Introduction
In general, the treatment of leukemia consists of
chemotherapy and bone marrow transplantation. Clinically, 60–80% of
acute myeloid leukemia (AML) patients experience complete remission
following routine chemotherapy treatment (1), however, numerous patients relapse. The
proportion of relapsed/refractory (RR) patients increases with age
(2). Gao et al observed that
the expression of P-glycoprotein (P-gp) in RR patients was higher
than that in treatment-sensitive patients (3). Therefore, the overexpression of P-gp
is considered to be the primary cause of multidrug resistance (MDR)
in patients with AML. P-gp functions as an ATP-dependent membrane
protein, and is principally expressed in excretory tissues located
in the placenta, kidneys, liver, intestines, blood brain barrier
and blood testes barrier. P-gp is involved in the absorption,
distribution and excretion of drugs, xenobiotics and endogenous
compounds (4). At present, research
into the reversal of MDR in leukemia is primarily aimed at
identifying reversal agents that target ATP-binding membrane
proteins. Verapamil (VRP) and cyclosporine A (CsA) have been
extensively studied in the clinic and the laboratory as potential
reversal agents. The therapeutic mechanism of VRP for MDR is the
competition with anticarcinogens for the associated binding sites
of P-gp, which increases the retention of anticarcinogens in cells
(5). CsA, and its derivative,
PSC-833, can bind to P-gp in a competitive manner to prevent MDR
(6). However, severe side-effects
are a common feature of these reversal agents, therefore, their
clinical application is restricted. Emerging biotechnologies such
as small interfering RNA and P-gp antibodies, which target the
function and expression of P-gp, have been successful, but are
currently in the early stages of research (7,8).
During the differentiation of leukemia cells, CD34 is expressed in
AML malignant cells, while the expression P-gp is decreased
(9,10). Whether CD34 levels correlate with
the expression or function of P-gp remains unclear (11). In recent years, the majority of
studies have focused on the treatment of tumors. Arsenic, also
known as arsenic trioxide in China, is a traditional Chinese
medicine that has been used for >2,000 years. The active
ingredient of arsenic, As2O3, was used for
the treatment of psoriasis, rheumatism, leukemia, syphilis and
hemorrhoids several centuries ago. The first report of
As2O3 therapy for acute promyelocytic
leukemia (APL), a subtype of AML, was in the early 1970s. A
research group from the Harbin Medical University of China (Harbin,
Heilongjiang, China) identified that intravenous infusions of
Ailing-1, a crude solution of As2O3 and trace
amounts of mercury, could be used as a treatment for APL (12–14).
Subsequently, researchers at the Shanghai Institute of Hematology
(Shanghai, China) demonstrated that As2O3
could induce APL cell apoptosis and differentiation (15–17).
Currently, As2O3 is used extensively as a
treatment for APL and solid tumors in the clinic. A Chinese study
that investigated the mechanism of As2O3 for
the treatment of APL was published in 2010 (18). The results revealed that
As2O3 upregulates the degradation of an
oncogenic protein involved in APL cell proliferation. In addition,
other previous studies have demonstrated that
As2O3 is effective in the treatment of cancer
stem cells, and reverses drug resistance in tumor cells (19,20).
However, the mechanism behind this action of
As2O3 remains unclear. At present, AML is
only treated with As2O3 when
chemotherapeutics are deemed ineffective. Furthermore,
As2O3 is not used as a resistance reversal
agent in clinical treatment. The present study aimed to investigate
the expression and functional changes of P-gp in AML cells, in
order to reveal the role of As2O3 in the
reversal of drug resistance in leukemia cells.
Materials and methods
Cell culture and patient samples
K562/D and K562/S drug-resistant cells were
maintained in RPMI 1640 medium (GIBCO, Los Angeles, CA, USA) with
10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml
streptomycin, in a humidified atmosphere at 37°C with 5%
CO2. The primary mononuclear cells were separated from
nine AML patients and maintained in RPMI 1640 medium in the
conventional manner (21). The
primary mononuclear and K562/D and K562/S cells were divided into
two groups; a medication group receiving 1μM
As2O3 and a control group receiving no
As2O3. In total, 85 AML patients (59 males
and 26 females) from the First Affiliated Hospital of China Medical
University (Shenyang, China), and the Department of Hematology in
the 202 Hospital of the People’s Liberation Army of China
(Shenyang, China), were included in the present study. The mean age
of the patients was 41.8 years (range, 18 to 79 years). The present
study was performed in accordance with the ethical standards of the
1975 Declaration of Helsinki, as revised in 2000, and was approved
by the Institutional Review Boards of the aforementioned hospitals.
