Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality, which accounts for 15% of all
new cases of cancer globally and this type of cancer has been
considered as nonimmunogenic (1).
In Europe, lung cancer accounts for ~3.2 million new cancer cases,
annually. The International Association for the Study of Lung
Cancer TNM staging system identified four major stages of lung
cancer, which correlate with significant differences in five-year
survival rates (2). The five-year
survival rate in patients with stage I cancer (70%) is
significantly higher than that of patients with stage III A cancer
(~25%)(3–5). Lymphoproliferative disorders (LPDs)
are a heterogeneous group of biologically and clinically distinct
neoplasms, including non-Hodgkin and Hodgkin’s lymphoma, which
originate from the lymphoid organs. Previous studies have
investigated the molecular pathogenesis of lymphoid malignancies
(6,7). Interactions between the immune system
and cancer development and progression have been previously
demonstrated. Lung tumors can evoke a cellular antitumor immune
response (8). High expression of
CD8 and CD4 T cells and macrophages in tumor tissue has been
demonstrated (9). A case of primary
lung cancer complicated by lymphoma was described by Ohno et
al (9). Additional cases of
primary cancers associated with LPDs have been reported, including
breast and non-small cell lung cancer, however, gastric cancer with
large B cell lymphoma was found to occur most frequently (10–12).
In this study, the possible interaction between the immune system
and solid tumors was investigated. In addition, two cases of LPD
diagnosed concurrently and successively to NSCLC are presented. The
first case presents a patient with the concurrent diagnosis of
lymphoma and lung cancer, who exhibited an hypersensitivity
reaction to bevacizumab, and the second case presents a patient
characterized by sequential diagnosis. Written informed consent was
obtained from both patients.
Case reports
Case one
The first patient was a 65-year-old female with a
smoking history of 40 pack years and a family history of blood
hypertension. The patient had previously suffered from pneumonia
one year prior to diagnosis and, clinically, the patient complained
of mild dyspnoea and was admitted to the Cardiopulmonary Department
of Sant’Andrea Hospital (Rome, Italy). Blood tests showed anemia
with hemoglobin levels, 9.9 g/dl (normal range, 12.5–15 g/dl);
platelet count, 360,000 μl (normal range, 150,000–450,000 μl;
relative lymphocytosis (70%; normal range, ≤20%); creatinine
levels, 1.3 mg/dl (normal range, 0.6–1 mg/dl); white blood cell
count, 18.0×103 μl (range, 4.3–10.8×103 μl);
and oncomarker carcinoembryonic antigen (CEA) levels, 7.5 ng/ml
(normal range, 2.5–5 ng/ml).
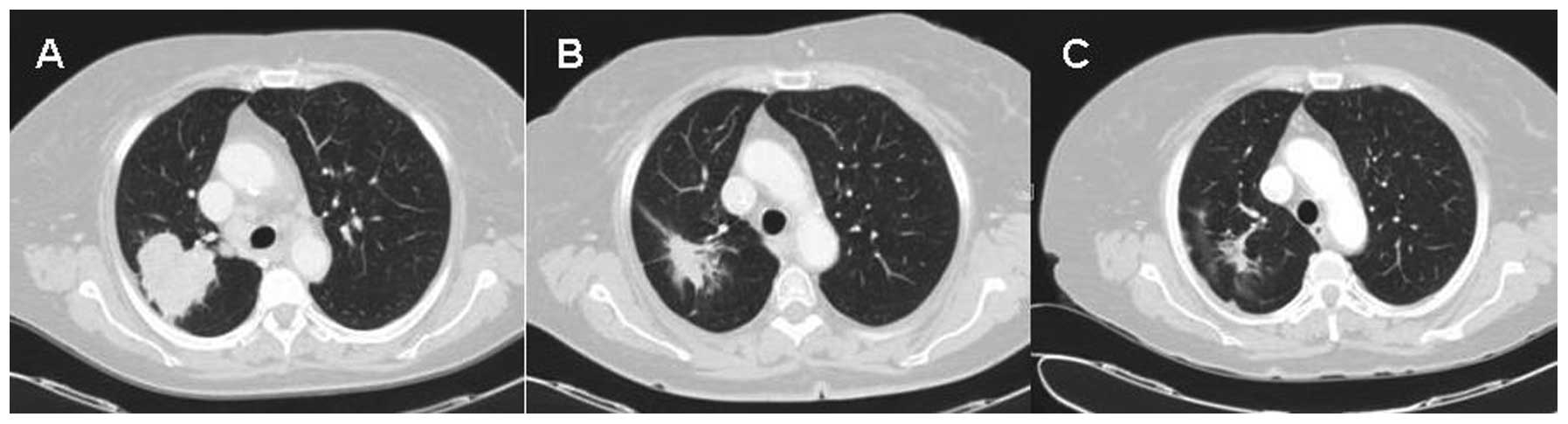
A computed tomography (CT) scan was performed
(Fig. 1A) and a right lung mass was
discovered, located in the upper right lobe and 5 cm in diameter,
with contrast enhancement. The mediastinal lymph nodes were found
to be involved and thrombi were present in the right renal
vein.
