempted to use the core
transcriptional network to describe the mechanism of RCC. In the
following section, the more complex regulatory network will be
discussed.
RCC-associated network
The associated regulatory network of RCC consists of
differentially expressed genes and miRNAs, associated genes and
miRNAs, targets of miRNAs and host genes of miRNAs. Naturally, the
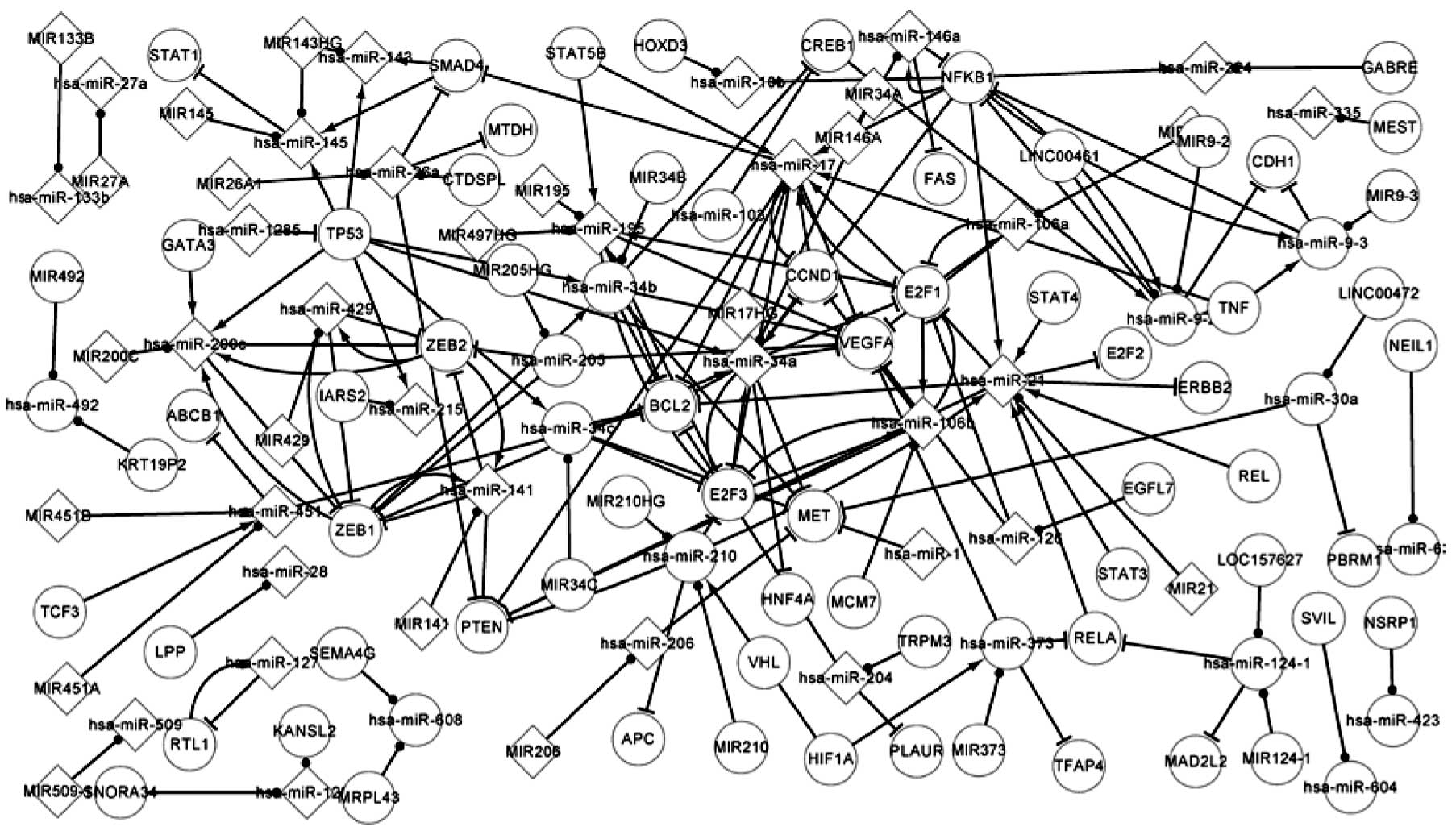
associated network includes the core network. Fig. 2 shows more complex regulatory
associations than Fig. 1.