Written informed consent was obtained from all patients.
Reagents
Rhodamine 123 (RH123) and CsA were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The mouse anti-human monoclonal
antibodies against UIC2 and cluster of differentiation 34 (CD34)
were purchased from Immunotech (Marseille, France). TRIzol and
Lipofectamine 2000 transfection reagents were purchased from
Invitrogen (Carlsbad, CA, USA). The diethylpyrocarbonate and
Reverse Transcription System were obtained from Promega (Madison,
WI, USA).
Separation of peripheral blood
mononuclear cells
In total, 85 AML patients consisting of 2, 8, 37,
15, 19, 3 and 1 case/s were classified with the M0, M1, M2, M4, M5,
M6, and M7 AML subtypes, respectively according to the
French-American-British classification system (22). The patients were divided into two
groups, consisting of either newly-treated (NT) patients (63 cases)
or RR patients (22 cases). Samples of 3–5 ml of blood or marrow
(immature leukemia cells >70%) were collected from the patients.
The mononuclear cells were prepared using the Ficoll-Hypaque
technique. Certain cells were used for detecting the expression of
P-gp and CD34 by immunocytochemistry, and others were used to
detect the function of P-gp.
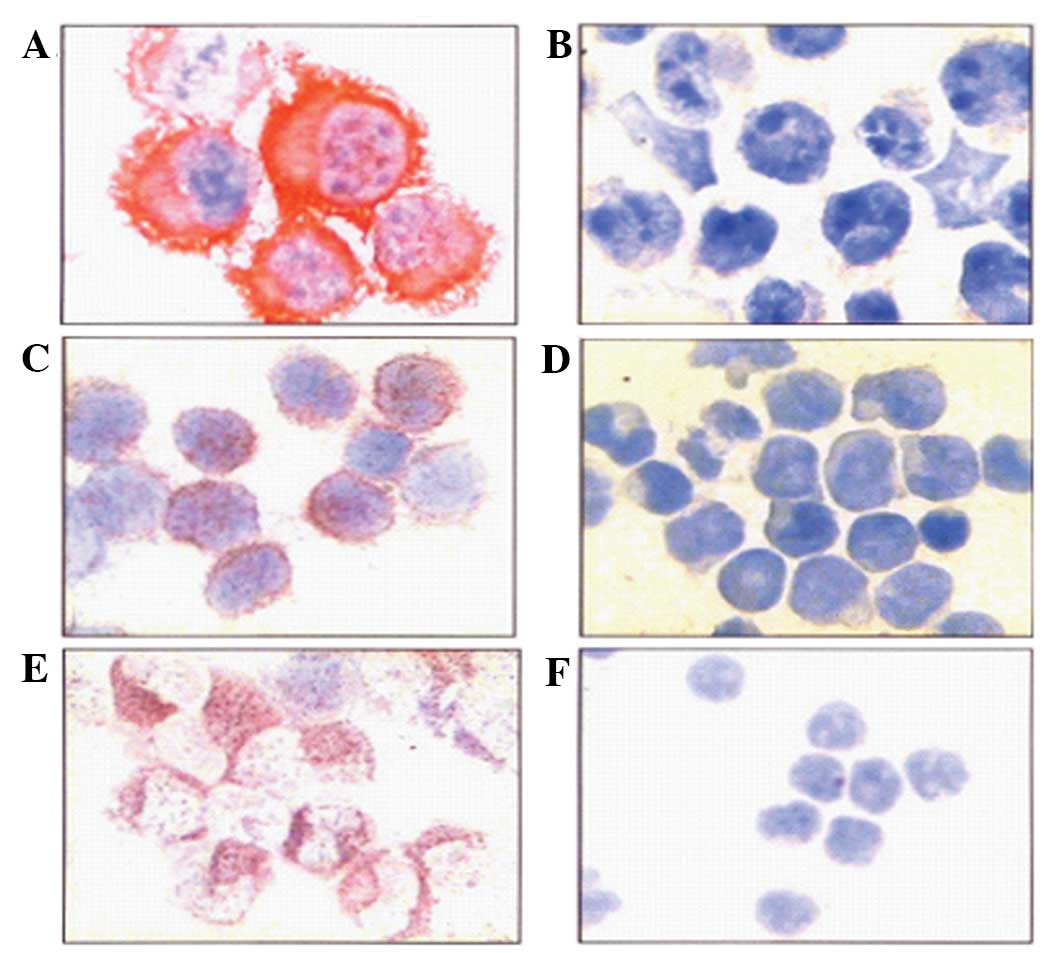
Expression of P-gp and CD34 detected by
immunocytochemistry
The K562/S and K562/D cells were fixed by incubation
in cold acetone for 10 min, and then incubated with 20 μl of mouse
