Introduction
Pancreatic cancer is a solid malignancy with one of
the highest current mortality rates. In spite of decades of effort,
the five-year survival rate remains at only ~5%. No early detection
tests have been developed, and the majority of patients with
localized disease exhibit no identifiable signs or symptoms.
Therefore, patients are often not diagnosed until the late stages
of disease, when the cancer has metastasized to other organs
(1). Fewer than 20% of patients are
eligible for potentially curative resection. However the majority
of these patients exhibit disease recurrence (2). Thus, effective adjuvant therapies are
urgently required.
Numerous studies support the hypothesis that
cantharidin and cantharidin derivatives exert marked in
vitro and in vivo antitumor activity against various
types of cancer cell (3–5). In previous studies, cantharidin was
found to repress cancer cell growth through cell cycle arrest and
the induction of apoptosis (6–9).
Norcantharidin is a derivate of cantharidin, which is more widely
used in clinical trials with less kidney toxicity. Cantharidin and
norcantharidin act as potent and selective inhibitors of protein
phosphatase 2A (PP2A), a multimeric serine/threonine phosphatase.
Inhibition of PP2A is considered to promote cancer development
through the induction of phosphorylation and activation of several
substrate kinases, including IκB kinase, c-Jun N-terminal kinase
(JNK), extracellular signal-related kinase, p38, Akt and protein
kinase C (PKC), the majority of which accelerate growth (10,11).
However, recent studies have shown that several kinase-dependent
growth inhibition pathways are induced by treatment with PP2A
inhibitors (12,13). We previously showed that cantharidin
exerts an anticancer effect through overactivation of the JNK
signaling pathway, while excessively activated PKC impaired the
anticancer effect of cantharidin (8). The combination of PP2A inhibitors and
PKC inhibitor was demonstrated to produce a synergistic effect
against pancreatic cancer cells (8). However, the PKC inhibitor used in our
previous study, GF109203X, has not been commonly used in clinic
trials. Thus, a PKC inhibitor with demonstrated clinical safety may
be more suitable for use in combination treatment with PP2A
inhibitors in future clinical trials.
Tamoxifen is a synthetic nonsteroidal antiestrogen
agent widely used for the endocrinotherapy of breast cancer.
Notably, tamoxifen also inhibits the growth of estrogen receptor
(ER)-negative cell lines (14,15).
Previous studies have demonstrated that inhibition of PKC may be
the underlying mechanism by which tamoxifen exerts
antiproliferative effects against ER-negative cell lines (16–19).
Thus, in the present study, tamoxifen-mediated inhibition of the
PKC signaling pathway and cell proliferation in pancreatic cancer
cells was investigated, together with the synergistic anticancer
effect using the combination of tamoxifen plus cantharidin or
norcantharidin.
Materials and methods
Cell lines and culture
MCF-7 and MDA-MB-231 breast cancer cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal calf serum (FCS; HyClone Laboratories,
Inc., Logan, UT, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2. PANC-1, BxPC-3, CFPAC-1, Capan-1, PL-45 and SW-1990
human pancreatic cancer cell lines were purchased from the American
Type Culture Collection and maintained in Dulbecco’s modified
Eagle’s medium (Gibco-BRL) supplemented with 10% FCS (HyClone
Laboratories, Inc., Logan, UT, USA), 100 U/ml penicillin and 100
mg/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2. The cells were passaged every 2–3 days to maintain
exponential growth.
Reagents
Cantharidin, tamoxifen and GF109203X were purchased
from Enzo Life Science International, Inc. (Plymouth Meeting, PA,
USA). Norcantharidin was purchased from Sigma-Aldrich (St. Louis,
MO, USA).
3-[4,5-dimethyltiazol-2-yl]
2,5-diphenyl-tetrazolium bromide (MTT) assay
Cellular viability and growth was evaluated by MTT
assay (20). The cells were seeded
into 24-well tissue culture plates at 5×104 cells/well.
