Introduction
Telomerase is a ribonucleoprotein complex, the
predominant function of which is to add six nucleotide repeats
(TTAGGG) to the end of chromosomes, in a mechanism that is
dependent on telomerase reverse transcriptase (TERT) and intrinsic
RNA template (TERC) activity, as well as additional associated
proteins. This process compensates for the telomere loss that
accompanies cell division and chromosome replication, and thus
prolongs the telomere length-restricted replicative lifespan of
cells (1,2). In contrast to the majority of normal
human somatic cells, which do not express telomerase and eventually
enter into senescence when telomeres shorten to a crucial point,
>80% of human cancers exhibit a high level of telomerase
activity, which maintains telomere length. TERC is ubiquitously
expressed in all normal and cancer cells, whereas TERT, which is
involved in cellular immortalization and carcinogenesis, acts as a
rate-limiting factor for the activation of telomerase (3,4).
Recently, several additional activities exhibited by TERT have been
identified, which indicates that TERT may exhibit
telomere-independent biological functions, including the promotion
of cell proliferation (5,6), extension of cell life (6,7),
delaying cell aging (8,9) and modulation of cell differentiation
(8). A number of these novel
functions do not rely on the reverse transcriptase activity of TERT
(7,10).
TERT protein expression is regulated by a
complicated system, predominantly involving transcriptional and
translational control (11–13). TERT translational control has been
demonstrated to be critical for functional regulation due to its
subcellular location (14). The
dynamic subcellular location of TERT is dependent on the cell
cycle, DNA damage or cellular transformation (15). Notably, a number of studies have
shown that TERT, which is regarded as a nuclear protein, has been
identified not only in the nucleus but also occasionally in the
cytoplasm (14,16,17).
However, at present, the biological significance of the TERT
subcellular location in the process of in vivo lymphatic
metastasis of nasopharyngeal carcinoma (NPC) remains unclear.
Materials and methods
Cell lines and reagents
The human NPC cell lines, 5–8F (high metastasis
capability) and 6–10B (low metastasis capability), were purchased
from China Center for Type Culture Collection (Wuhan, China) and
conserved at Renmin Hospital of Wuhan University (Wuhan, China) and
stored in liquid nitrogen. Fetal bovine serum was obtained from
Thermo Fisher Scientific (Waltham, MA, USA). RPMI 1640 medium and
0.25% trypsin solution were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The TERT antibody (AB5181) was
purchased from Abcam (Cambridge, UK) and the glyceraldehyde
3-phosphate dehydrogenase (GAPDH) antibody (5174) was purchased
from Cell Signaling Technology, Inc., (Beverly, MA, USA). The
Quantum dots (QDS) immunofluorescence detection kit was purchased
from Wuhan Jiayuan Quantum Dots Co., Ltd., (Wuhan, China).
Cell culture
The 5–8F and 6–10B cell lines were cultured in RMPI
1640 medium supplemented with 10% fetal bovine serum, 10 μg/ml
ampicillin and 10 μg/ml kanamycin, and incubated at 37°C in a
humidified atmosphere of 5% CO2.
Human NPC tissue samples
A total of 39 human NPC tissue samples and 13 lymph
nodes were obtained from NPC cancer patients undergoing biopsy at
Renmin Hospital of Wuhan University and the diagnosis of NPC was
confirmed by pathological examination. Paraffin blocks created from
these biopsies were used to construct tissue microarrays. Written
informed consent was obtained from all patients.