Fig. 1 shows that
there are two TFs, PTEN and TP53, in the regulatory network.
Therefore, these two TFs were regarded as the essential regulatory
elements. In Fig. 2, there are 20
TFs in the regulatory network. These 20 TFs include the two
essential TFs presented in Fig. 1
and regulate 22 mutated miRNAs. The TFs in the network, TP53, ZEB1
and NFKB1, regulate more miRNA expression and have a high potential
for being more influential with regard to the overall behavior of
the network compared with others (13,14).
NFKB1 regulates hsa-miR-146a expression, and hsa-miR-146a targets
FAS and NFKB1 itself. Therefore, NFKB1 and hsa-miR-146a constitute
a feedback loop.
Fig. 2 shows
additional pathways that affect the progression of RCC compared
with Fig. 1. In order to briefly
explain this, only certain additional pathways for differentially
expressed TFs and additional miRNAs are described. ZEB1 encodes a
zinc finger TF. The encoded protein likely plays a role in the
transcriptional repression of interleukin 2 (15). Through this picture it can be
predicted that mutations in this gene may associate with RCC. ZEB1
regulates hsa-miR-141, hsa-miR-200c, hsa-miR-34a, hsa-miR-34b and
hsa-miR-429 expression. Liu et al identified that miR-200c
microRNAs and E-cadherin maintain a higher level of expression by
repressing ZEB1 (16). These five
miRNAs target 12 relevant genes, including PTEN, MET and CCND1. It
has been demonstrated that an ESR1-mediated decrease in hsa-miR-21
expression correlates with increased protein expression of
endogenous hsa-miR-21 targets, including PDCD4, PTEN and BCL2
(17).
NFKB1 is another important TF that regulates the
expression of eight miRNAs, including hsa-miR-21, hsa-miR-17 and
hsa-miR-224. In the present network, a total of 16 feedback loops,
consisting of seven genes and 12 miRNAs, were identified. Two
differentially expressed genes were involved in the feedback
loops.
Global network of RCC
The global regulatory network shows additional
comprehensive regulatory associations of RCC, including all the
associations in U1, U2 and
U3, and contains a larger number of TFs, targets,
miRNAs and miRNA host genes compared with the associated network.
The global regulatory network also includes the differentially
expressed and associated networks.
Comparison and analysis of the genetic
role of differentially expressed genes
Nodes were classed according to the regulatory
association of adjacent nodes in the three network levels for
comparing and analyzing the interacting features of each
differentially expressed gene. Among these genes, two genes, PTEN
and TP53, demonstrated the particular feature of regulating miRNA
and being targeted by the miRNA.
Initially, the present study focused on the genes.
The first class of gene possesses six types of adjacent nodes,
consisting of three types of successors and three types of
predecessors. This class of gene includes PTEN and TP53. Table I shows PTEN, predecessors of PTEN
and successors of PTEN as well as their regulatory
associations.
 | Table IRegulatory associations between miRNAs
and PTEN. |
Table I
Regulatory associations between miRNAs
and PTEN.