The patient subsequently underwent a transthoracic
CT-guided needle biopsy. Immunohistochemistry revealed that the
tissue was positive for thyroid transcription factor 1, CK67 and
p63. Therefore, the patient was diagnosed with an adenosquamous
tumor.
Lymphocyte typing and serum immunofixation tests
performed on the patient’s blood sample showed, respectively, a
high level of B cells 41% 1318 (up to 400) and B lymphocyte
CD19+FMC+ presence, with an associated high
intensity of light λ-chains. Therefore, the diagnosis was
lymphoproliferative syndrome B, LNH type (Table I).
 | Table ICitofluorimetric analysis of lymphoid
population in Case 1. |
Table I
Citofluorimetric analysis of lymphoid
population in Case 1.
| Antigene | Frequency (%) |
|---|
| CD3+ | 54 |
|
CD5+ | 54 |
| CD3+
CD5+ | 54 |
| CD3−
CD5+ | 0 |
|
CD19+ | 34 |
|
CD20+ | 27a |
|
CD19+
CD5+ | 0.7 |
| CD19+
CD10+ | 0 |
| CD19+
CD23+ | 2 |
|
CD19+
FMC7+ | 26 |
|
CD19+
CD38+ | NS |
|
CD19+
CD49d+ | NS |
| CD19+
CD20+ | 27 |
| CD19+
CD103+ | 0 |
|
CD19+
CD11c+ | 5 |
| CD19+
sκ+ | 1 |
|
CD19+
sλ+ | 33 |
The patient was treated for lung cancer, with a
first line therapy including six cycles of gemcitabine (1,000
mg/m2, days 1 and 8) and cisplatin (70 mg/m2,
day 1) for 12 days, showing a good response (Fig. 1B). The patient had an adverse
reaction to bevacizumab during the first cycle, which was
adminstered after gemcitabine and prior to cisplatin, and for this
reason it was not administered further. The symptoms of bevacizumab
treatment were itching, urticaria, erythematous rash and facial
swelling. Second line therapy was initiated with six cycles of
vinorelbine (60 mg/m2, days 1 and 8) as a single agent
for 12 days.
Blood samples after each course revealed a grade 4
neutropenia with a high level of lymphocytes (lymphocytes, 70%;
neutrophils, 12%). The total white blood cell count was 4,800/μl. A
CT scan performed after the second line therapy showed a partial
response with lymph node down-staging (Fig. 1C). Following multidisciplinary
evaluation by radiotherapists and hematologists, the case was
considered amenable for local radiotherapy. Treatment of LPD,
including chemotherapy and steroidal therapy, was delayed until the
termination of this course of radiotherapy, and a follow-up
strategy has been adopted until the time of writing, with a
survival from diagnosis of ~23 months without objective signs of
hematological disease.