Xenograft model
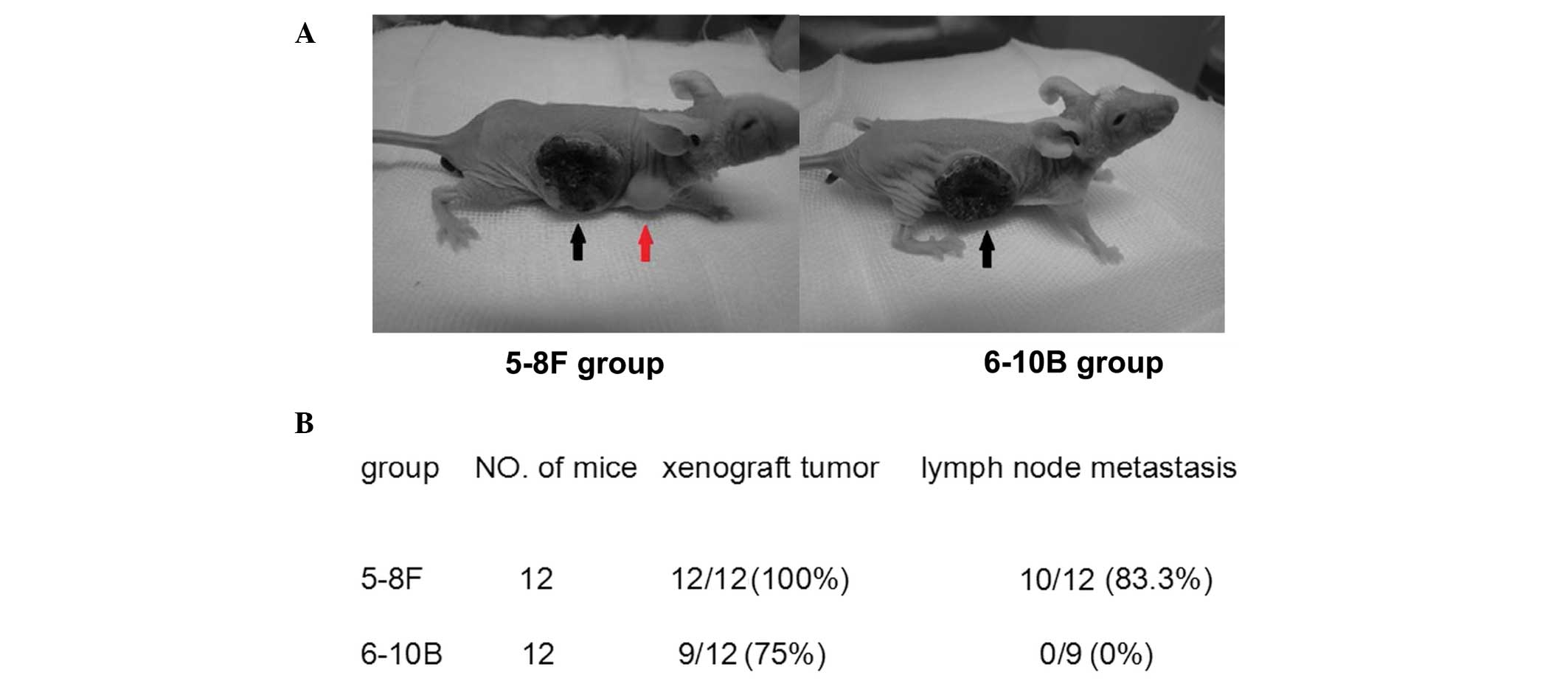
Female BALB/c nude mice (four to six weeks old) were
obtained from Beijing HFK Bioscience Co., Ltd., (Beijing, China)
and quarantined for one week prior to tumor implantation. The
xenograft tumor model was established by subcutaneously injecting
5–8F and 6–10B cells (2×106) suspended in 0.2 ml RPMI
1640 medium into the right flank of the mice. Twelve weeks
following implantation, the mice were sacrificed and the primary
tumors and the draining lymph nodes were collected for western blot
analysis. Animal welfare and experimental procedures were followed
strictly. This study was approved by the ethics committee of Renmin
Hospital of Wuhan University.
Western blot analysis
Total cell lysate was performed according to
standard instructions. Cytosol and nuclear extracts were prepared
following the manufacturer’s instructions. The lysates were
resolved using 10% SDS-PAGE, transferred to nitrocellulose
membranes and immunoblotted with primary antibodies against TERT
and GAPDH. Following incubation with secondary antibodies, the
protein bands were detected using an enhanced chemiluminescence
reagent (Thermo Fisher Scientific, Rockford, IL, USA).