| miRNAs that target
gene | | miRNA that is
regulated by gene |
|---|
| |
|
|---|
| Differentially
expressed miRNAs | Associated
miRNAs | Global miRNAs | Gene | Differentially
expressed miRNAs | Associated
miRNAs | Global miRNAs |
|---|
| hsa-miR-106b | hsa-miR-106b | hsa-miR-106b | PTEN | hsa-miR-21 | hsa-miR-21 | hsa-miR-19a |
| hsa-miR-141 | hsa-miR-141 | hsa-miR-141 | PTEN | 0 | 0 | hsa-miR-21 |
| hsa-miR-17 | hsa-miR-17 | hsa-miR-17 | PTEN | 0 | 0 | hsa-miR-22 |
| hsa-miR-21 | hsa-miR-21 | hsa-miR-18a | PTEN | 0 | 0 | hsa-miR-25 |
| hsa-miR-26a | hsa-miR-26a | hsa-miR-19a | PTEN | 0 | 0 | hsa-miR-302 |
| hsa-miR-494 | 0 | hsa-miR-19b | PTEN | 0 | 0 | hsa-miR-302a |
| 0 | 0 | hsa-miR-19b-1 | PTEN | 0 | 0 | hsa-miR-302b |
| 0 | 0 | hsa-miR-19b-2 | PTEN | 0 | 0 | hsa-miR-302c |
| 0 | 0 | hsa-miR-20 | PTEN | 0 | 0 | hsa-miR-302d |
| 0 | 0 | hsa-miR-20a | PTEN | 0 | 0 | hsa-miR-302f |
| 0 | 0 | hsa-miR-21 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-214 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-216 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-216a | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-217 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-221 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-222 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-26a | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-26a-1 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-26a-2 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-494 | PTEN | 0 | 0 | 0 |
| 0 | 0 | hsa-miR-91 | PTEN | 0 | 0 | 0 |
Table I shows six
miRNAs that target PTEN. In the core, associated and global
networks, PTEN regulates one, one and 10 miRNAs, respectively.
Additionally, in the associated and global networks, PTEN is
targeted by five and 22 miRNAs, respectively. These predecessors
indirectly affect successors through PTEN. Among all the miRNAs, it
was found that hsa-miR-21 targets and is regulated by PTEN in the
three networks. PTEN and hsa-miR-21 form a self-adaption
association. They are each differentially expressed elements in
RCC. Therefore, PTEN and hsa-miR-21 must play a key role in the
progression of RCC. Dey et al revealed that miR-21 targets
the PTEN mRNA 3′ untranslated region to decrease PTEN protein
expression and augments Akt phosphorylation in renal cancer cells,
and also revealed that downregulation of PTEN and overexpression of
constitutively active Akt kinase prevented miR-21 Sponge-induced
inhibition of renal cancer cell proliferation and migration
(18).
Secondly, the remaining genes that do not regulate
any miRNA were focused on. The first class of gene possesses three
types of adjacent nodes, consisting of three types of predecessors,
including APC, FAS, HNF4A and MET. They are only targeted by
certain miRNAs, but do not regulate any miRNA. It was suggested
that these may be the last nodes in the pathway.
The second class of gene possesses one type of
adjacent node, a type of predecessor, such as GRB2. GRB2 is
targeted by two miRNAs in the global network and it does not
regulate any miRNA. It was suggested that GRB2 has the least effect
compared with other differentially expressed genes.
The third class of gene possesses two types of
adjacent nodes, two types of successors or two types of
predecessors, including PBRM1 and VHL. PBRM1 has predecessor in the
associated network and global network, and it does not regulate any
miRNA. VHL possesses successors in the associated and global
networks and it does not target any miRNA.
The final class of gene only possesses a type of
adjacent node, such as HSPA1B or IL6. There are also certain genes
that possess no adjacent nodes, including CA9 and FH, that are not
in the present discussion.
Comparison and analysis of the features
of differentially expressed miRNAs
Similar to analyzing differentially expressed genes,
the same method was used to analyze 38 differentially expressed
miRNAs. In Table II, hsa-miR-34a
was set as an example and the precursors and successors of
hsa-miR-34a in the differentially expressed, associated and global
networks are listed. hsa-miR-34a possesses six types of adjacent
nodes, three types of predecessors and three types of
successors.
 | Table IIPartially shows regulatory relations
between hsa-miR-143 and genes in three networks. |
Table II
Partially shows regulatory relations
between hsa-miR-143 and genes in three networks.