Subsequent to treatment, MTT (Sigma-Aldrich) at a final
concentration of 0.5 mg/ml was added to each well and the cells
were incubated at 37°C for 4 h. The medium was then removed and 800
μl dimethyl sulfoxide was added to each well. The absorbance of the
mixture was measured at 490 nm using a microplate ELISA reader
(Model 680; Bio-Rad, Hercules, CA, USA). The relative cell
viability was calculated as follows: Relative cell viability =
(mean experimental absorbance/mean control absorbance) × 100%. The
growth inhibition rate was calculated as follows: Inhibition rate =
[(mean control absorbance − mean experimental absorbance)/mean
control absorbance] × 100%.
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was performed to estimate the expression
levels of ER mRNA. In brief, total RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. Following spectrophotometric
quantification, 1 μg total RNA in a final volume of 20 μl was used
for reverse transcription using Avian Myeloblastosis Virus reverse
transcriptase (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. The RT-PCR reaction products were
electrophoresed on 1.5% agarose gels, visualized using ethidium
bromide staining and quantified using Quantity One software
(Bio-Rad). β-actin served as the internal positive control and as
the reference gene for PCR cycle number normalization. This ensured
linear amplification of the templates in each experiment. The
primers used in PCR were as follows: Forward,
5′-AGGGTAAATGGTAGTTGAAAGGA-3′ and reverse,
5′-ACGCTGGGAAATGAAGAAGA-3′ for ER-1 (product, 280 bp); forward,
5′-TTTAGTGGTCCATCGCCAGTTA-3′ and reverse, 5′-CAGCTCTTGCGCCGGTTT-3′
for ER-2 (product, 339 bp); and forward,
5′-TCATGAAGTGTGACGTGGACAT-3′ and reverse,
5′-CTCAGGAGGAGCAATGATCTTG-3′ for β-actin (product, 158 bp).
Western blot analysis
Monoclonal mouse anti-PKCα and mouse anti-human
β-actin antibodies were purchased from Santa Cruz Biotechnologies
(Santa Cruz, CA, USA), and polyclonal rabbit anti-human
phospho-PKCα (Thr638) antibodies were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Total protein was extracted
using a lysis buffer containing 50 mm Tris-HCl (pH 7.4), 150 mm
NaCl, 1% Triton X-100, 0.1% SDS and 1 mm EDTA, supplemented with
protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and
phosphatase inhibitor cocktail (Roche). The protein extract was
loaded onto an SDS-polyacrylamide gel, size-fractionated by
electrophoresis and then transferred to polyvinylidene fluoride
membranes (Bio-Rad Laboratories). Subsequent to blocking in 5%
non-fat milk for 1 h, the membranes were incubated overnight with
the primary antibodies at 4°C. Protein expression was determined
using horseradish peroxidase-conjugated secondary monoclonal goat
anti-rabbit IgG and goat anti-mouse IgG antibodies (sc-2004 and
sc-2005, respectively; Santa Cruz Biotechnologies) followed by
enhanced chemiluminescence detection (Amersham Pharmacia Biotech,
Amersham, UK). β-actin served as the internal control. Analysis of
grays was performed using Quantity One 4.6.2 software
(Bio-Rad).
Statistical analysis
Each experiment was performed a minimum of three
times. The results are expressed as the mean± standard deviation.
