Introduction
Gastric cancer is the fourth most common malignant
tumor (1) and has a poor prognosis;
the 5-year survival rate is usually ≤20% (2). Invasion and metastasis are the main
reasons for clinical treatment failure and patient mortality
(3–5). In addition, the current clinical
therapies do not obtain good outcomes. Therefore, novel therapies
for the diagnosis and treatment of gastric cancer are required. It
has been shown that gastric cancer metastasis or invasion of the
adjacent tissues via penetration of the stomach wall is a complex
process, which is associated with numerous factors and includes
steps, such as tumor cell invasion, breaking through the cellular
matrix, entering the blood, reaching distant organs, settling and
proliferating (6). As an important
intracellular signaling molecule, focal adhesion kinase (FAK)
mediates the crosslink of the intracellular signaling networks,
playing a key role in cell migration and invasion. Su et al
(7) found that compared with
non-cancerous tissues, FAK expression in gastric cancer tissues
increased. In particular, FAK expression in poorly differentiated
gastric cancer tissues was increased compared with that in
well-differentiated cancer tissues; FAK expression in the tumors
with lymph node metastasis was enhanced compared with that in those
without lymph node metastasis; and the deeper the extent of tumor
invasion, the stronger the FAK expression. Numerous studies have
reported that the expression of FAK is increased in the invasive
and metastatic tumors (8–15). The high expression of FAK is
suggested to promote the migration and metastasis of tumor cells,
therefore, interfering with the functions of FAK may provide new
insights into gastric cancer therapy.
In the present study, RNA interference (RNAi)
technology and novel lentiviral vector were used to prepare the
recombinant FAK-shRNA lentivirus for infecting the human metastatic
gastric cancer cells. In vivo and in vitro
investigations were performed to observe the growth and metastasis
of gastric tumors following the intervention of FAK functions,
aiming to provide the basis for new gastric cancer therapies. This
study was approved by the ethics committee of the First Affiliated
Hospital of Xiamen University (Xiamen, China).
Materials and methods
Construction of FAK interference
vector
The pLentilox3.7 plasmid was kindly provided by
Professor Boan Li, Xiamen University (Xiamen, China). The primer
design and vector construction used to intervene with FAK gene
expression were as described previously (16). The positive plasmids identified by
enzyme-cutting were sent to Shanghai Invitrogen Biotechnology Co.,
Ltd. (Shanghai, China) for the nucleotide sequencing.
Lentiviral packaging and titer
determination
According to reported methods (17), SGC7901 cells (Shanghai Institute of
Biochemistry and Cell Biology, Shanghai, China) were cultured, and
the cells with 80% density were used for transfection. A total of 5
ml of serum-free Dulbeccos’s modified Eagle’s medium (DMEM) was
added to the cells, followed by the addition of the transfection
reagent system (Shanghai Run-Biotech Co., Ltd., Shanghai, China)
and incubation for 6 h. Serum-free DMEM was then replaced with DMEM
with 2% fetal bovine serum (Shanghai Jiang Lai Biotechnology Co.,
Ltd., Shanghai, China), followed by incubation for 48 h. Next, the
cell supernatant was collected, followed by centrifugation at 1,030
× g for 5 min at 4°C (3–18K; Sigma-Aldrich, St. Louis, MO, USA) and
filtration. The supernatant was removed, and 1 ml of medium was
added. The mixture was placed in a refrigerator at 4°C until the
virus was dissolved completely. After sub-packaging, the virus
liquid was placed in a refrigerator (Qingdao Haier Co., Ltd.,
Qingdao, China) at −80°C for use. The virus supernatant was diluted
with serum-free DMEM. The diluted virus liquid was then mixed with
the SGC7901 cells with 90–100% density, followed by incubation in
5% CO2 at 37°C. The cells were counted under a
fluorescent microscope (DMS-853; Shenzhen Boyu Instrument Co.,
Ltd., Shenzhen, China). The virus titer was determiend using the
following formula: Virus titer (IU/mL) = number of fluorescent
cells/ml suspension × dilution multiple.
Establishment of stably transfected
gastric cancer cells SGC-7901
Recombinant lentivirus
plasmid-transfected SGC-7901 cells
The cells in the logarithmic growth phase were
inoculated into a 10 cm-dish and grown to ~70% confluence for the
transfection. According to the different structures of the
lentiviruses, the cells were divided into three groups: The
interference experimental group was infected with FAK-shRNA
lentivirus, the negative control group was infected with empty
lentiviral vector and the blank control group received no
treatment. The constructed lentiviruses were used to transfect the
SGC-7901 cells (Cell Bank of Shanghai Institute of Cell Biology,
Chinese Academy of Sciences, Shanghai, China) in the logarithmic
growth phase according to the multiplicity of infection (MOI), 10
μl of 1,000X polybrene (Gibco Inc., Billings, MT, USA) was added
and the medium was replaced after 24 h. Subsequently, RPMI-1640
medium (Gibco Inc.) was added to complete medium for another 48 h
of incubation, prior to using a fluorescence microscope (BX51;
Olympus Corporation, Tokyo, Japan) to observe the transfection
efficiency. Western blotting was used to analyze the FAK
expression.
Screening of stable recombinant
lentiviral plasmid-transfected SGC-7901 cells
SGC-7901 cells were counted and 1×105
cells were plated in 10 wells of a 24-well plate, which were
sequentially labeled as 1–10. The medium was replaced on the
following day and, when the cells reached the logarithmic growth
phase, different concentrations of neomycin G418 (Gibco Inc.) were
added. The G418 concentration was 100 μg/ml in well no. 1 and
increased to 1,000 μg/ml in well no. 10, arithmetically. The medium
was replaced after 24 h and the cell growth was observed. The
lowest concentration that could completely kill the cells on the
14th day was used as the screening concentration.