| Genes that regulate
miRNA | | Target genes of
miRNA |
|---|
| |
|
|---|
| Differentially
expressed network | Associated
network | Global network | miRNA | Differentially
expressed network | Associated
network | Global network |
|---|
| TP53 | E2F3 | CEBPA | hsa-miR-34a | HNF4A | E2F1 | AXIN2 |
| 0 | NFKB1 | E2F3 | hsa-miR-34a | MET | E2F3 | BCL2 |
| 0 | TP53 | MYC | hsa-miR-34a | 0 | HNF4A | BIRC3 |
| 0 | ZEB1 | NFKB1 | hsa-miR-34a | 0 | MET | CCND1 |
| 0 | 0 | NR1H4 | hsa-miR-34a | 0 | CCND1 | CCND3 |
| 0 | 0 | SNAI1 | hsa-miR-34a | 0 | BCL2 | CCNE2 |
| 0 | 0 | TP53 | hsa-miR-34a | 0 | VEGFA | CD44 |
| 0 | 0 | ZEB1 | hsa-miR-34a | 0 | 0 | CDC25A |
| 0 | 0 | 0 | hsa-miR-34a | 0 | 0 | CDC25C |
| 0 | 0 | 0 | hsa-miR-34a | 0 | 0 | CDK4 |
| 0 | 0 | 0 | hsa-miR-34a | 0 | 0 | CDK6 |
| 0 | 0 | 0 | hsa-miR-34a | 0 | 0 | CEBP |
In the differentially-expressed network, TP53
regulates hsa-miR-34a and hsa-miR-34a targets HNF4A and MET. In the
associated network, E2F3, NFKB1, TP53 and ZEB1 regulates
has-miR-34a, while hsa-miR-34a targets E2F1, E2F3, HNF4A, MET,
CCND1, BCL2 and VEGFA. In the associated network, there is a
specific gene, E2F3, which forms a self-adaption association with
hsa-miR-34a.
The method that was used for the differentially
expressed genes was used to compare and analyze each differentially
expressed miRNA. Among the present miRNAs, there were 38
differentially expressed miRNAs. The first class of miRNA possessed
six types of adjacent nodes, three types of predecessors and three
types of successors, including hsa-miR-143. The second class of
miRNA possessed five types of adjacent nodes, including
hsa-miR-141. Hsa-miR-141 is targeted by three genes and is
regulated by two genes. The classification method used for miRNAs
was similar to that used for the genes and is therefore not
explained.
Comparison and analysis of the features
of popular TFs
The same method that was used for the genes and
miRNA was used to compare and analyze each popular TF in the
associated network. Numerous TFs, including CREB1, E2F1, NFKB1 and
ZEB1, and the corresponding miRNAs were found to form self-adaption
associations. Table III uses ZEB1
as an example.
 | Table IIIRegulatory relation between miRNAs
and ZEB1. |
Table III
Regulatory relation between miRNAs
and ZEB1.
| miRNA that targets
gene | | miRNA that is
regulated by gene |
|---|
| |
|
|---|
| Differentially
expressed miRNAs | Associated
miRNAs | Global miRNAs | Gene | Differentially
expressed miRNAs | Associated
miRNAs | Global miRNAs |
|---|
| 0 | hsa-miR-141 | hsa-miR-141 | ZEB1 | 0 | hsa-miR-141 | hsa-let-7 |
| 0 | hsa-miR-200c | hsa-miR-200a | ZEB1 | 0 | hsa-miR-200c | hsa-let-7a |
| 0 | hsa-miR-205 | hsa-miR-200b | ZEB1 | 0 | hsa-miR-34a | hsa-let-7a-1 |
| 0 | hsa-miR-429 | hsa-miR-200c | ZEB1 | 0 | hsa-miR-34b | hsa-let-7a-2 |
| 0 | 0 | hsa-miR-205 | ZEB1 | 0 | hsa-miR-429 | hsa-let-7a-3 |
| 0 | 0 | hsa-miR-429 | ZEB1 | 0 | 0 | hsa-let-7b |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7c |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7d |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7e |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7f |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7f-1 |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7f-2 |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7g |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-let-7i |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-141 |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-200a |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-200b |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-200c |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-34 |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-34a |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-34b |
| 0 | 0 | 0 | ZEB1 | 0 | 0 | hsa-miR-429 |
In the differentially expressed network, ZEB1 is not
targeted by any miRNAs and does not regulate any miRNAs. In the
associated network, ZEB1 regulates hsa-miR-141, hsa-miR-200c,
hsa-miR-34a, hsa-miR-34b, hsa-miR-429 and hsa-miR-141.