Case two
A 74-year-old male was referred to the
Cardiopulmonary Department, Sant’Andrea Hospital, Sapienza
University (Rome, Italy) for dyspnoea, weakness and chest pain. The
patient was subsequently admitted to the Department of Lung
Oncology (Sant’Andrea Hospital, Sapienza University). Analysis of
the patient’s blood sample revealed lymphocytosis. Further
investigation included a bone marrow biopsy, revealing an
infiltration of large B-cell lymphoma CD20/79a+, stage
IV (due to hepatic involvement) according to the European-American
classification of lymphoid neoplasms (13).
The patient underwent chemotherapy with
cyclophosphamide, prednisone, vincristine and doxorubicine. A
positron emission tomography (PET)-CT scan following diagnosis
revealed partial remission. Two months after diagnosis, due to
persisting metabolic uptake shown by PET, a second-line therapy was
started with cisplatin, dexhamethasone and rituximab. Subsequently,
ibritumomab was used for the maintenance therapy and periodic
follow-ups revealed the patient was in remission.
The following year, the patient was diagnosed with
squamous cell lung cancer (p63- and CEA-positive). The patient
underwent a right superior lung lobectomy. The lung cancer stage
was determined as stage Ib, according to the most recent staging
system (14). A subsequent CT scan
was performed after surgery (Fig.
2A), which revealed the results of the right superior lung
lobectomy.
The patient acheived remission for three years,
however, was then referred to the Cardiopulmonary Department,
Sant’Andrea Hospital, Sapienza University for chest pain.
Laboratory tests revealed leukocytosis with a high level of white
blood cells (14,000 μl; normal range, 4,5000–10,000 μl) and an
increased level of C-reactive protein (16 mg/dl; normal range,
0–0.5 mg/dl). Protein electrophoresis analysis revealed that γ
globulin levels were also increased (normal range, 0.7–1.4 g/dl),
and κ (normal range, 3.9–19.4 mg/l) and λ (normal range, 5.7–26.3
mg/ml) light chains were lower.
A chest CT scan revealed an irregular profile mass
(Fig. 2B), which was confirmed as
recurrent lung cancer after fine needle aspiration biopsy. A brain
CT scan also revealed a metastatic lesion.
The patient underwent stereotactic radiotherapy over
one day, 20 Gy of the total dose. Subsequently, six cycles of oral
chemotherapy with 60 mg/m2 vinorelbine was initiated for
12 days. Restaging three months after the beginning of therapy
showed a minimal response (Fig.
2C). The patient remained alive at the follow-up after three
years, with pulmonary stable disease. Follow-up hematological and
radiological CT scans were also planned.
Discussion
It is well demonstrated that the population of
cancer survivors has an increased risk of developing secondary
cancers (8–16%). Leukemias and lymphomas generally arise in the
first five years of treatment, whereas solid cancers such as
breast, lung, gastrointestinal, brain, genitourinary cancers
usually arise after five years (15–18).
LPDs have three major categories: B-cell, NK/T-cell neoplasms and
Hodgkin lymphoma (HL), according to the World Health Organization
classification (19). Recently, a
marked improvement in survival was achieved for patients with LPD,
thus increasing the likelihood of secondary cancer (20,21).
LPDs are the neoplasms most frequently involved in cases of
multiple primary cancers (MPCs), as primary or subsequent (1).
The etiology of second primary cancers may be
attributed to the interaction between the immune system and
carcinogenetic factors; numerous risk factors have been described
and identified as contributing to the development of LPD. These may
act in association to result in a second malignancy, and include
immunodeficiency states (22,23),
viruses (particularly the Epstein-Barr virus), chemotherapeutic
agents, pesticides and organic solvents, and exposure to
ultraviolet radiation (23,24), A family history of LPD (24–28),
but also genetic susceptibility (29), may be found. Shared risk factors,
such as smoking and genetics, may cause certain malignancies to
occur more frequently than expected. The study of multiple primary
malignancies following LPD therefore has etiological and clinical
importance. In a recent retrospective study on MPC by Demirci et
al (30), the ability to
diagnose a second cancer was shown to be earlier and more accurate
than in past reports. In a population of 242 patients, the mean
time for diagnosis of second malignancies was 55.5 months, whereas
a third cancer was diagnosed after 22 months of the second
neoplasm. In the same study, the incidence of solid tumors compared
with LPD as a second cancer was equal, contrary to the results of
previous studies (20,31,32).