Statistical analysis was performed using an unpaired Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tamoxifen represses growth of pancreatic
cancer cells in a hormone receptor-independent manner
As hormone receptors are the main target of
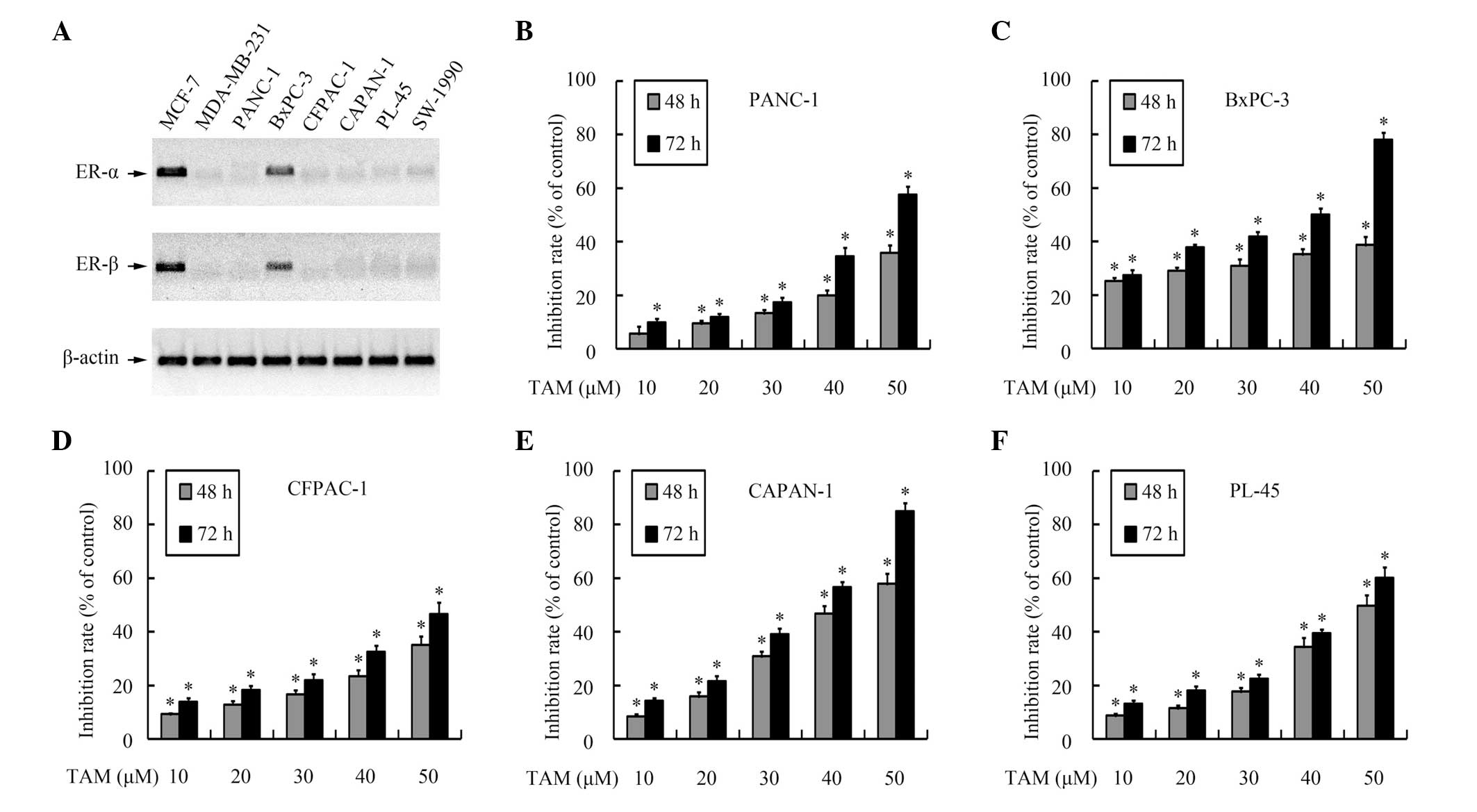
tamoxifen, the expression of ER-α and ER-β in the pancreatic cancer
cell lines was evaluated using RT-PCR. As shown in Fig. 1A, hormone receptor expression was
detected in the BxPC-3 cells, but the other pancreatic cell lines
were observed to be hormone receptor-negative.
The effects of tamoxifen on the growth of pancreatic
cancer cells were then analyzed using MTT assays. As shown in
Fig. 1B–F, tamoxifen inhibited cell
growth in a dose- and time-dependent manner, not only in the
hormone receptor-positive cell line, but also in the hormone
receptor-negative cells lines, which suggests that tamoxifen
repressed growth in pancreatic cancer cells independently of the
hormone receptor status.
Tamoxifen inhibits proliferation of
pancreatic cancer cells through PKC suppression
PKC inhibition has been previously shown to be the
predominant mechanism involved in the ER-independent anticancer
effect of tamoxifen (21). As
tamoxifen-mediated cytotoxicity was demonstrated in ER-positive and
-negative pancreatic cancer cells, whether this hormone
receptor-independent inhibition effect was mediated through
inhibition of PKC was then investigated.