Hsa-miR-200c, hsa-miR-205 and hsa-miR-429 target ZEB1. ZEB1 forms
self-adaption associations with hsa-miR-141, hsa-miR-200c and
hsa-miR-429.
Analysis of host genes and miRNA in
RCC
Host genes and their miRNA demonstrate certain
important features in the present study. Although these host genes
are not differentially expressed in RCC, they are considered to be
differentially expressed genes when the miRNAs is differentially
expressed. Fig. 1 shows certain
host gene and miRNA pathways. For example, the MCM7 gene codes for
hsa-miR-106b, which targets PTEN. There is a notable association
between hsa-miR-21 and PTEN. The PTEN gene includes hsa-miR-21 and
is also targeted by hsa-miR-21. A host gene includes several
miRNAs. For example, the MIR143HG gene includes hsa-miR-143 and
hsa-miR-145. One miRNA can also be located in several genes. For
example, two host genes, KANSL2 and SNORA34, include hsa-miR-1291.
It was suggested that host genes and their miRNAs may aid the
understanding of the pathogenesis of RCC.
Transcriptional network of TFs and
differentially expressed miRNAs
Fig. 3 shows the
transcriptional network of popular TFs and differentially expressed
miRNAs. There are 32 different miRNAs in the differentially
expressed network. In total, 16 differentially expressed miRNAs are
included in Fig. 3. These miRNAs
and popular TFs construct a transcriptional network, which presents
several significant characters in the progression of RCC. TFs and
miRNAs exhibit several types of regulatory associations that
precisely affect the expression of their targeted elements.
Fig. 3 shows that PTEN regulates
one miRNA and is targeted by six miRNAs. The five miRNAs,
hsa-miR-143, hsa-miR-145, hsa-miR-200c, hsa-miR-215 and
hsa-miR-34a, are regulated by TP53 and can target HNF4A and MET.
PTEN regulates and is targeted by hsa-miR-21. The aforementioned
and differentially expressed miRNAs interact with each other to
affect the progression of RCC. Fig.
3 also shows that one differentially expressed miRNA can be
regulated by several TFs and can indirectly affect another miRNA
through TFs, and Fig. 3 also shows
that one TF can be targeted by several differentially expressed
miRNAs and indirectly affect another TF through differentially
expressed miRNA.
Conclusion
All the currently validated genes and miRNAs
associated with RCC were collected in the present study and three
regulatory networks were used to analyze the complex regulatory
associations of the differentially expressed elements in RCC. The
present study extracted and compared the similarities and
differences of all the differentially expressed elements in the
three networks to distinguish the key nodes and pathways that
contribute to understanding the mechanism of the carcinogenicity
and the therapy of RCC. It was found that certain pathways of
differentially expressed elements have been validated in RCC and
other pathways that have not been validated in RCC affect other
cancers. Certain associated pathways in the associated network and
in additional pathways in the global network were also found to
affect the progression of other cancers. Pathways of differentially
expressed elements must be involved in RCC, but the majority of
these mechanisms remain unclear. The pathways that are not
validated in RCC can affect the progression of cancer whether they
play similar or novel roles in RCC. Additional research on these
pathways in RCC is required. The present study supplied
comprehensive data associated with RCC that will guide medical
investigators and biologists to further achieve pertinent research
about the mechanisms of differentially expressed genes and miRNAs
in RCC. In future studies, transcription co-factors and the
interaction between proteins may be considered in the present
network, which may derive a more comprehensive and extensive
network for RCC. In-depth research into the pathogenesis and
treatment of RCC using such ample data may also be a focus of
future studies.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 60973091) and
Science and Technology Development Plan of Jilin Province (no.
20130101166JC).