anti-human monoclonal JSB-1 (Maixin Biotechnology, Fuzhou, China)
and mouse anti-human monoclonal CD34 antibodies (Immunotech) (5
μg/ml) at 4°C overnight. The following day, the biotin-conjugated
rabbit anti-mouse IgG secondary antibody (Maixin Biotechnology) and
alkaline phosphatase-conjugated streptavidin complex were added,
and the samples were incubated at 37°C for 30 min. The samples were
then stained with hematoxylin for 2 min, and the slides were
examined using an optical microscope. The cells positive for the
expression of P-gp and CD34 exhibited a red color in the cell
membrane and cytoplasm, and the cells negative for P-gp and CD34
expression were counterstained blue. Positive P-gp and CD34
expression was confirmed by a positive cell number of >20% (in
500 cells).
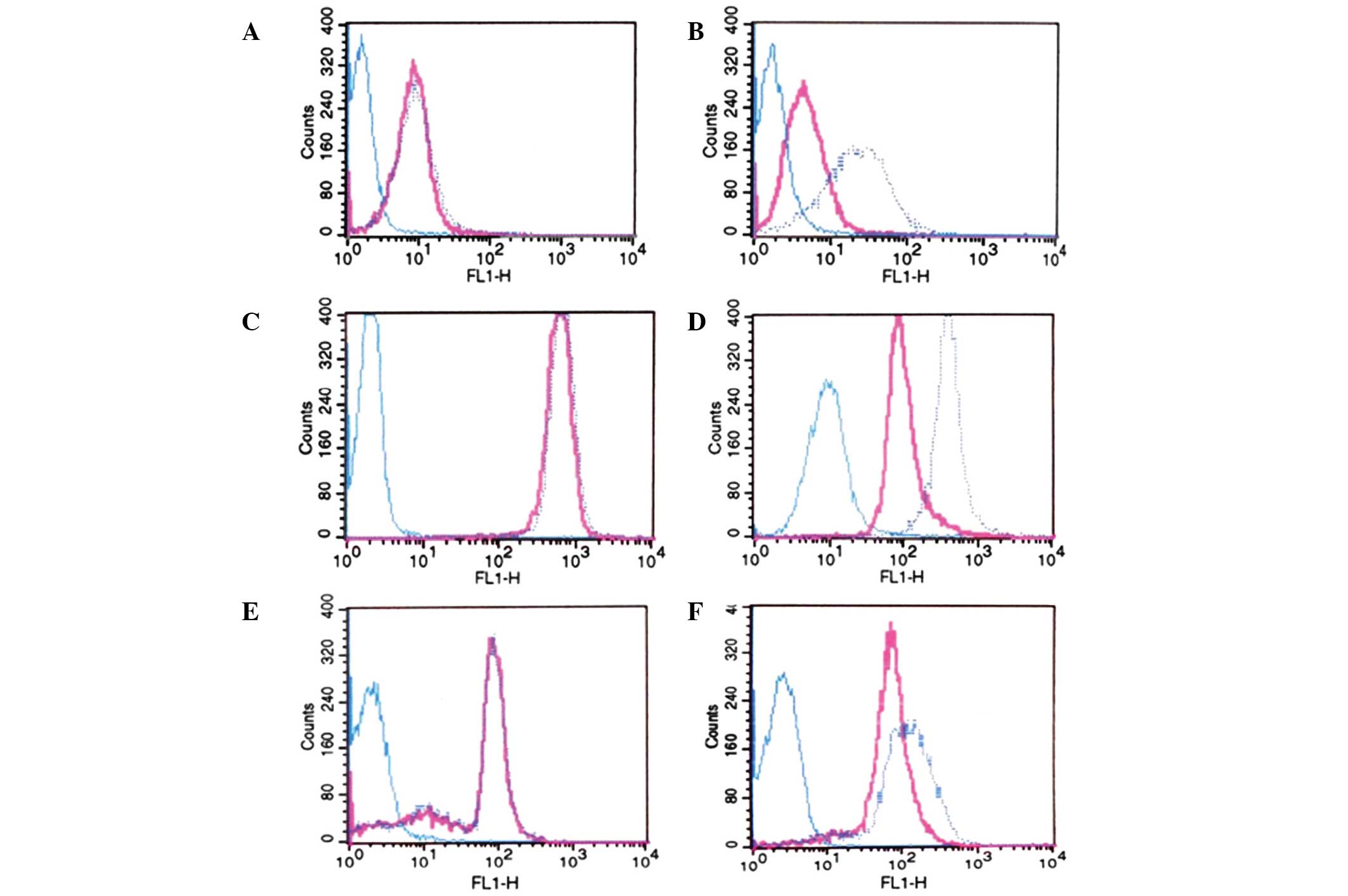
P-gp function detected by flow cytometry
(FCM)
The leukemia cells isolated from the AML patients,
and the K562/D cells, were divided into two groups, receiving
either treatment with 200 ng/ml RHl23 and 5 μg/ml CsA or treatment
with 200 ng/ml RHl23 only, as a control. Subsequent to the
incubation of all cells with RH123 at 37°C for 60 min, 10,000 cells
were analyzed by FCM (FACScan; Becton Dickinson, Franklin Lakes,
NJ, USA) at a wavelength of 490 nm. An accumulation of >30%
indicated a positive result.
Expression of P-gp in patients treated
with As2O3
In three cases, the patients were treated with a
dose of 10 mg As2O3 daily. The mononuclear