PKC repression by tamoxifen was confirmed using
western blotting. As shown in Fig.
2A, treatment with tamoxifen reduced PKCα phosphorylation. The
time-dependent repression of cell viability induced by tamoxifen
was attenuated by pretreatment with GF109203X, an inhibitor of PKC
(Fig. 2B–F), which suggests that
tamoxifen repressed pancreatic cancer cell viability in a PKC
pathway-dependent manner.
PP2A inhibitors suppress the growth of
pancreatic cancer cells
Cantharidin has been previously reported to repress
the growth of pancreatic cancer cell lines (6). However, whether norcantharidin also
exerts a comparable cytotoxicity effect against pancreatic cancer
cells remains unknown. To investigate the cytotoxic effect of
norcantharidin, MTT assays were performed. As presented in Fig. 3, both cantharidin and norcantharidin
treatment markedly repressed the growth of PANC-1, BxPC-3, CFPAC-1,
CAPAN-1, PL-45 and SW-1990 cells in a dose- and time-dependent
manner.
Tamoxifen represses the PKC
phosphorylation induced by PP2A inhibitors and increases the
cytotoxicity mediated by PP2A inhibitors
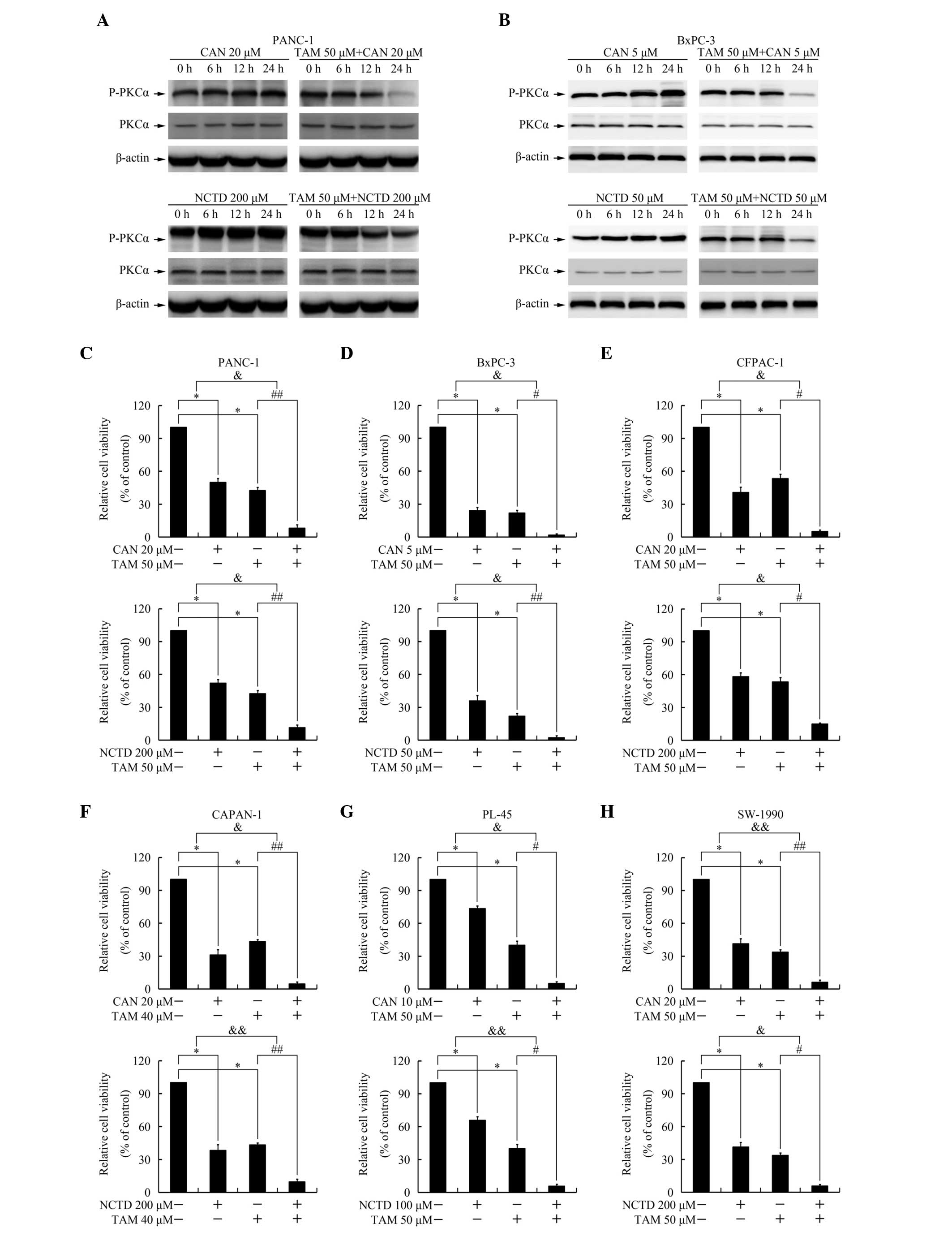
As shown in Fig. 4A and
B, cantharidin and norcantharidin induced persistent and
excessive phosphorylation of PKCα, an effect repressed by
pretreatment with tamoxifen, which suggests that tamoxifen may act
as a PKC inhibitor and inhibit the activation of PKC induced by the
PP2A inhibitors. Administration of a combination of PKC inhibitors
has previously been reported to increase cantharidin-mediated
cytotoxicity (8). To investigate
whether tamoxifen also exerts a similar effect, an MTT assay was
performed. As shown in Fig. 4C–H,
combination treatment with tamoxifen increased the cytotoxicity of
cantharidin and norcantharidin, exerting a synergistic effect.
Discussion
Exocrine pancreatic cancer is significantly more
frequent in young males than in young females. The male-to-female
ratio is 1.25–1.75:1, but is reduced with increasing age (22). ERs and estrogen-binding proteins are