In vitro experimental grouping
SGC7901 gastric cancer cells were divided into the
interference group (IG; infected with FAK-shRNA lentivirus), the
transfected negative control group (TN; infected with empty
lentiviral vector) and the untreated group (UG).
In vitro invasion and migration
assay
After 48 h transfection, the cells were digested
with trypsin (Beijing CellChip Biotechnology Co., Ltd., Beijing,
China) and, following this, the culture medium was removed by
centrifugation at 716 × g for 5 min. Subsequently, the cells were
resuspended with serum-free medium for cell counting and the cell
density was adjusted to 5×104 cells/well. The Matrigel
basement membrane matrix (BD Biosciences, Franklin Lakes, NJ, USA)
was added to the Transwell chamber, followed by incubation at 37°C
for 2 h. A total of 1 ml cell suspension was then added into a
Transwell chamber and the cells were cultured at 37°C and 5%
CO2 for 48 h. Following this, the Transwell chamber was
removed, and a cotton swab was used to remove the cells on the
Matrigel side of the Transwell chamber. Next, 0.1% crystal violet
(Shanghai Zerun Biotechnology Co., Ltd., Shanghai, China) was used
for staining for 1 h. The film of the Transwell chamber was rinsed
with double distilled water, and removed for affixation to the back
of the slide for cell counting under an inverted microscope (CKX31;
Olympus Corporation, Tokyo, Japan). The procedures of the migration
assay were the same as the invasion assay, with the exception of
using the Matrigel basement membrane matrix.
In vivo cell lines and experimental
animals
SGC-7901 gastric cancer cells were purchased from
the Cell Bank of Shanghai Institute of Cell Biology, Chinese
Academy of Sciences. The SGC-7901 cells were divided into the same
three groups as previously: IG, TN and UG. BALB/C female nude mice
were purchased from Shanghai SLAC Laboratory Animal Co. Ltd.
(Shanghai, China), were aged 4 to 6 weeks and weighed 16–19 g
[license no. SCXK (Hu) 2007-0005]. The mice were fed under SPF
conditions in the Cancer Research Center of Xiamen University for 1
week before the experiment.
Establishment of in vivo animal
model
Animal grouping and cell
preparation
60 BALB/C/nu mice were randomly divided into six
groups and labeled as Groups 1–6 (n=10 for each group). Groups 1–3
were the subcutaneous tumor transplantation models and Groups 4–6
were the orthotopic tumor transplantation models. IG, TN and UG
cells were trypsin-digested into a single cell suspension, washed
three times with RPMI 1640 medium and then counted under microscope
(CKX41; Olympus Corporation).
Establishment of the subcutaneous
tumor transplantation model
The cell density was adjusted to 1×107,
then 0.2 ml IG, TN and UG cells were inoculated subcutaneously into
the armpits of mice in Groups 1–3, respectively. The injection
method involved conventional disinfection of the skin with alcohol,
and then the needle was inserted in the chest ~2 cm from the right
armpit, reaching the right armpit along the subcutaneous tissue.
The cell suspension was slowly injected and, on removal of the
needle, an alcohol swab was compressed on the area to avoid leakage
of the injected tumor cells.
Establishment of the orthotopic tumor
transplantation model
Mice in Groups 4–6 were initially fasted for 12 h.
IG cells were inoculated first: Following anesthesia with ether,
the abdominal cavity was opened to reveal the stomach and 0.1 ml
cell suspension was inoculated under the mucous membrane of the
greater curvature of the gastric antrum. The bulge under the serous
membrane of the stomach was used to confirm successful vaccination.
A sterile cotton ball was pressed onto the injection site for 1 min
following the removal of the needle, to ensure that the cells did
not leak out into the abdominal cavity. Suture of the peritoneum,
muscle and skin was performed layer by layer. Postoperative
disinfection with 75% alcohol and iodine was conducted and then the
mice were put back into the cages. The same method was used to
inoculate the TN and UG cells.
Rearing observation
Following inoculation, both the subcutaneous and
orthotropic mice were bred in a SPF animal room, and regular
observation of activity and eating habits were performed. The body
weights and armpit tumor volumes in the nude mice were measured and
evaluated. The long diameter (a) and short diameter (b) of tumors
were measured with a vernier caliper for the calculation of the
approximate tumor volume (V) (17),
according to the formula V (mm3) = a × b2/2.
Through observation, all mice were alive and exhibited a tumor 1
week after the inoculation, with the tumor formation rate as 100%.
During the rearing process, there was no mortality in Groups 1–3,
while two mice in each of Groups 4–6 died on the 10th day after
inoculation. All mice were sacrificed (by decapitation) after 4
weeks of feeding.
Tumor resection and tissue analysis
The tumors were resected and their size and weights
were measured. The Carestream imaging system (Eastman Kodak
Company, Rochester, NY, USA) was used for fluorescence detection of
tumor in vitro. Simultaneously, laparotomy and thoracotomy
were performed to obtain the liver and lungs of the mice. The
thoracic cavity was dissected to investigate whether tumor
metastasis had occurred. The tumor tissue paraffin sections were
prepared and dewaxed followed by antigen retrieval. The primary
rabbit anti-human FAK monoclonal antibody (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) was added, followed by incubation
at room temperature for 90 min. After two washes with PBS, the
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibody (Fuzhou Maixin Biotechnology Development Co., Ltd.,
Fuzhou, China) was added, followed by incubation at room
temperature for 15 min and two washes with PBS. After coloration,
counterstaining and mounting, the sections were observed using the
Q550CW image acquisition and analysis system (Leica, Mannheim,
Germany). The primary antibody was replaced with PBS, to serve as a
negative control and the known positive tissue sample was used as
positive control. Each section was divided into five fields of
vision (×200), with 100 counted cells in each field using the
Q550CW image acquisition and analysis system (Leica). The
percentage of positive cells was scored as follows: 0, 0–5%; 1,
6–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. The staining intensity
was scored as follows: 0, no staining; 1, weak staining intensity;
2, moderate staining intensity; and 3, strong staining intensity.