The frequencies of primary and secondary diagnoses of LPD were the
same (27). NSCLC was the second
most frequently diagnosed solid tumor after LPD, the first being
breast cancer. Diffuse large B-cell lymphoma as a subsequent cancer
was the most frequent subtype of non-HL (NHL). Markedly high
incidences of lung cancer, melanoma, soft tissue sarcoma, NHL,
acute myeloid leukemia, genitourinary cancers, and head and neck
cancer have been reported in lymphoma survivors (1,30,33).
In the present case, the patient in case two had a synchronous
diagnosis of LDP and lung cancer. Tumors are considered synchronous
when the period of time between the diagnoses of the primary and
subsequent malignancies is less than or equal to six months
(10).
A synchronous diagnosis of thoracic MPC with
lymphoma and myeloma has previously been reported (10). BALT lymphoma has been found in
patients affected by lung adenocarcinoma in six cases and by
squamous carcinoma in one case (10,30,33–37).
One case of lung adenocarcinoma and mantle cell lymphoma of the
pleura, and one case of unspecified lung cancer associated with
tracheal mucosa-associated lymphoid tissue lymphoma have also been
reported. The most common type of primary pulmonary lymphoma is
bronchus-associated lymphoid tissue in the lungs (38). The concurrent presence of two solid
and hematic neoplasms may affect treatment choice, as solid tumors
may cause a greater sensitivity to anticancer drugs and may lead to
a worse prognosis. Numerous chemotherapeutic agents, including
vincristine, carboplatin and cyclosporine are used as a single
treatment, as well as in combination with other drugs. However,
these drugs may induce hypersensitivity reactions.
Immediate hypersensitivity reactions for the
majority of chemotherapeutic agents were described in a review by
Visittsunthorn et al in the pediatric population (39). Symptoms were either moderate or
mild, and early reaction onset is generally a result of
IgE-mediated hypersensitivity. Delayed reactions could be explained
by a release of TNF-α and interleukin 6 during treatment (40).
Vincristine as well as carboplatin,
cychlophosphamide and cyclosporine may be used as monotherapies, as
well as for combined treatments. Bevacizumab has not been yet
presented in these settings, but the unusual manifestation we
observed while using this drug in a ‘double-cancer’ patient could
hide more complex mechanisms than simple immediate
hypersensitivity. Immune interaction between coexisting neoplasms
could lead to an increased susceptibility to develop side effects
to specific chemotherapy agents. Relative lymphocytosis constantly
shown in case 1 may be representative of such a ‘hypersensitive
state’.
In conclusion, lymphoproliferative disorders can be
associated with solid tumors and complicate their disease course.
The time of manifestation with respect to the primary cancer and
the type of LPD will determine the optimal treatment type.
Treatment can often favor management of the cancer, which has a
poorer prognosis. Early detection and specific diagnosis programs
should be therefore perceived for cancer survivors, life-long. In
addition, careful attention should be paid to the choice of the
chemotherapy agents used in association, due to the potentially
increased risk of drug reactivity.
References
|
1
|
Dong C and Hemminki K: Second primary
neoplasms among 53 159 haematolymphoproliferative malignancy
patients in Sweden, 1958–1996: a search for common mechanisms. Br J
Cancer. 85:997–1005. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giroux DJ, Rami-Porta R, Chansky K, et al;
International Association for the Study of Lung Cancer
International Staging Committee. The IASLC Lung Cancer Staging
Project: data elements for the prospective project. J Thorac Oncol.
4:679–683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sano H and Marugame T: International
comparisons of cumulative risk of lung cancer, from cancer
incidence in five continents. Vol VIII. Jpn J Clin Oncol.