cells were isolated from the peripheral blood or bone marrow of the
patients following As2O3 treatment for five
days. The total RNA was isolated by TRIzol reagent according to the
manufacturer’s instructions (Invitrogen), and cDNA was reverse
transcribed from the isolated mRNA using an AMV RNA PCR kit (Takara
Bio, Inc., Shiga, Japan), in line with the standard operating
procedure. The MDR1 mRNA primers and probe were as follows:
Forward, 5′-CCCTTCAGTGGCTGGTACAT-3′ and reverse,
5′-ACCGCGATATTGATCTCCAC-3′; and TaqMan probe,
5′-FCCGATCCATGCTCAGACAGGATGTGAP-3′. Each polymerase chain reaction
(PCR) (25 μl) contained 5 μl 5× buffer, 0.5 μl MgCl2
(250 mmol/l), 0.75 μl dNTPs (10 mmol/l), 1 μl TaqMan primers (10
μmol/l, each), 0.6 μl probe (5 μmol/l), Taq polymerase (1.25 units,
ABI; Life Technologies, Paisley, UK), 1 μl cDNA sample and 14.9 μl
ddH2O. The PCR parameters were as follows: 50°C for 2
min and 94°C for 2 min, followed by 40 cycles at 94°C for 15 sec
and 60°C for 40 sec. The standard recombinant plasmid was diluted
into five gradients and constructed as the reference standard, and
the PCR without a patient sample was used as the negative control.
The results were analyzed using the standard software provided with
the ABI 7900HT Fast Real-Time PCR System (Life Technologies). All
measurements were performed at least three times.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Statistical significance was
determined by a one-way analysis of variance and Student’s t-test.