The percentage score and staining intensity scores were then added
to obtain a total staining score, which was scored as follows:
<2, negative (−); 2–3, weakly positive (+); 4–5, positive (++);
and 6–7, strong positive (+++). Quantitative polymerase chain
reaction (qPCR) was conducted according to a previously reported
method (18). The total RNA was
extracted from the tumor tissue. The PCR-reaction mixture
containing 1 μg total RNA was added to a 200 μl PCR tube. The
primer sequences for FAK (125 bp) were as follows: Forward,
5′-ACATTATTGGCCACTGTGGATGAG-3′ and reverse, anti-sense primer:
5′-GGCCAGTTTCATCTT GTTGATGAG-3′ for FAK (125 bp); and forward,
5′-GATGCAGAAGGAGATCACTG-3′ and reverse, 5′-GGGTGTAACGCAACTAAGTC-3′
for the reference, β-actin (222 bp). All primers were synthesized
by Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai, China).
The PCR steps were as follows: initial denaturation for 3 min at
94°C, denaturation for 30 sec at 94°C, annealing for 30 sec at 59°C
and elongation for 1 min at 72°C for 35 cycles, followed by
continued elongation at 72°C for 7 min.
Statistical processing
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for data processing. The tumor cell proliferation and
tumor weight in the different groups were compared using
single-factor analysis of variance, while FAK expression and tumor
metastasis were compared using χ2 and Fisher’s exact
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction, identification and viral
packaging of vector
The amplified plasmid was identified by XbaI
and NotI digestion electrophoresis. As shown in Fig. 1, the FAK-RNAi recombinant plasmid
obtains a 504-bp product by double digestion (insert fragment size,
55 bp); while the pLentilox3.7 empty vector, which was inserted,
obtains a 449-bp product after the double digestion (lane 1). Lane
2 revealed that the target gene was not successfully inserted,
while lanes 6 and 7 showed that the enzyme digestion of recombinant
plasmid was not successful. The results revealed that the product
identification results of lanes 3, 4, 5 and 8 were the same as the
expectations, i.e. plasmids from the positive colonies. Lanes 3 and
4 indicated the successful construction of the negative control
plasmids, while lanes 5 and 8 exhibited the positive colony
plasmids. Certain plasmids from the positive colonies were
sequenced by Shanghai Invitrogen Biotechnology Co., Ltd., and the
results showed that they contained the correct target gene sequence
(Fig. 1). The results showed that
FAK shRNA and control shRNA were constructed in the pLentilox3.7
vector, and the recombinant was constructed successfully. After
packaging with 293T cells, the titer of the recombinant virus group
was 4×107 pfu/ml.
Screening concentrations of G418
After 14 days, cells in well nos. 7, 8, 9 and 10,
i.e. wells with a G418 concentration of 700, 800, 900 and 1,000
μg/ml, respectively, were all dead. Additionally, only trace cells
survived in the 600 μg/ml G418 group.
Establishment of a stable FAk-silencing
SGC-7901 cell line
During the cultivation of the transfected
recombinant lentivirus SGC-7901 cells in each group, the G418
concentration was maintained at 600 μg/ml for consecutive 14 days,
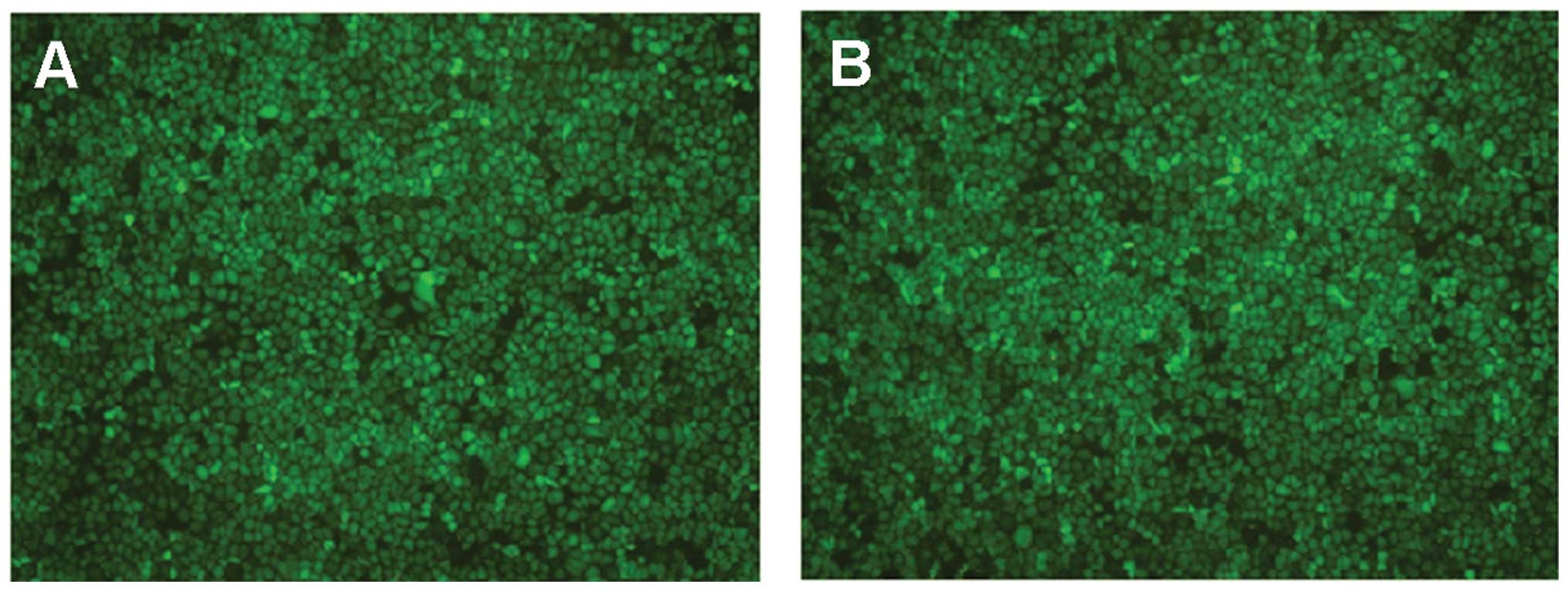
the transfected cells grew well and the fluorescence expression
increased (Fig. 2).