present in the human healthy pancreas, and experimental pancreatic
cancer has been shown to be influenced by estrogens (22). These investigations have raised
interest in sex hormones in the development of pancreatic cancer
and in the application of endocrinotherapy in the treatment of
pancreatic cancer (22).
Tamoxifen is a prototypical drug that targets the
ER. Tamoxifen exerts potent antiestrogenic activity and has been
used extensively for the past 40 years to treat and prevent breast
cancer (23). Although tamoxifen
administered alone has repeatedly been shown to not exert a
significant effect against pancreatic cancer in clinical studies
(22), combination therapy
comprising tamoxifen with other chemotherapeutic agents has been
shown to be effective in phase II trials, regardless of the hormone
receptor status of the pancreatic tumor (24,25).
These studies suggest that tamoxifen may be able to increase the
cytotoxicity of other agents against pancreatic cancer in a hormone
receptor-independent manner.
The inhibition of ER-negative breast cancer cells
and other cell types by tamoxifen has been previously investigated.
Studies have hypothesized that this off-target effect of tamoxifen
involves PKC inhibition (18). The
present study found that tamoxifen treatment repressed the growth
of pancreatic cancer cell lines, independent of the hormone
receptor status. This tamoxifen-mediated cytotoxicity was
attenuated by the PKC inhibitor, GF109203X. Thus, the
growth-inhibitory activity of tamoxifen on pancreatic cancer cells
may partially be due to PKC inhibition. Western blot analyses
revealed that tamoxifen significantly repressed the phosphorylation
of PKCα stimulated by cantharidin and norcantharidin. Furthermore,
co-treatment with tamoxifen increased cantharidin- and
norcantharidin-mediated cytotoxicity, exerting a synergistic
effect. Thus, tamoxifen, as a PKC inhibitor widely used in clinical
practice, may increase the cytotoxic effect of cantharidin and
norcantharidin treatment, regardless of the hormone receptor
status.