Correlation between CD34 expression, P-gp expression and patient
age was analyzed by Spearman’s rank correlation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CD34 and P-gp, and
functional analysis of P-gp in AML
The expression and function of P-gp in the leukemia
cells from the AML patients was assessed using immunocytochemistry
(Figs. 1 and 2). In total, 23 of 63 (36.5%) NT patients
and 11 of 22 (50%) RR patients were
P-gpexpression+. However, no statistically
significant difference was identified between the two groups.
Furthermore, 18 of 63 (28.6%) NT patients and 13 of 22 (59.1%) RR
patients were P-gpfunction+. The positivity
rate of P-gpfunction was significantly higher in the RR
group than in the NT group (P<0.05). In addition,
P-gpexpression demonstrated a positive correlation with
P-gpfunction (P=0.0001, r=0.579).
In order to analyze the correlation between CD34 and
P-gp expression, the expression of CD34 was investigated using
immunocytochemistry (Fig. 1E and
F). The results demonstrated that 20 of 63 (31.7%) patients in
the NT group, and 13 of 22 (59.1%) in the RR group, were
CD34+. The expression of CD34 in the RR group was
significantly higher than that in the NT group (P<0.05). In
addition, CD34 expression was positively correlated with
P-gpfunction (P=0.0001, r=0.579) and
P-gpexpression (P=0.0001, r=0.483).
To analyze the correlation between CD34 expression,
P-gp expression and function, and patient age, a total of 85 AML
patients were divided into two groups, consisting of patients
either <50 years old or ≥50 years old. The results revealed that
8 of 46 (17.4%), 12 of 46 (26.1%) and 9 of 46 (19.6%) NT patients
in the <50 years group were CD34+,
P-gpexpression+ and
P-gpfunction+, respectively. Furthermore, 11
CD34+ cases (64.7%), 11
P-gpexpression+ cases (64.7%) and nine
P-gpfunction+ cases (52.9%) were found in 17
NT patients from the ≥50 years group. Notably, those patients ≥50
years had a significantly higher CD34+,
P-gpexpression+ and
P-gpfunction+ values compared with those
<50 years (P<0.05).
Effect of MDR1 mRNA in K562/D cells and
AML patients treated with As2O3
Out of a total of 85 AML patients, MDR1 mRNA
was detected in three patients positive for P-gp expression
following As2O3 treatment, and in nine cases
of primary mononuclear leukemia cells following
As2O3 treatment. The quantitative PCR results
demonstrated that the expression of MDR1 mRNA in the three
patients was significantly downregulated following treatment with
As2O3 (P<0.05; Table I). Significant downregulation of the
MDR1 mRNA expression was also observed in eight cases of
primary mononuclear leukemia cells following treatment with
As2O3 (P<0.05). Furthermore, the
MDR1 mRNA expression level decreased 2.3-fold in the K562/D
cells following treatment with As2O3.
 | Table IExpression of MDR1 mRNA before
and after As2O3 treatment. |
Table I
Expression of MDR1 mRNA before
and after As2O3 treatment.
| MDR1 mRNA
expression |
|---|
|
|
|---|
| Samples | Before | After |
|---|
| 1 |
5.7×10−3 |
3.8×10−3 |
| 2 |
2.5×10−4 |
2.4×10−4 |
| 3 |
8.0×10−2 |
1.4×10−2 |
| 4 |
9.0×10−4 |
3.7×10−4 |
| 5 |
1.3×10−3 |
1.2×10−3 |
| 6 |
1.7×10−3 |
7.8×10−3 |
| 7 |
2.2×10−3 |
3.7×10−3 |
Effect of P-gp expression and function in
K562/D cells following treatment with
As2O3
Using FCM, it was observed that the expression of
P-gp decreased following four days of treatment with 1μM
As2O3. The fluorescence intensity of P-gp
decreased by 30.5%, whereas the intensity of RH123 increased by
38.1% (P<0.05). Therefore, it was concluded that
As2O3 could prolong drug release in the
K562/D cells by inhibiting the expression of P-gp and attenuating
drug elimination, which is attributable to the function of
P-gp.