Western blot analysis of FAK
expression
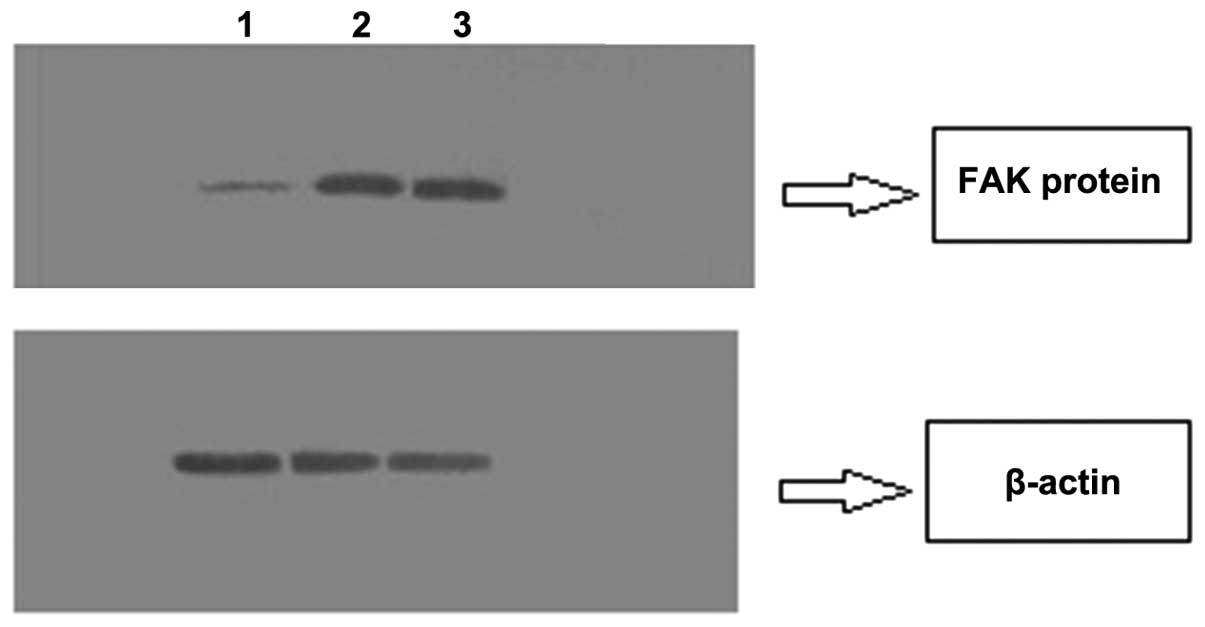
Compared with the TN and NG SGC-7901 cells, the FAK
protein expression in the IG SGC-7901 cells significantly reduced,
suggesting that the transfected recombinant lentivirus plasmid
could effectively silence the FAK gene (Fig. 3).
Effect of different MOIs on SGC-7901
cells
Following the infection with lentivirus, with
increasing MOIs, the proportion of the infected cells also
increased. When the MOI was 20, ~60% of cells were infected; when
the MOI was 30, ~90% of cells were infected; and when the MOI was
40, a large number of cells began to die. The cell transfection
experiments were therefore performed with an MOI of 30.
The optimal MOI of 30 was used to infect cells of
each group, and it was found that compared with the TN and UG
groups, the cell proliferation ability in IG cells (3.75±0.01) was
significantly inhibited compared with that in TN (8.29±0.32) and NG
(8.16±0.29) cells, respectively (P<0.05), and, over time, the
difference gradually increased.
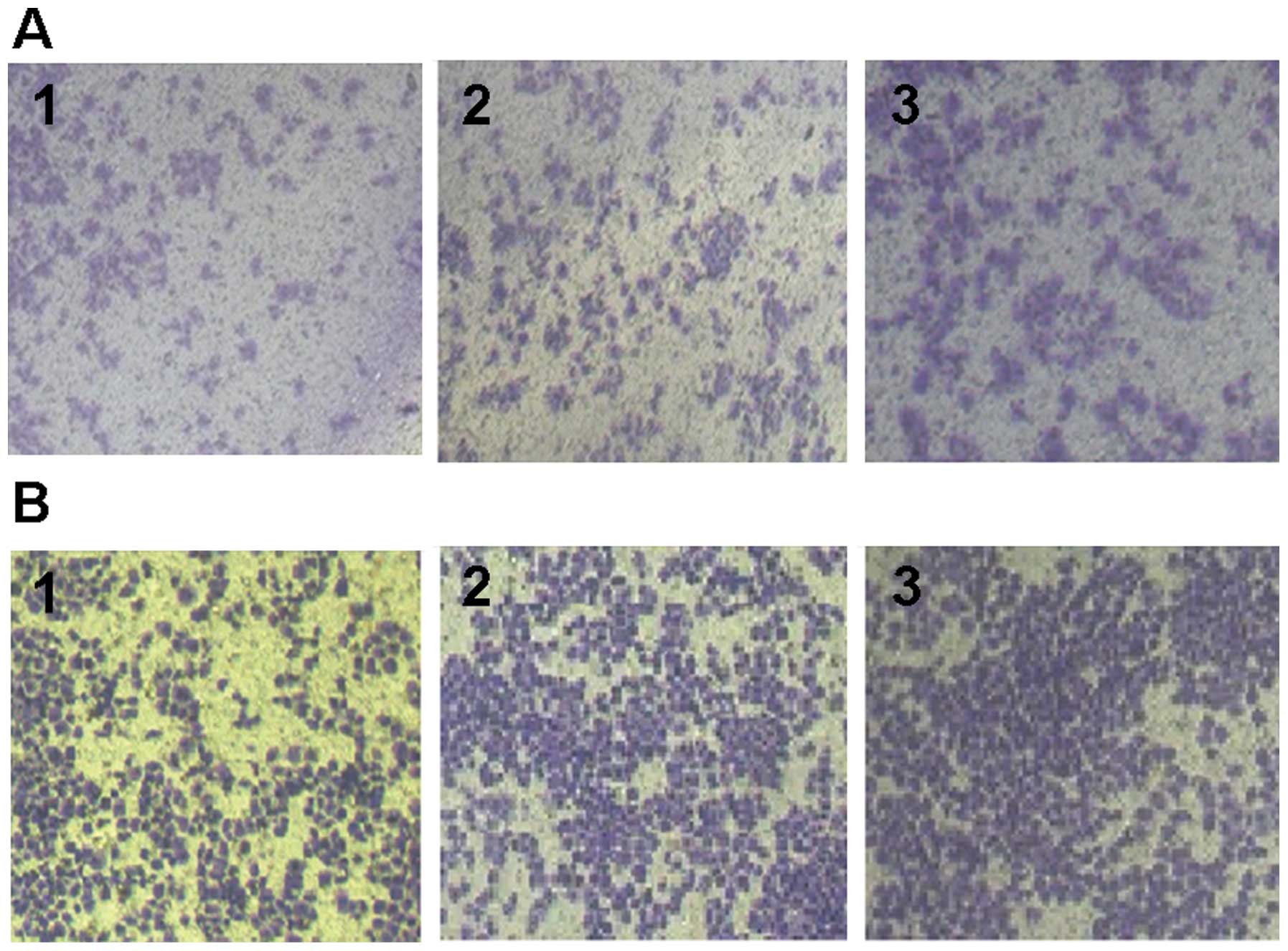
In the Transwell chamber invasion assay, the number
of membrane-penetrating IG cells (59.3±4.1) was significantly lower
than that of TN (105.1±3.7) (P<0.05) and UG (103.8±8.3)
(P<0.05) cells. There was no significant difference between that