QDs based immunofluorescence
TERT immunofluorescence staining using a 545-QD-SA
probe (Wuhan Jiayuan Quantum Dot Technological Development Co.,
Ltd., Wuhan, China) was performed on the NPC tissue and metastatic
lymph nodes. The slide was deparaffinized, antigen retrieval was
performed, blocked with 3% bovine serum albumin and incubated with
primary mouse anti-human TERT monoclonal antibody. The slide was
then washed and incubated with biotinylated goat anti-mouse IgG,
washed, blocked and incubated with 545-QD-SA, mounted and observed
by fluorescence microscopy. Images were captured and analyzed by
Nuance 2.10 software (CRi, Woburn, MA, USA) (18).
Telomerase repeat amplification protocol
(TRAP) assay of telomerase activity
TRAP assays were performed using the Telo TAGGG
Telomerase polymerase chain reaction ELISA kit (Roche, Mannheim,
Germany) according to the manufacturer’s instructions. The relative
telomerase activity was calculated using the following formula:
Relative telomerase activity (%) = sample A450 nm-A690 nm
unit/positive control A450 nm-A690 nm unit. The mean value was
calculated from three independent experiments.
Statistical analysis
All data are expressed as the mean ± standard
deviation. One-way analysis of variance was performed using SPSS
version 13.0 (SPSS Inc., Chicago, IL, USA) and P<0.05 was
considered to indicate a statistically significant difference.
Results
Nuclear translocation of TERT is
associated with the lymphatic metastasis of NPC
To investigate the subcellular localization of the
TERT protein and determine whether lymphatic metastasis of NPC is
accompanied by changes in TERT localization, the TERT protein
expression was analyzed by QDS-based immunofluorescence and western
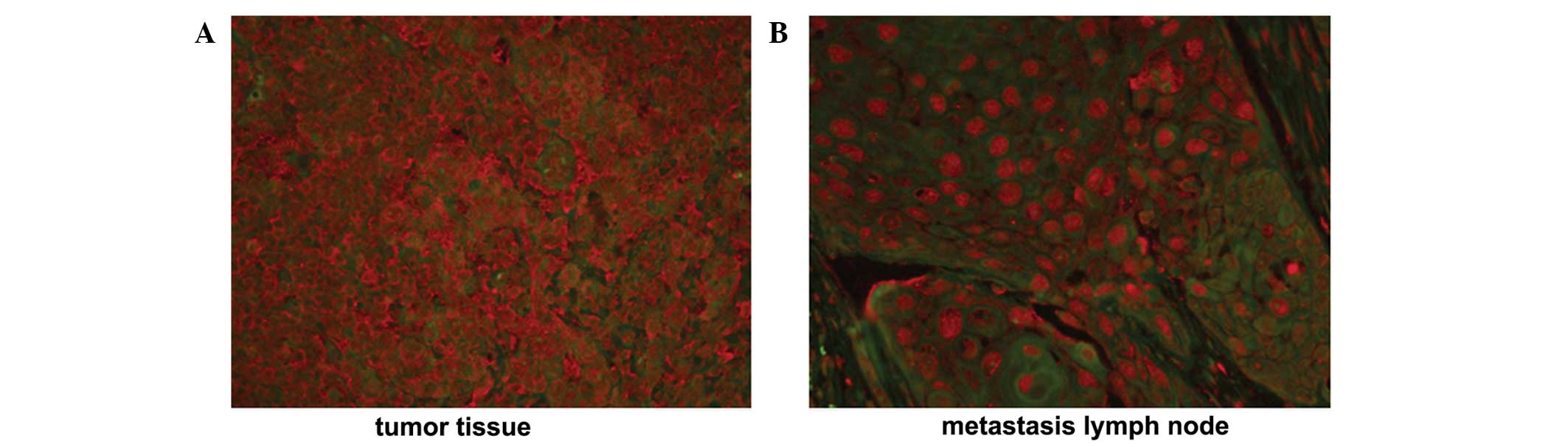
blot analysis, respectively. A positive TERT staining signal was
detected in 34/39 NPC tissue samples and 13/13 metastatic lymph
nodes, identified in the cytoplasm and nucleus. In NPC tissue
samples, TERT protein was exclusively localized to the cytoplasm,
with a weak positive signal identified in the nucleus. (Fig. 1A) By contrast, the TERT protein was
translocated to the nucleus from the cytoplasm when NPC cells
metastasized to lymph nodes (Fig.