Discussion
The present study identified that P-gp, involved in
drug resistance, was expressed in AML cells, and that
As2O3 could reverse the drug resistance in
P-gp-expressing leukemia cells. A previous study demonstrated that
the expression of P-gp in RR AML patients was higher than that in
NT patients (23). However, using
FCM, the present study identified extremely few cases of positive
P-gp expression (data not shown). P-gpfunction, however,
was found to be higher in RR patients than in the NT patients. The
expression of P-gp, detected by immunocytochemistry, demonstrated
no significant difference between the two patient groups, however,
P-gpexpression was revealed to be positively correlated
with P-gpfunction. Therefore, it was hypothesized that
immunocytochemistry and FCM may not have been sensitive enough to
detect the expression of P-gp in the present study.
Previous studies identified that leukemia cells
exist as differentially-phased subgroups of cells (24–26).
Certain leukemia cells can reproduce and have long-term
proliferation abilities. Others possess a finite capacity to
replicate and eventually differentiate into immature leukemia
cells, which account for the majority of malignant cells; these
express the CD34 in the AML subgroup cells (1). The positivity rate of P-gp expression,
and the function of P-gp, demonstrated a downward trend during the
CD34+/CD33−,
CD34+/CD33+ and
CD34−/CD33+ cell maturation processes. The
present study identified that the expression of CD34 was positively
correlated with P-gpfunction and
P-gpexpression. Furthermore, CD34 had a higher P-gp
positivity rate in the RR patient group. Therefore, it was
concluded that the reason for the increase in the P-gp positivity
rate observed in the RR patient group was due not to induction by
chemotherapeutics, but to the elimination of leukemia cells that
were sensitive to treatment, which allowed the
CD34+/P-gp+ subgroup of cells to
preferentially proliferate. Therefore, it is important to determine
whether it is feasible to use an inhibitor of P-gp during the
initial treatment with chemotherapy, so as to eliminate the
malignant clone of P-gp+ cells and avoid MDR caused by
long-term chemotherapy. A previous study (27) found that the positivity rates of
P-gp expression and function increased with increasing patient age.
In the present study, it was revealed that the expression of CD34
and P-gp, and the function of P-gp, were significantly different
between the <50-year-old and ≥50-year-old patient groups. In
addition, older patients demonstrated an increased P-gp positivity
rate. Older patients are often treated with lower doses of drugs in
order to reduce side-effects and complications in clinical therapy.
Unfortunately, these lower doses can affect treatment outcomes and
prognosis. Therefore, it will be critical to detect the function of
P-gp as early as possible in order to provide a rational and
efficacious treatment.
Previous studies have demonstrated that
As2O3 could reverse anticancer drug
resistance by inhibiting the expression of P-gp (28,29).
The present study investigated the expression of MDR1 using
quantitative PCR prior to and subsequent to treatment with
As2O3 in three RR AML patients. The
expression of MDR1 was inhibited by
As2O3 in vivo. Due to the small number
of As2O3 treatment cases in clinical therapy,
primary mononuclear leukemia cells positive for P-gp expression
were cultured from nine AML patients and analyzed for changes in
MDR1 expression following treatment with
As2O3. According to quantitative PCR, the
expression of MDR1 was significantly downregulated following
treatment with As2O3.
The drug-resistant cell line used in the present
study, K562/D, was induced by Adriamycin (ADM). This cell line has
typical MDR characteristics and is resistant to a number of
chemotherapeutics, such as ADM, daunorubicin and vincristine (VCR).
Compared with K562 wild-type cells, K562/D cells possess ~160 and
500 times greater drug resistance to ADM and VCR, respectively
(30). Therefore, the K562/D cell
line is an appropriate model to study the role of P-gp expression
in AML drug resistance. In the present study, the expression and
function of P-gp were significantly downregulated in the K562/D
cells following As2O3 treatment, as detected
by FCM. The K562/D cell line has commonly been used to examine the
function of the P-gp ion pump (31). The overexpression of P-gp can result
in a lower drug concentration in the cells, and as observed in the
present study, the RH123 fluorochrome is pumped out the cells as
the substrate. The experimental results demonstrated that
As2O3 could inhibit the expression of P-gp in
primary mononuclear and drug-resistant cells. The results of the
present study demonstrate that As2O3 has the
potential to reverse drug resistance, and therefore should not only
be used when chemotherapeutics prove ineffective, but also as a
resistance reversal agent used in coordination with other
chemotherapeutics. These results provide support for the
therapeutic development and application of
As2O3. The mechanism of
As2O3-mediated P-gp inhibition warrants
further investigation.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (no. 30928008) and the Science and
Technology Projects of Liaoning Province (no. 2008408002-2).