of TN and UG (P>0.05) cells, indicating that the cell
invasiveness in IG cells was significantly inhibited (Fig. 4).
In vivo tumor formation rate of each
subcutaneous tumor transplantation group
Observation of the subcutaneous and orthotopic tumor
transplantation groups revealed that on the seventh day of tumor
cell inoculation, tumors of 2 mm in diameter were visible in each
group, and the tumor formation rate was 100%.
Comparison of tumor formation in vivo in
each subcutaneous tumor transplantation group
The mice were sacrificed 4 weeks following
inoculation and the mean±standard deviation (SD) average tumor
weight in the IG, TN and UG inoculation groups was 1.474±0.9840,
3.134±0.3299 and 2.687±0.3827 g, respectively. The mean (±SD) tumor
weight in the IG inoculation group was significantly smaller than
that of the remaining two groups (F=18.09, P=0.0017).
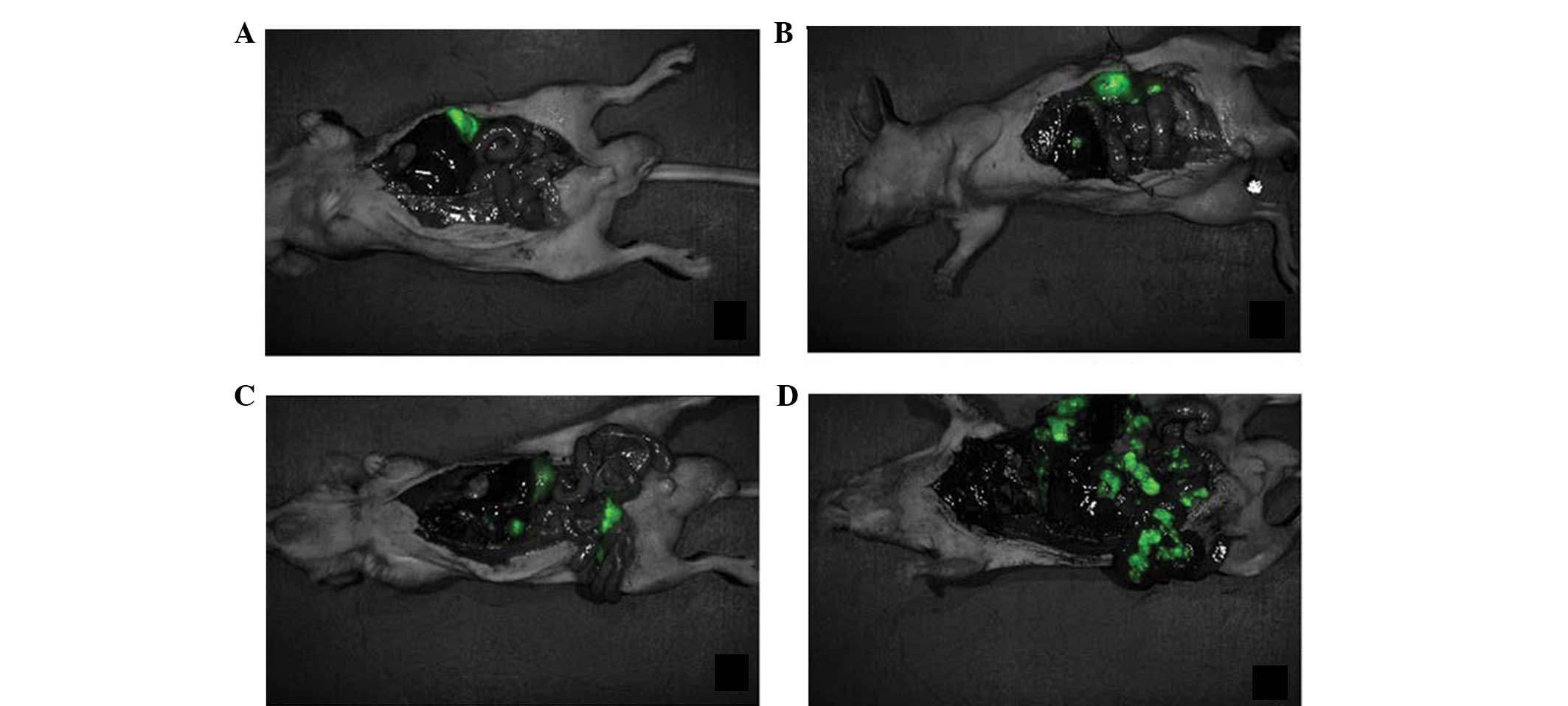
Fluorescence detection of the tumor in vitro
revealed that there was no strong fluorescence expression in the IG
and TN inoculation groups, while no fluorescence expression was
identified in mice inoculated with UG cells. This indicated that
the virus-packaged plasmid, labeled with green fluorescent protein,
infected the SGC-7901 gastric cancer cells, the cells repeatedly
proliferated in nude mice and the target gene expression remained
strong (Fig. 5).