Multi-component therapy, termed herbal formulae in
traditional Chinese medicine, in which two or more agents interact
with multiple targets simultaneously, is considered as a rational
and efficient medicinal system designed to treat various illnesses,
including cancer (26,27). As determined by the symptoms and
characteristics of patients, herbal formulae are designed to
contain a combination of different types of plants or minerals in
the order of ‘Jun (monarch)-Chen (assistant)-Zuo (minister)-Shi
(guide)’ (28). In a traditional
formula, the ‘Jun’ (monarch) drug is an required ingredient in a
prescription, and exerts a leading curative role aimed at the cause
or the predominant syndrome of a disease. The ‘Chen’ (minister)
drug strengthens the curative effect of the ‘Jun’ drug or treats
the accompanying symptoms, if applicable. The ‘Zuo’ (assistant)
drug mainly coordinates the formula, increasing the therapeutic
effects of ‘Jun’ and ‘Chen’ and reducing the side-effects. The
‘Shi’ (guide) drug directs the other drugs in the prescription to
the affected area or regulates the properties of the other
components. Over thousands of years, almost 100,000 formulae have
been recorded by practitioners according to experience and heritage
from ancestors, but the mechanisms involved in the majority of
these formulae remain unclear. With the development of molecular
biology techniques, an increasing number of composition principles
of ‘Jun-Chen-Zuo-Shi’ Chinese compound prescriptions have been
analyzed, examining the chemical components, precise mechanisms of
action, clinical application and efficacy validation (29–32).
For example, the Chinese medicinal formula Realgar-Indigo naturalis
has been shown to be effective in treating promyelocytic leukemia,
in which tetraarsenic tetrasulfide is the ‘Jun’ drug, and ndirubin
and tanshinone IIA act as the ‘Chen’ drugs. The combination of
these three drugs yields synergy in the treatment of a murine acute
promyelocytic leukemia (29). These
findings not only demonstrate the molecular mechanism of formula at
the molecular biology level, but also indicate that formulae may
exert therapeutic effects through mechanisms beyond each individual
component.
Ginseng and Astragalus membranaceus are two
Chinese medicinal herbs commonly used in herbal formulae that
contain mylabris. In these formulae, mylabris is considered to be
the ‘Jun’ drug, and Ginseng and Astragalus membranaceus are
known as the ‘Chen’ drugs. Notably, studies have demonstrated that
Ginsenosides, the predominant active constituent of ginseng, and
Astragaloside, a saponin purified from Astragalus
membranaceus, are capable of inhibiting PKC (33,34).
Thus, the regulation of the PKC signaling pathway by these herbs
may be involved in the synergistic mechanism of these formulae.
However, Ginsenosides and Astragaloside are also able to repress
the JNK signaling pathway (35,36),
the activation of which is the main mechanism of cantharidin
cytotoxicity (6,8). Repression of JNK by Ginsenosides and
Astragaloside may impair the anticancer effect of cantharidin,
which suggests that a relatively specific PKC inhibitor may be a
more effective choice in a multi-component therapy.
In our previous study, the addition of a PKC
inhibitor, GF109203X, to treatment regimes was found to increase
the cytotoxicity of cantharidin (8). However, GF109203X has not been applied
in clinical trials. Furthermore, cantharidin cytotoxicity to the
normal hepatic and urinary system tissues restricts the clinical
application of this drug (9). Thus
combination treatment with PKC inhibitors and PP2A inhibitors
requires practical candidate therapeutic agents. As the PKC
inhibitor, tamoxifen, and the demethylated form of cantharidin,
norcantharidin, have been shown to exhibit adequate efficacy,
safety and compliance in the clinical setting, the combination of
these agents may become a promising adjuvant therapy formula in the
treatment of pancreatic cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81472296, 81101867,
81272542, 81200369 and 81372443), the China International Medical
Foundation (grant no. CIMF-F-H001-057), the Scientific Research
Project of Jiangsu Provincial Bureau of Traditional Chinese
Medicine (grant no. L213236), the Medical Scientific Research
Project of Jiangsu Provincial Bureau of Health (grant no. Z201206),
the Special Foundation of Wu Jieping Medical Foundation for
Clinical Scientific Research (grant nos. 320.6753.1225 and
320.6750.12242), Natural Science Foundation for Colleges and
Universities in Jiangsu Province (grant no. 11KJB320013), the
Science and Education for Health Foundation of Suzhou for Youth
(grant nos. SWKQ1003 and SWKQ1011) and the Science and Technology
Project Foundation of Suzhou (grant nos. SYS201112, SYSD2012137 and
SYS201335).