References
|
1
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: an update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165.
2003.PubMed/NCBI
|
|
2
|
Leith CP, Chen IM, Kopecky KJ, Appelbaum
FR, Head DR, Godwin JE, Weick JK and Willman CL: Correlation of
multidrug resistance (MDR1) protein expression with functional
dye/drug efflux in acute myeloid leukemia by multiparameter flow
cytometry: identification of discordant
MDR−/efflux+ and
MDR1+/efflux− cases. Blood. 86:2329–2342.
1995.PubMed/NCBI
|
|
3
|
Gao FZM, Liu Y and Sun K: Analyses of
expression and function of P-glycoprotein in relapsed/refractory
acute myelogenous leukemia patients. Journal of Leukemia &
Lymphoma. 15:419–421. 2006.(In Chinese).
|
|
4
|
Huls M, Russel FG and Masereeuw R: The
role of ATP binding cassette transporters in tissue defense and
organ regeneration. J Pharmacol Exp Ther. 328:3–9. 2009. View Article : Google Scholar
|
|
5
|
Mahadevan D and List AF: Targeting the
multidrug resistance-1 transporter in AML: molecular regulation and
therapeutic strategies. Blood. 104:1940–1951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopes EC, Scolnik M, Alvarez E and Hajos
SE: Modulator activity of PSC 833 and cyclosporin-A in vincristine
and doxorubicin-selected multidrug resistant murine leukemic cells.
Leuk Res. 25:85–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malmo J, Sandvig A, Vårum KM and Strand
SP: Nanoparticle mediated P-glycoprotein silencing for improved
drug delivery across the blood-brain barrier: a siRNA-chitosan
approach. PLoS One. 8:e541822013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aliabadi HM, Mahdipoor P and Uludağ H:
Polymeric delivery of siRNA for dual silencing of Mcl-1 and
P-glycoprotein and apoptosis induction in drug-resistant breast
cancer cells. Cancer Gene Ther. 20:169–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haase D, Feuring-Buske M, Könemann S,
Fonatsch C, Troff C, Verbeek W, Pekrun A, Hiddemann W and Wörmann
B: Evidence for malignant transformation in acute myeloid leukemia
at the level of early hematopoietic stem cells by cytogenetic
analysis of CD34+ subpopulations. Blood. 86:2906–2912.
1995.PubMed/NCBI
|
|
10
|
Feller N, Schuurhuis GJ, van der Pol MA,
Westra G, Weijers GW, van Stijn A, Huijgens PC and Ossenkoppele GJ:
High percentage of CD34 positive cells in autologous AML peripheral
blood stem cell products reflects inadequate in vivo purging and
low chemotherapeutic toxicity in a subgroup of patients with poor
clinical outcome. Leukemia. 17:68–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samdani A, Vijapurkar U, Grimm MA, Spier
CS, Grogan TM, Glinsmann-Gibson BJ and List AF: Cytogenetics and
P-glycoprotein (PGP) are independent predictors of treatment
outcome in acute myeloid leukemia (AML). Leuk Res. 20:175–180.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu J, Chen Z, Lallemand-Breitenbach V and
de Thé H: How acute promyelocytic leukaemia revived arsenic. Nat
Rev Cancer. 2:705–713. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun HD, Ma L, Hu XC and Zhang TD: Ai-ling
1 treated 32 cases of acute promyelocytic leukemia. Chin J Integrat
Trad Chin West Med. 12:21992.(In Chinese).
|
|
14
|
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ,
Si GY, Jin XL, Tang W, Li XS, Xong SM, et al: In vitro studies on
cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia: As2O3 induces NB4
cell apoptosis with downregulation of Bcl-2 expression and
modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996.PubMed/NCBI
|
|
15
|
Zhang PWS, Hu LH, Shi FD, Qiu FQ, Hong GJ,
Han XY, Yang HF, Sun YZ, Liu YP, Zhao J and Jin ZJ: Treatment of 72
cases of acute promyelocytic leukemia with intravenous arsenic
trioxide. Chin J Hematol. 5:41996.(In Chinese).
|
|
16
|
Chen Z, Wang ZY and Chen SJ: Acute
promyelocytic leukemia: cellular and molecular basis of
differentiation and apoptosis. Pharmacol Ther. 76:141–149. 1997.