qPCR revealed statistically significant difference
in FAK mRNA expression levels between the IG and TN or UG
inoculation groups (P<0.05; Table
I).
 | Table IExpression of FAK protein in tumor
specimens of each group. |
Table I
Expression of FAK protein in tumor
specimens of each group.
| FAK mRNA
expression | |
|---|
|
| |
|---|
| Group | + | ++ | +++ | P-value |
|---|
| IG | 7 | 2 | 1 | 0.034 |
| TN | 2 | 4 | 4 | |
| UG | 1 | 4 | 5 | |
Comparison of stained tumor sections in
vivo in each subcutaneous tumor transplantation group
Positive expression of FAK was identified in the TN
and UG groups, while almost no expression of FAK was observed in
the IG group (Fig. 6).
Comparison of metastasis in vivo in each
orthotopic tumor transplantation group
Eight nude mice survived in each group: One mouse
exhibited hepatic metastasis and no mice exhibited peritoneal
metastasis in the IG inoculation group; six mice demonstrated
hepatic metastasis, while four had peritoneal metastasis in the TN
inoculation group; five mice had hepatic metastasis and four showed
peritoneal metastasis in the UG inoculation group. The difference
was statistically significant between Group 1 and Groups 2 and 3
(P<0.05). The FAK mRNA levels in Group 4 were significantly
lower than those in Groups 5 and 6.
3D fluorescence imaging could better display the
fluorescence in the liver and peritoneal metastases. A significant
difference was identified in the levels of liver and peritoneal
metastasis of the mice inoculated with IG and TN or UG cells
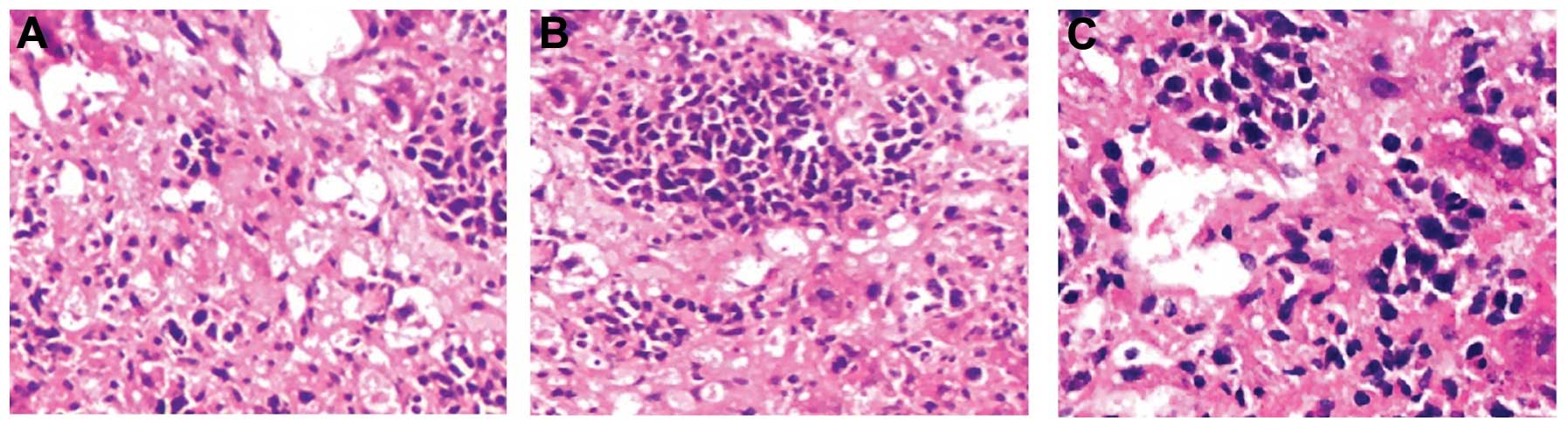
(Figs. 7 and 8). The immunohistochemical staining of
metastatic lesions revealed liver metastasis of gastric cancer in
mice inoculated with TN and UG cells (Fig. 9).
Discussion
The expression of FAK increases in the early stage
of tumorigenesis, resulting in the potential of invasion and
metastasis of tumor cells. When the expression of FAK is blocked,
the apoptosis of tumor cells is then induced (18). Therefore, the present study aimed to
investigate the changes in the biological behavior of gastric
cancer cells in which FAK function had been knocked down or out.
Firstly, high-efficiency transfection technology and hairpin RNA
was used to build the FAK interference vector (shRNA lentivirus).