1B).
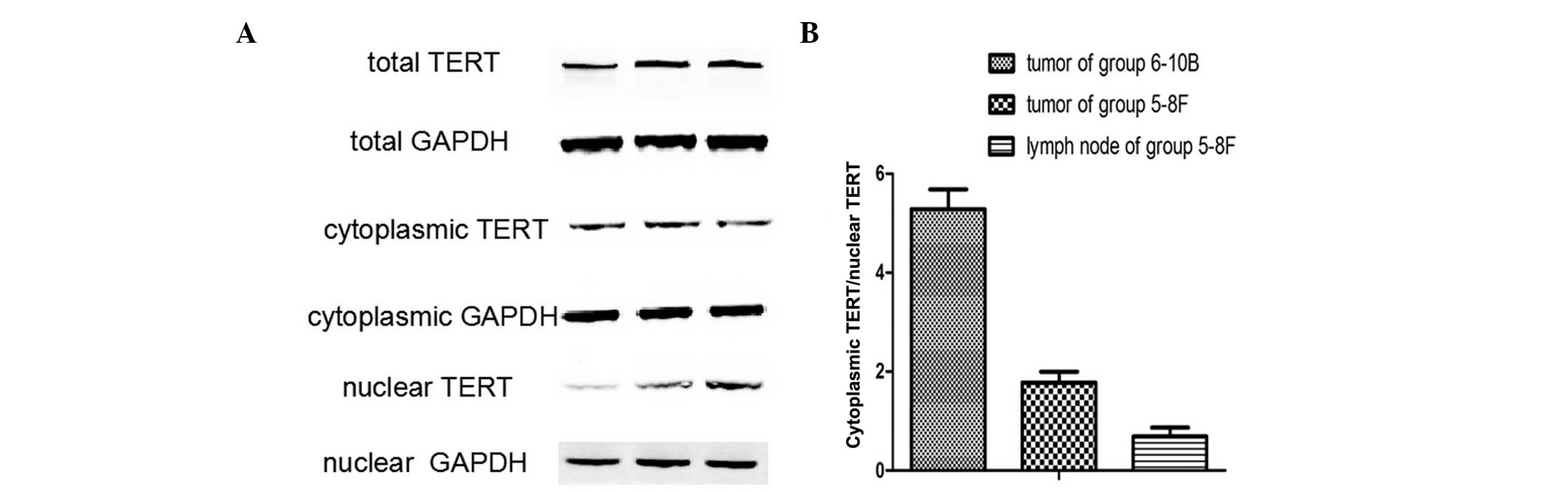
To further confirm the subcellular localization of
TERT in NPC metastasis, TERT protein expression was analyzed in
non-metastatic xenograft tumor tissues, metastatic tumor tissues
and metastatic lymph nodes by western blot analysis. The TERT
protein was detected in all samples (Fig. 2), and the ratio of cytoplasmic
TERT/nuclear TERT differed between the three groups. (Fig. 3A) A significant difference was
identified between the ratios of cytoplasmic TERT/nuclear TERT in
non-metastatic xenograft tumor tissues, metastatic tumor tissues
and metastatic lymph nodes (Fig.
3B). Thus, nuclear translocation of TERT was closely associated
with the lymphatic metastasis of NPC.