View Article : Google Scholar
|
|
17
|
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J,
Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): I. As2O3 exerts
dose-dependent dual effects on APL cells. Blood. 89:3345–3353.
1997.PubMed/NCBI
|
|
18
|
Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY,
Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, et
al: Arsenic trioxide controls the fate of the PML-RARalpha
oncoprotein by directly binding PML. Science. 328:240–243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB,
Liu W, Qi Q, Lu N, Tao L, Wang XT, et al: Isolation and
characterization of cancer stem like cells in human glioblastoma
cell lines. Cancer Lett. 279:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao YFJ, Liang J, Chen B, Yao R, Shang Q
and Wang S: MDR reversing and apoptosis inducing effects on
treatment with arsenic trioxide in gastric cancer cell cine
SGC7901/ADM. Clin Oncol Cancer Res. 32:32005.(In Chinese).
|
|
21
|
Fuss IJ, Kanof ME, Smith PD and Zola H:
Isolation of whole mononuclear cells from peripheral blood and cord
blood. Curr Protoc Immunol. Chapter 7:2000.
|
|
22
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abd El-Ghaffar HA, Aladle DA, Farahat SE
and Abd El-Hady N: P-glycoprotein (P-170) expression in acute
leukemias. Hematology. 11:35–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Li J, Du W, Zhang J, Liu W, Chen X,
Li H, Huang S and Li X: Relevance of immunophenotypes to prognostic
subgroups of age, WBC, platelet count, and cytogenetics in de novo
acute myeloid leukemia. APMIS. 119:76–84. 2011. View Article : Google Scholar
|
|
25
|
Rao J, Xu DR, Zheng FM, Long ZJ, Huang SS,
Wu X, Zhou WH, Huang RW and Liu Q: Curcumin reduces expression of
Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute
myeloid leukemia cell lines and primary sorted CD34+ acute myeloid
leukemia cells. J Transl Med. 9:712011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oelschlaegel U, Mohr B, Schaich M, Schäkel
U, Kroschinsky F, Illmer T, Ehninger G and Thiede C: HLA-DRneg
patients without acute promyelocytic leukemia show distinct
immunophenotypic, genetic, molecular, and cytomorphologic
characteristics compared to acute promyelocytic leukemia. Cytometry
B Clin Cytom. 76:321–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao FLY, Hou K and Sun K: The relationship
between age of AML patients and expression of P-gp. Chin J Prac Int
Med. 27:22007.(In Chinese).
|
|
28
|
Xue YW, Han JG, Li BX and Yang BF:
Reversal effect and mechanism of arsenic trioxide on multidrug
resistance of gastric carcinoma cells SGC7901. Yao Xue Xue Bao.
42:949–953. 2007.(In Chinese). PubMed/NCBI
|
|
29
|
Wang T, Ma LM, Zhang HP, Wang HW, Yang LH
and Qiao ZH: The effect of arsenic trioxide (As2O3) combined with
BSO on K562/ADM cell and its mechanisms. Zhonghua Xue Ye Xue Za
Zhi. 28:438–443. 2007.(In Chinese). PubMed/NCBI
|
|
30
|
Yang C and Lui S: The clinical
significance of overexpression of drug-resistance genes and
reversal of multidrug resistance in cancer. Bulletin of Chinese
Cancer. 10:132–136. 2001.(In Chinese).
|
|
31
|
Praet M, Stryckmans P and Ruysschaert JM:
Cellular uptake, cytotoxicity, and transport kinetics of
anthracyclines in human sensitive and multidrug-resistant K562
cells. Biochem Pharmacol. 51:1341–1348. 1996. View Article : Google Scholar : PubMed/NCBI
|