References
|
1
|
Mulders PF, Brouwers AH, Hulsbergen-van
der Kaa CA, van Lin EN, Osanto S and de Mulder PH: Guideline ‘Renal
cell carcinoma’. Ned Tijdschr Geneeskd. 152:376–380. 2008.(In
Dutch). PubMed/NCBI
|
|
2
|
Bruyère F, Hovens CM, Marson MN, d’Arcier
BF, Costello AJ, Watier H, Linassier C and Ohresser M: VEGF
polymorphisms are associated with an increasing risk of developing
renal cell carcinoma. J Urol. 184:1273–1278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y, Saini
S, Majid S, Deng G, Ishii N and Dahiya R: Wnt antagonist DKK1 acts
as a tumor suppressor gene that induces apoptosis and inhibits
proliferation in human renal cell carcinoma. Int J Cancer.
128:1793–1803. 2011. View Article : Google Scholar
|
|
4
|
Hobert O: Gene regulation by transcription
factors and microRNAs. Science. 319:1785–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baskerville S and Bartel DP: Microarray
profiling of microRNAs reveals frequent coexpression with
neighboring miRNAs and host genes. RNA. 11:241–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulos GL, Reczko M, Simossis VA,
Sethupathy P and Hatzigeorgiou AG: The database of experimentally
supported targets: a functional update of TarBase. Nucleic Acids
Res. 37:D155–D158. 2009. View Article : Google Scholar :
|
|
8
|
Wang J, Lu M, Qiu C and Cui Q: TransmiR: a
transcription factor-microRNA regulation database. Nucleic Acids
Res. 38:D119–D122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Q, Wang Y, Hao Y, et al: miR2
Disease: a manually curated database for microRNA deregulation in
human disease. Nucleic Acids Res. 37:D98–D104. 2009. View Article : Google Scholar
|
|
10
|
Wirsing A, Senkel S, Klein-Hitpass L and
Ryffel GU: A systematic analysis of the 3′UTR of HNF4A mRNA reveals
an interplay of regulatory elements including miRNA target sites.
PloS One. 6:e274382011. View Article : Google Scholar
|
|
11
|
Baytekin F, Tuna B, Mungan U, Aslan G and
Yorukoglu K: Significance of P-glycoprotein, p53, and survivin
expression in renal cell carcinoma. Urol Oncol. 29:502–507. 2011.
View Article : Google Scholar
|
|
12
|
Petrella BL and Brinckerhoff CE: PTEN
suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null
renal-cell carcinoma. Cancer Biol Ther. 8:1389–1401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada K, Miyake H, Kusuda Y and Fujisawa
M: Expression of epithelial-mesenchymal transition markers in renal
cell carcinoma: impact on prognostic outcomes in patients
undergoing radical nephrectomy. BJU Int. 110:E1131–E1137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai H, Sun L, Cui L, Cao Q, Qin C, Zhang
G, Mao X, Wang M, Zhang Z, Shao P and Yin C: A functional
insertion/deletion polymorphism (-94 ins/del ATTG) in the promoter
region of the NFKB1 gene is related to the risk of renal cell
carcinoma. Urol Int. 91:206–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Postigo AA and Dean DC: Independent
repressor domains in ZEB regulate muscle and T-cell
differentiation. Mol Cell Biol. 19:7961–7971. 1999.PubMed/NCBI
|
|
16
|
Liu H, Brannon AR, Reddy AR, Alexe G,
Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, Ganesan S,
Levine AJ, Rathmell WK and Bhanot GV: Identifying mRNA targets of
microRNA dysregulated in cancer: with application to clear cell
renal cell carcinoma. BMC Syst Biol. 4:512010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kompier LC, Lurkin I, van der Aa MN, van
Rhijn BW, van der Kwast TH and Zwarthoff EC: FGFR3, HRAS, KRAS,
NRAS and PIK3CA mutations in bladder cancer and their potential as
biomarkers for surveillance and therapy. PloS One. 5:e138212010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dey N, Das F, Ghosh-Choudhury N, Mandal
CC, Parekh DJ, Block K, Kasinath BS, Abboud HE and Choudhury GG:
microRNA-21 governs TORC1 activation in renal cancer cell
proliferation and invasion. PloS One. 7:e373662012. View Article : Google Scholar : PubMed/NCBI
|