36:334–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakelee HA, Chang ET, Gomez SL, et al:
Lung cancer incidence in never smokers. J Clin Oncol. 25:472–478.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chansky K, Sculier JP, Crowley J, et al;
International Staging Committee and Participating Institutions. The
International Association for the Study of Lung Cancer Staging
Project: prognostic factors and pathologic TNM stage in surgically
managed non small cell lung cancer. J Thorac Oncol. 4:792–801.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Swerdlow SH, Campo E, Harris NL, et al:
World Health Organization Classification of Tumours of
Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2008
|
|
7
|
Klein U and Dalla-Favera R: Germinal
centres: role in B-cell physiology and malignancy. Nat Rev Immunol.
8:22–33. 2008. View
Article : Google Scholar
|
|
8
|
Chawla-Sarkar M, Lindner DJ, Liu JF, et
al: Apoptosis and interferons: role of interferon-stimulated genes
as mediators of apoptosis. Apoptosis. 8:237–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohno Z, Tamaki H, Ohsuga T, et al: Primary
lung carcinoma complicated by malignant lymphoma in two cases of
Epstein Barr virus infection. Case Rep Oncol. 5:367–372. 2012.
View Article : Google Scholar
|
|
10
|
Cui Y, Liu T, Zhou Y, et al: Five cases
report of solid tumors synchronously with hematologic malignancy.
Cancer Res Treat. 44:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Compostella A, Toffolutti T, Soloni P, et
al: Multiple synchronous tumors in a child with Fanconi Anemia. J
Pediatr Surg. 45:e5–e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneko S and Yamaguchi N: Epidemiological
analysis of site relationships of synchronous and metachronous
multiple primary cancers in the National Cancer Centers, Japan,
1962–1996. Jpn J Clin Oncol. 29:96–105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris NL, Jaffe ES, Stein H, et al: A
revised European-American classification of lymphoid neoplasms: a
proposal from the International Lymphoma Study Group. Blood.
84:1361–1392. 1994.PubMed/NCBI
|
|
14
|
Goldstraw P, Crowley J, Chansky K, et al:
The IASLC Lung Cancer Staging Project: Proposal for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung CY and Kwon KY: A case of synchronous
lung adenocarcinoma and extranodal marginal zone B-cell lymphoma of
mucosa-associated lymphoid tissue (MALT) type. Tuber Resp Dis
(Seoul). 73:61–66. 2012. View Article : Google Scholar
|
|
16
|
Dores GM, Metayer C, Curtis RE, et al:
Second malignant neoplasms among long-term survivors of Hodgkin’s
disease: a population-based evaluation over 25 years. J Clin Oncol.
20:3484–3494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodgson DC, Koh ES, Tran TH, et al:
Individualized estimates of second cancer risks after contemporary
radiation therapy for Hodgkin lymphoma. Cancer. 110:2576–2586.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Groves FD, Linet MS, Travis LB and Devesa
SS: Cancer surveillance series: non-Hodgkin’s lymphoma incidence by
histologic subtype in the United states from 1978 through 1995. J
Natl Canc Instit. 92:1240–1251. 2000. View Article : Google Scholar
|
|
19
|
Harris NL, Jaffe E, Diebold J, et al:
World Health Organization classification of neoplastic diseases of
the hematopoietic and lymphoid tissues: Report of the Clinical
Advisory Committee Meeting-Arlie House, Virginia, November 1997. J
Clin Oncol. 17:3835–3849. 1999.PubMed/NCBI
|
|
20
|
Schmitz N, Pfistner B, Sextro M, et al:
Aggressive conventional chemotherapy compared with high-dose
chemotherapy with autologous haematopoietic stem-cell
transplantation for relapsed chemosensitive Hodgkin’s disease: a
randomised trial. Lancet. 359:2065–2071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enblad G, Glimelius B and Sundström C:
Treatment outcome in Hodgkin’s disease in patients above the age of
60: a population-based study. Ann Oncol. 2:297–302. 1991.PubMed/NCBI
|
|
22
|
Adami J, Frisch M, Yuen J, et al: Evidence
of an association between non Hodgkin’s lymphoma and skin cancer.