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, et al:
Recent progress in pancreatic cancer. CA Cancer J Clin. 63:318–348.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paulson AS, Tran Cao HS, Tempero MA and
Lowy AM: Therapeutic advances in pancreatic cancer.
Gastroenterology. 144:1316–1326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng F, Wei YQ, Tian L, et al: Induction
of apoptosis by norcantharidin in human colorectal carcinoma cell
lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin
Oncol. 128:223–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen YN, Chen JC, Yin SC, et al: Effector
mechanisms of norcantharidin-induced mitotic arrest and apoptosis
in human hepatoma cells. Int J Cancer. 100:158–165. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huan SK, Lee HH, Liu DZ, Wu CC and Wang
CC: Cantharidin-induced cytotoxicity and cyclooxygenase 2
expression in human bladder carcinoma cell line. Toxicology.
223:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Xie L, Chen Z, et al: Cantharidin, a
potent and selective PP2A inhibitor, induces an oxidative
stress-independent growth inhibition of pancreatic cancer cells
through G2/M cell-cycle arrest and apoptosis. Cancer Sci.
101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Chen Z, Zong Y, et al: PP2A
inhibitors induce apoptosis in pancreatic cancer cell line PANC-1
through persistent phosphorylation of IKKalpha and sustained
activation of the NF-kappaB pathway. Cancer Lett. 304:117–127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Chen Z, Gong FR, et al: Growth of
the pancreatic cancer cell line PANC-1 is inhibited by protein
phosphatase 2A inhibitors through overactivation of the c-Jun
N-terminal kinase pathway. Eur J Cancer. 47:2654–2664. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Li DM, Chen K, et al: Development of
a gene therapy strategy to target hepatocellular carcinoma based
inhibition of protein phosphatase 2A using the alpha-fetoprotein
promoter enhancer and pgk promoter: an in vitro and in vivo study.
BMC Cancer. 12:5472012. View Article : Google Scholar
|
|
10
|
Millward TA, Zolnierowicz S and Hemmings
BA: Regulation of protein kinase cascades by protein phosphatase
2A. Trends Biochem Sci. 24:186–191. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janssens V, Goris J and Van Hoof C: PP2A:
the expected tumor suppressor. Curr Opin Genet Dev. 15:34–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YJ, Kuo CD, Tsai YM, et al:
Norcantharidin induces anoikis through Jun-N-terminal kinase
activation in CT26 colorectal cancer cells. Anticancer Drugs.
19:55–64. 2008. View Article : Google Scholar
|
|
13
|
Schweyer S, Bachem A, Bremmer F, et al:
Expression and function of protein phosphatase PP2A in malignant
testicular germ cell tumours. J Pathol. 213:72–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka T, Masuda H, Naito M and Tamai H:
Pretreatment with 5-fluorouracil enhances cytotoxicity and
retention of DNA-bound platinum in a cisplatin resistant human
ovarian cancer cell line. Anticancer Res. 21:2463–2469.
2001.PubMed/NCBI
|
|
15
|
McClay EF, McClay MT, Monroe L, Jones JA
and Winski PJ: A phase II study of high dose tamoxifen and weekly
cisplatin in patients with metastatic melanoma. Melanoma Res.