The study found that FAK-shRNA lentivirus could stably infect the
SGC790 gastric cancer cells, and then significantly inhibit the
expression of FAK following the infection. Then, through Transwell
chamber migration and invasion assays, it was found that the
membrane-penetrating IG cells significantly decreased compared with
that of TN and UG cells, while there was no significant difference
between the UG and TN cells, indicating that the inhibition of FAK
expression could significantly decrease the invasion and migration
of SGC7901 cells. The results are similar to those in a study by
Ren et al (19), where siRNA
was used to inhibit the expression of myofibrillogen-esis regulator
1 in human laryngeal carcinoma Hep-2 cells, thereby significantly
reducing FAK phosphorylation at Tyr-925, and significantly
inhibiting the invasion and metastasis of the Hep-2 cells.
Additionally, Tan et al (18) found that the increased invasiveness
of human chondrosarcoma cells by Cyr61 was likely through the
signaling pathway that was dependent on ανβ integrin, FAK, ERK and
AP-1. Furthermore, Hauck (21)
found that the inhibition of FAK activity or blocking the
expression of FAK could inhibit the invasion of tumor cells.
Additional studies have confirmed that the inhibition of FAK
expression could effectively reduce the adhesion and invasion of
tumor cells (22,23), which is consistent with the results
of the present study, suggesting that in vitro inhibition of
FAK expression inhibits the invasion and migration of tumor
cells.
To investigate the effects of FAK interference in
vivo, a stably transfected gastric cancer cell model was
established in this experiment. The monoclonal cells with a good
interference effect and vigorous growth were selected and largely
proliferated in vitro to be used as the in vivo
interference group of the present study. Simultaneously, a negative
control group (empty plasmid-transfected group) and a blank control
group were also established, and cells of all three groups were
transplanted into nude mice, respectively. A subcutaneous tumor
transplantation model and an orthotopic tumor transplantation model
were also established. The results showed that in each subcutaneous
group, there were significant differences in the tumor volume and
weight between mice inoculated with IG and TN/UG cells; while there
was no significant difference in the tumor volume and weight
between mice inoculated with TN and UG cells.
Furthermore, qPCR detection revealed that there was
a significant difference in the FAK mRNA levels between the IG and
TN/UG cell-inoculated mice, while there was no statistical
significance between the latter two groups. Immunohistochemical
staining showed that the expression of FAK in mice inoculated with
IG cells was significantly lower than that of mice inoculated with
TN and UG cells, which was consistent with the in vitro
western blot results. Analysis of the results suggested that the
shRNA in IG cells could effectively degrade FAK mRNA, thereby
reducing or inhibiting the expression of FAK. This study showed
that, in the subcutaneous tumor transplantation group of gastric
cancer nude mice, the tumor growth was successfully inhibited, and
FAK gene transcription and protein expression in tumor tissues were
reduced. In the treatment of melanoma, Li et al(22) directly injected siRNA to intervene
the intratumoral FAK plasmids, and it was found that when the FAK
expression reduced, the average tumor weight in the mouse tumor
model also decreased.
In the orthotopic tumor transplantation group, the
gross anatomy of the nude mice was combined with an in vitro
fluorescence imaging system to locate the metastatic lesions. The
positioning was accurate and therefore a more accurate
understanding of the tumor metastasis situation in nude mice was
achieved. It was identified that there were significant differences
in the levels of liver metastasis among IG, TN and UG
cell-inoculated mice. There were significant differences in the
levels of peritoneal metastasis between IG and TN/UG
cell-inoculated mice, while there was no significant difference in
the levels of peritoneal metastasis between TN and UG
cell-inoculated mice. The H&E staining of the metastatic
lesions showed that there was gastric cancer metastasis in the
liver tissues. The orthotopic tumor transplantation model showed
that when the FAK gene expression was silenced by RNAi technology,
the metastasis of gastric cancer in nude mice was inhibited, which
further confirmed that FAK plays an important role in the process
of tumor metastasis, suggesting that orthotopic tumor
transplantation may better present the biological characteristics
of SGC-7901 cells in the event of metastasis. Further study with an
increased sample size is required to investigate the significant
difference in the peritoneal metastasis between IG and UG
cell-inoculated mice that was identified in the present study.
Notably, during the establishment process of the
subcutaneous tumor model, in order to avoid premature necrosis and
ulceration of the tumors, careful observation was paid to the
injected densities of SGC-7901 cells. It was found that when the
inoculated concentration was 1×108 cells/well, the
grain-sized mass in the injection site was palpable 5 days after
inoculation and the subcutaneous tumor would appear ulcerated or
bleed 15 days following the inoculation. Additionally, the
ulceration would form a scab if feeding was continued, the
longitudinal growth rate would be lower than previously, and the
tumor may grow around the vaccination site. By contrast, when the
inoculated concentration of SGC-7901 was 1×107
cells/well, a tumor was palpable in the injection site 7 days after
inoculation. After 21 day, the skin covering the surface of the
tumor was rosy and smooth, without rupture and bleeding. However,
when the inoculated concentration was 1×106 cells/well,
tumor formation occurred 15 days after inoculation and the growth
was slow. Therefore, the appropriate inoculation concentration was
determined to be 1×107 cells/well. These observations
were similar to those identified previously (25,26),
which provided a valuable insight into the establishment of animal
gastric cancer models.
In summary, the present study successfully
constructed a pLentilox3.7 FAK lentiviral vector and, through
establishing the stably transfected cell lines, the in vivo
and in vitro studies confirmed that the expression of FAK in
SGC-7901 gastric cancer cells was reduced and the FAK-based signal
transduction pathway was blocked, which could effectively inhibit
the growth and metastasis of cancer cells. Therefore, the current
study has provided new insights into clinical gene therapy for
gastric cancer.