BMJ. 310:1491–1495. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shugart YY, Heminki K, Vaittinen P, et al:
A genetic study of Hodgkin’s lymphoma: an estimate of heritability
and anticipation based on the familial cancer database in Sweden.
Hum Genet. 106:553–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Capalbo S, Trerotoli P, Ciancio A, et al:
Increased risk of lymphoproliferative disorders in relatives of
patients with B-cell chronic lymphocitic leukemia: relevance of the
degree of familial linkage. Eur J haematol. 65:114–117. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cardous-Ubbink MC, Heinen PC, Bakker PJ,
et al: Risk of second malignancies in long-term survivors of
childhood cancer. Eur J Canc. 43:351–362. 2007. View Article : Google Scholar
|
|
26
|
Wiernik PH, Wang SQ, Hu XP, et al: Age of
onset evidence for anticipation in familial non Hodgkin’s lymphoma.
Br J Haematol. 108:72–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Travis LB, Curtis RE, Bennett WP, et al:
Lung cancer after Hodgkin’s disease. J Natl Cancer Inst.
87:1324–1327. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hemminki K and Vaittinen P: National
database of familial cancer in Sweden. Genet Epidemiol. 15:225–236.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milatovich A, Travis A, Grosschedl R and
Francke U: Gene for lymphoid-enhancer-binding factor 1 (LEF1)
mapped to human chromosome 4 (q23–q25) and mouse chromosome 3 near
Egf. Genomics. 11:1040–1048. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Demirci U, Ozdemir N, Benekli M, et al:
Lymphoproliferative disorders in multiple primary cancers. Asian
Pac J Cancer Prev. 13:383–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caillard S, Lelong C, Pessione F, et al:
Post-transplant lymphoproliferative disorders occurring after renal
transplantation in adults: report of 230 cases from the French
Registry. Am J Transplant. 6:2735–2742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Enblad G, Glimelius B and Sundström C:
Treatment outcome in Hodgkin’s disease in patients above the age of
60: a population-based study. Ann Oncol. 2:297–302. 1991.PubMed/NCBI
|
|
33
|
Bassal M, Mertens AC, Taylor L, et al:
Risk of selected subsequent carcinomas in survivors of childhood
cancer: a report from Childhood Cancer Survivor Study. J Clin
Oncol. 24:476–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chanel S, Burke L, Fiche M, et al:
Synchronous pulmonary adenocarcinoma and extranodal marginal
zone/low-grade B-cell lymphoma of MALT type. Hum Pathol.
32:129–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oikonomou A, Astrinakis E, Kotsianidis I,
et al: Synchronous BALT Lymphoma and Squamous Cell Carcinoma of the
Lung: Coincidence or Linkage? Case Rep Oncol Med Volume.
2013:4203932013.
|
|
36
|
Cui Y, Liu T, Zhou Y, et al: Five cases
report of solid tumor synchronously with hematologic malignancy.
Cancer Res Treat. 44:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung CY and Kwon KY: A Case of Synchronous
Lung Adenocarcinoma and Extranodal Marginal Zone B-Cell Lymphoma of
Mucosa-Associated Lymphoid Tissue MALT type. Tuberc Respir Dis
(Seoul). 73:61–66. 2012. View Article : Google Scholar
|
|
38
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. European Respiratory Journal.
3:750–762. 2002. View Article : Google Scholar
|
|
39
|
Visittsunthorn N, Utsawapreechawong W,
Pacharn P, et al: Immediate type hypersensitivity to
chemotherapeutic agents in pediatric patients. Asian Pac J Allergy
Immunol. 27:191–197. 2009.
|
|
40
|
Ganz PA: Late effects of cancer and its
treatment. Semin Oncol Nurs. 17:241–248. 2001. View Article : Google Scholar
|