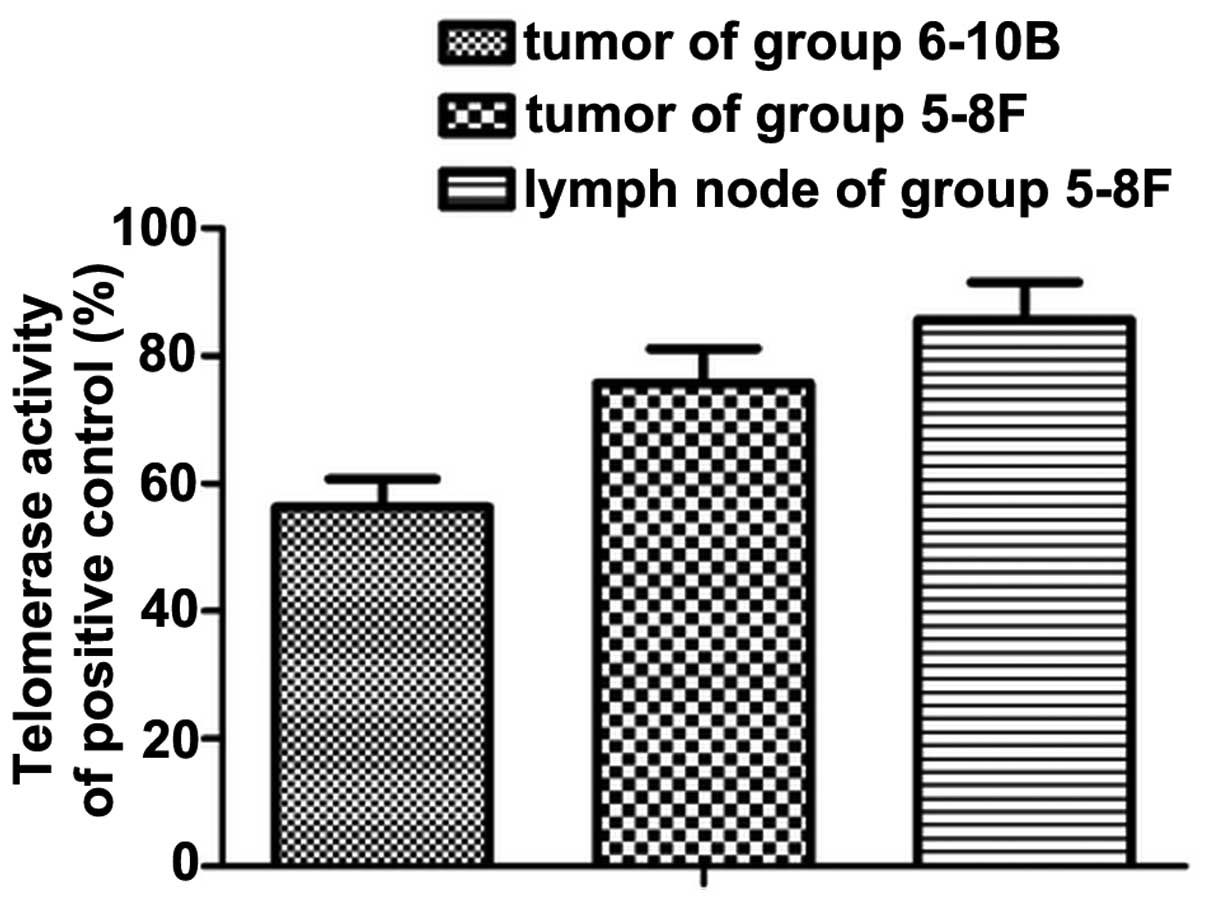
Increased telomerase activity in the
translocation of TERT
Telomerase activity in all three groups was detected
using TRAP-ELISA. Consistent with the levels of nuclear TERT
protein identified, telomerase activity was low in the
non-metastatic primary tumor. In addition to the translocation of
the TERT protein, high telomerase activity was maintained and
reached a level, which was significantly different to that detected
in metastatic lymph nodes (P<0.05; Fig. 4).