11:309–313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sudo K, Monsma FJ Jr and Katzenellenbogen
BS: Antiestrogen-binding sites distinct from the estrogen receptor:
subecellular localization, ligand specificity, and distribution in
tissues of the rat. Endocrinology. 112:425–434. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lam HY: Tamoxifen is a calmodulin
antagonist in the activation of cAMP phosphodiesterase. Biochem
Biophys Res Commun. 118:27–32. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Brian CA, Liskamp RM, Solomon DH and
Weinstein IB: Inhibition of protein kinase C by tamoxifen. Cancer
Res. 45:2462–2465. 1985.PubMed/NCBI
|
|
19
|
Su HD, Mazzei GJ, Vogler WR and Kuo JF:
Effect of tamoxifen, a nonsteroidal antiestrogen, on
phospholipid/calcium-dependent protein kinase and phosphorylation
of its endogenous substrate proteins from the rat brain and ovary.
Biochem Pharmacol. 34:3649–3653. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
21
|
Mandlekar S and Kong AN: Mechanisms of
tamoxifen-induced apoptosis. Apoptosis. 6:469–477. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andrén-Sandberg A, Hoem D and Bäckman PL:
Other risk factors for pancreatic cancer: hormonal aspects. Ann
Oncol. 10(Suppl 4): 131–135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jordan VC and Morrow M: Tamoxifen,
raloxifene, and the prevention of breast cancer. Endocr Rev.
20:253–278. 1999.PubMed/NCBI
|
|
24
|
Tomao S, Romiti A, Massidda B, et al: A
phase II study of gemcitabine and tamoxifen in advanced pancreatic
cancer. Anticancer Res. 22:2361–2364. 2002.PubMed/NCBI
|
|
25
|
Eckel F, Lersch C, Lippl F, Assmann G and
Schulte-Frohlinde E: Phase II trial of cyclophosphamide,
leucovorin, 5-fluorouracil 24-hour infusion and tamoxifen in
pancreatic cancer. J Exp Clin Cancer Res. 19:295–300. 2000.
|
|
26
|
Efferth T, Fu YJ, Zu YG, et al: Molecular
target-guided tumor therapy with natural products derived from
traditional Chinese medicine. Curr Med Chem. 14:2024–2032. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan E, Tan M, Xin J, Sudarsanam S and
Johnson DE: Interactions between traditional Chinese medicines and
Western therapeutics. Curr Opin Drug Discov Devel. 13:50–65.
2010.PubMed/NCBI
|
|
28
|
Jiang JC: English translation of terms of
prescription-monarch, minister, assistant and guide. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 30:11052010.(In Chinese).
|
|
29
|
Wang L, Zhou GB, Liu P, et al: Dissection
of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edmond KM, Kortsalioudaki C, Scott S, et
al: Group B streptococcal disease in infants aged younger than 3
months: systematic review and meta-analysis. Lancet. 379:547–556.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu JJ, Ai CZ, Liu Y, et al: Interactions
between phytochemicals from traditional Chinese medicines and human
cytochrome P450 enzymes. Curr Drug Metab. 13:599–614. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tseng YP, Wu YC, Leu YL, Yeh SF and Chou
CK: Scutellariae radix suppresses hepatitis B virus production in
human hepatoma cells. Front Biosci (Elite Ed). 2:1538–1547. 2010.
View Article : Google Scholar
|
|
33
|
Li HB, Ge YK, Zhang L and Zheng XX:
Astragaloside IV improved barrier dysfunction induced by acute high
glucose in human umbilical vein endothelial cells. Life Sci.
79:1186–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lan TH, Xu ZW, Wang Z, et al: Ginsenoside
Rb1 prevents homocysteine-induced endothelial dysfunction via
PI3K/Akt activation and PKC inhibition. Biochem Pharmacol.
82:148–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zong Y, Ai QL, Zhong LM, et al:
Ginsenoside Rg1 attenuates lipopolysaccharide-induced inflammatory
responses via the phospholipase C-gamma1 signaling pathway in
murine BV-2 microglial cells. Curr Med Chem. 19:770–779. 2012.
View Article : Google Scholar
|
|
36
|
He CL, Yi PF, Fan QJ, et al: Xiang-Qi-Tang
and its active components exhibit anti-inflammatory and
anticoagulant properties by inhibiting MAPK and NF-kappaB signaling
pathways in LPS-treated rat cardiac microvascular endothelial
cells. Immunopharmacol Immunotoxicol. 35:215–224. 2013. View Article : Google Scholar
|