Acknowledgements
This study was supported by a grant from the Xiamen
Municipal Science and Technology Bureau Project (project no.
3502z20089006).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomassen I, van Gestel YR, van Ramshorst
B, et al: Peritoneal carcinomatosis of gastric origin: a
population-based study on incidence, survival and risk factors. Int
J Cancer. 134:622–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiang CY, Huang KH, Fang WL, et al:
Factors associated with recurrence within 2 years after curative
surgery for gastric adenocarcinoma. World J Surg. 35:2472–2478.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brancato S and Miner TJ: Surgical
management of gastric cancer: review and consideration for total
care of the gastric cancer patient. Curr Treat Options
Gastroenterol. 11:109–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leung WK, Wu MS, Kakugawa Y, et al; Asia
Pacific Working Group on Gastric Cancer. Screening for gastric
cancer in Asia: current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie ZJ, Liu Y, Jia LM and He YC:
Heparanase expression, degradation of basement membrane and low
degree of infiltration by immunocytes correlate with invasion and
progression of human gastric cancer. World J Gastroentero.
14:3812–3818. 2008. View Article : Google Scholar
|
|
7
|
Su JM, Gui L, Zhou YP and Zha XL:
Expression of focal adhesion kinase and alpha5 and beta1 integrins
in carcinomas and its clinical significance. World J Gastroenterol.
8:613–618. 2002.PubMed/NCBI
|
|
8
|
Golubovskaya VM, Ho B, Zheng M, et al:
Disruption of focal adhesion kinase and p53 interaction with small
molecule compound R2 reactivated p53 and blocked tumor growth. BMC
Cancer. 13:3422013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hao HF, Naomoto Y, Bao XH, et al: Progress
in researches about focal adhesion kinase in gastrointestinal
tract. World J Gastroenterol. 15:5916–5923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JH, Lee BL, Yoon J, et al: Focal
adhesion kinase (FAK) gene amplification and its clinical
implications in gastric cancer. Hum Pathol. 41:1664–1673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Li X, Sunkara M, et al: PIPKIγ
regulates focal adhesion dynamics and colon cancer cell invasion.
PLoS One. 6:e247752011. View Article : Google Scholar
|
|
12
|
Kamo N, Naomoto Y, Shirakawa Y, et al:
Involvement of focal adhesion kinase in the progression and
prognosis of gastrointestinal stromal tumors. Hum Pathol.
40:1643–1649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dy GK: The role of focal adhesion kinase
in lung cancer. Anticancer Agents Med Chem. 13:581–583. 2013.
View Article : Google Scholar
|
|
14
|
Mitra SK, Lim ST, Chi A and Schlaepfer DD:
Intrinsic focal adhesion kinase activity controls orthotopic breast
carcinoma metastasis via the regulation of urokinase plasminogen
activator expression in a syngeneic tumor model. Oncogene.
25:4429–4440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dassie JP, Liu XY, Thomas GS, et al:
Systemic administration of optimized aptamer-siRNA chimeras
promotes regression of PSMA-expressing tumors. Nat Biotechnol.
27:839–849. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zagzag D, Friedlander DR, Margolis B, et
al: Molecular events implicated in brain tumor angiogenesis and
invasion. Pediatr Neurosurg. 33:49–55. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu B, Huang J, Zhou Y, et al:
Lentivirus-mediated LOX-1-RNA interference attenuates oxidative
stress-induced apoptosis in myocardial cells. Nan Fang Yi Ke Da Xue
Xue Bao. 32:165–168. 2012.(In Chinese). PubMed/NCBI
|
|
18
|
Tan TW, Yang WH, Lin YT, et al: Cyr61
increases migration and MMP-13 expression via alphavbeta3 integrin,
FAK, ERK and AP-1-dependent pathway in human chondrosarcoma cells.
Carcinogenesis. 30:258–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren K, Jin H, Bian C, et al: MR-1
modulates proliferation and migration of human hepatoma HepG2 cells
through myosin light chains-2 (MLC2)/focal adhesion kinase
(FAK)/Akt signaling pathway. J Biol Chem. 283:35598–35605. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sonoda Y, Hada N, Kaneda T, et al: A
synthetic glycosphingolipid-induced antiproliferative effect in
melanoma cells is associated with suppression of FAK, Akt, and Erk
activation. Biol Pharm Bull. 31:1279–1283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hauck CR, Sieg DJ, Hsia DA, Loftus JC,
Gaarde WA, Monia BP and Schlaepfer DD: Inhibition of focal adhesion
kinase expression or activity disrupts epidermal growth
factor-stimulated signaling promoting the migration of invasive
human carcinoma cells. Cancer Res. 61:7079–7090. 2001.PubMed/NCBI
|
|
22
|
Li S, Dong W, Zong Y, et al:
Polyethylenimine-complexed plasmid particles targeting focal
adhesion kinase function as melanoma tumor therapeutics. Mol Ther.
15:515–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Megison ML, Stewart JE, Nabers HC, et al:
FAK inhibition decreases cell invasion, migration and metastasis in
MYCN amplified neuroblastoma. Clin Exp Metastasis. 30:555–568.
2013. View Article : Google Scholar :
|
|
24
|
Tsutsumi K, Kasaoka T, Park HM, et al:
Tumor growth inhibition by synthetic and expressed siRNA targeting
focal adhesion kinase. Int J Oncol. 33:215–224. 2008.PubMed/NCBI
|
|
25
|
Nakanishi H, Yasui K, Ikehara Y, et al:
Establishment and characterization of three novel human gastric
cancer cell lines with differentiated intestinal phenotype derived
from liver metastasis. Clin Exp Metastasis. 22:137–147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mei LJ, Yang XJ, Tang L, et al:
Establishment and identification of a rabbit model of peritoneal
carcinomatosis from gastric cancer. BMC Cancer. 10:1242010.
View Article : Google Scholar : PubMed/NCBI
|