Discussion
In this study, the TERT protein expression level,
subcellular localization and telomerase activity in the process of
NPC lymphatic metastasis were investigated. It was demonstrated
that TERT protein expression level was increased in NPC tumor
tissue and metastatic lymph nodes. Furthermore, the TERT
subcellular location was associated with telomerase activity, and
nuclear translocation of TERT was associated with lymphatic
metastasis of NPC.
As hypothesized, increased TERT protein expression
was observed in NPC tumor tissue and metastatic lymph nodes.
Furthermore, nuclear translocation of TERT may be involved in the
regulation of telomerase activity and the lymphatic metastasis of
NPC.
As telomerase activity is controlled by TERT, and
the association between NPC metastasis and TERT expression levels
and TERT subcellular localization remains unclear (14), the cellular localization of TERT in
the process of NPC metastasis was investigated. In the present
study, TERT protein expression levels and telomerase activity were
increased significantly in NPC tissues and metastatic lymph nodes
and lymphatic metastasis was observed to be closely associated with
the nuclear translocation of TERT, which was detected by QDS-based
immunofluorescence and western blot analysis.
In the xenograft tumor model of NPC, TERT protein
expression levels and telomerase activity were analyzed in all
xenograft tumor tissue and metastasis lymph nodes, TERT was
predominantly distributed in the cytoplasm in xenograft tumor
tissues of the non-metastatic group, which is important for
protecting cells from apoptosis stimuli (19–21).
TERT was predominantly distributed in the nucleus in metastatic
lymph nodes. These results indicated that nuclear translocation of
TERT increases the telomerase activity and lymphatic metastasis of
NPC cells. In metastatic lymph nodes, nuclear translocation of TERT
may be recharacterized to promote invasion and metastasis of NPC
cells. It has been proposed that TERT may be involved in altering
gene expression of proteins associated with invasion and metastasis
of NPC, including TGF-β and β-catenin (22).
In normal cells, only phosphorylated TERT regulates
telomerase activity following nuclear translocation, however, tumor
cells that constitutively exhibit high levels of telomerase
activity express the TERT protein in the phosphorylated form, which
is located in the nucleus (16).
Recent studies have shown that following phosphorylation by Akt and
protein kinase C, TERT was exported to the nucleus, playing its
role in maintaining telomerase activity (23). No methods were available to directly
detect phosphorylated nuclear TERT in tissue samples, therefore,
additional studies are required to confirm the expression of
nuclear TERT identified in the study. However, the potential
involvement of phosphorylation during the process of lymphatic
metastasis of NPC must not be ignored.
In conclusion, TERT protein expression and
telomerase activity are increased in NPC tissues. In comparison
with the TERT protein expression levels, the nuclear translocation
of TERT may be more important in the regulation of telomerase
activity and lymphatic metastasis of NPC. Therefore, TERT nuclear
translocation alone or in combination with telomerase activity or
TERT expression level may present an appropriate biomarker for
predicting the lymphatic metastasis of NPC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30901662 and
30872851), the Science and Technology Program of Hubei Province of
China (grant no. 2007AA302B08), the Science and Technology Program
of Wuhan City (grant nos. 200951199455 and 200950431168) and the
Self-Research Program for Doctoral Candidates of Wuhan University
(grant no. 2042011KF0138).
References
|
1
|
Harley CB: Telomerase and cancer
therapeutics. Nat Rev Cancer. 8:167–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JP, Chen SM, Cong YS, Nicholls C, Zhou
SF, Tao ZZ and Li H: Regulation of telomerase activity by
apparently opposing elements. Ageing Res Rev. 9:245–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodnar AG, Ouellette M, Frolkis M, et al:
Extension of life-span by introduction of telomerase into normal
human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang C, Przyborski S, Cooke MJ, et al: A
key role for telomerase reverse transcriptase unit in modulating
human embryonic stem cell proliferation, cell cycle dynamics, and
in vitro differentiation. Stem Cells. 26:850–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blagoev KB: Cell proliferation in the
presence of telomerase. PLoS One. 4:e46222009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang B, Myatt L and Cui XL: Loss of
proliferative capacity in a retroviral immortalized human uterine
smooth muscle cell line derived from leiomyoma is restored by hTERT
overexpression. Reprod Sci. 16:1062–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bollmann FM: The many faces of telomerase:
emerging extratelomeric effects. Bioessays. 30:728–732. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Yang R, Zhang W, Dorfman H, Rao P
and Gorlick R: Genetically transforming human mesenchymal stem
cells to sarcomas: changes in cellular phenotype and multilineage
differentiation potential. Cancer. 115:4795–4806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Techangamsuwan S, Haas L, Rohn K,
Baumgärtner W and Wewetzer K: Distinct cell tropism of canine
distemper virus strains to adult olfactory ensheathing cells and
Schwann cells in vitro. Virus Res. 144:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pellicciotta I, Cortez-Gonzalez X, Sasik
R, Reiter Y, Hardiman G, Langlade-Demoyen P and Zanetti M:
Presentation of telomerase reverse transcriptase, a self-tumor
antigen, is down-regulated by histone deacetylase inhibition.
Cancer Res. 68:8085–8093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S and Zhu J: The hTERT gene is
embedded in a nuclease-resistant chromatin domain. J Biol Chem.
279:55401–55410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Guo X, Jiang G, et al: CpG island
methylator phenotype association with upregulated telomerase
activity in hepatocellular carcinoma. Int J Cancer. 123:998–1004.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu K, Hodes RJ and Weng Np: Cutting edge:
telomerase activation in human T lymphocytes does not require
increase in telomerase reverse transcriptase (hTERT) protein but is
associated with hTERT phosphorylation and nuclear translocation. J
Immunol. 166:4826–4830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong JM, Kusdra L and Collins K:
Subnuclear shuttling of human telomerase induced by transformation
and DNA damage. Nat Cell Biol. 4:731–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyo S, Masutomi K, Maida Y, et al:
Significance of immunological detection of human telomerase reverse
transcriptase: re-evaluation of expression and localization of
human telomerase reverse transcriptase. Am J Pathol. 163:859–867.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lepreux S, Doudnikoff E, Aubert I,
Bioulac-Sage P, Bloch B and Martin-Negrier ML: Cytoplasmic
expression of human telomerase catalytic protein (hTERT) in
neutrophils: an immunoelectron microscopy study. Ultrastruct
Pathol. 32:178–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen C, Xia HS, Gong YP, et al: The
quantitative detection of total HER2 load by quantum dots and the
identification of a new subtype of breast cancer with different
5-year prognosis. Biomaterials. 31:8818–8825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haendeler J, Hoffmann J, Rahman S, Zeiher
AM and Dimmeler S: Regulation of telomerase activity and
anti-apoptotic function by protein-protein interaction and
phosphorylation. FEBS Lett. 536:180–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawauchi K, Ihjima K and Yamada O: IL-2
increases human telomerase reverse transcriptase activity
transcriptionally and posttranslationally through
phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and
mammalian target of rapamycin in transformed NK cells. J Immunol.
174:5261–5269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saretzki G: Telomerase, mitochondria and
oxidative stress. Exp Gerontol. 44:485–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maida Y, Yasukawa M, Furuuchi M, et al: An
RNA-dependent RNA polymerase formed by TERT and the RMRP RNA.
Nature. 461:230–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minamino T, Mitsialis SA and Kourembanas
S: Hypoxia extends the life span of vascular smooth muscle cells
through telomerase activation. Mol Cell Biol. 21:3336–3342. 2001.
View Article : Google Scholar : PubMed/NCBI
|