Introduction
Cervical cancer is the second most common type of
cancer in females worldwide, particularly in numerous developing
countries (1). To reduce the high
morbidity rate, the development of effective prevention and gene
therapy is needed. With the advancement of molecular biology, gene
therapy can be developed for a variety of cancers (1,2). Human
papillomaviruses (HPVs) are double-stranded DNA viruses and, at
present, >200 genotypes have been identified (3,4).
Infection with high-risk human papillomaviruses is known to be the
predominant risk factor for cervical cancer. HPVs can be detected
in >99% of cervical cancer patients (5). HPVs encode two proteins, E6 and E7,
which are important for cell immortalization. E6 and E7 interact
with p53 and pRb, respectively (6,7), and
induce growth inhibition via apoptosis (8). E7 leads to the functional inactivation
of these proliferation regulators and uncontrolled cell
proliferation (9). A previous study
has revealed that the expression of HPV16 E6 may play an important
role in cell transformation and cancer development (10). The tissue-specific promoter, also
termed the organ specific promoter, can control the expression of
downstream genes in tissues or organs and is closely associated
with the serotonin receptor gene, similar to tissue-specific
alternative promoters (11). Human
involucrin (hINV) is selectively expressed in the stratified
squamous epithelium, where it is associated with membrane protein
(12,13). The region that can regulate
tissue-specific expression is the hINVpromoter (pINV), which
contains 2474 bp of hINV upstream sequence (14). pINV was cloned using polymerase
chain reaction (PCR) and the HPV16 E6/7 was extracted from cervical
cancer tissues obtained from patients at the Yangzhou Maternal and
China Health-Care Center of Jinagsu Province (Yangzhou, China).
First, the carcinogenic fraction was removed from the E6/7 gene and
the remaining section was cloned into T vectors, correctly
sequenced and then cloned into the eukaryotic expression vector
pCEP4, which was separated from the CMV promoter. The tissue
specificity of the recombinant pINV-HPV16E6/7 plasmid was detected
in the present study.
Materials and methods
Materials
Surgical excisions of cervical cancer tissue samples
were obtained from 13 patients (age range, 41–58 years) at the
Yangzhou Maternal and China Health-Care Center of Jiangsu Province.
The pathological diagnoses of these specimens were cervical
squamous-cell carcinoma; 10 of which were stage II and three of
which were stage III. The study was approved by the ethics
committee of Yangzhou University (Yangzhou, China) and written
informed consent was obtained from all participants. The pMD18-T
vector, DNA Ligation kit and Recmobinant DNase I, RNase-free were
purchased from Takara Biotechnology (Dalian) Co., Ltd. (Dalian,
China). The expression vector pCEP4 was kindly provided by
Professor Huaichang Sun, College of Veterinary Medicine, Yangzhou
University and the host bacterium DH5α was obtained from the
Comparative Medicine Center (College of Veterinary Medicine,
Yangzhou University). DNA polymerase, the NotI, XhoI,
HindIII and BamHI restriction enzymes and DEPC were
the products of Shanghai Sangon Biological Engineering Technology
& Services Co., Ltd (Shanghai, China). QIAquick Gel Extraction
kits were obtained from Qiagen (Hilden, Germany). The DNAExtraction
Kit Ver.3.0 [Takara Biotechnology (Dalian) Co., Ltd.] and cell
transfection kit (Fugene 6 transfection reagent) were purchased
from Roche (Basel, Switzerland). The Eastep Universal RNA
Extraction Kit was purchased from Promega (Madison, WI, USA). The
yeast extracts and peptones for the bacterial culture were
purchased from Oxoid (Basingstoke, Hampshire, UK). The reagents for
microinjection, including M16, pregnant mare serum and human
chorionic gonadotropin and the hyaluronic acid enzymes were
purchased from Sigma-Aldrich (St. Louis, MO, USA). All other
reagents were analytical reagents made in China.
Cloning and sequence determination of the
tissue-specific promoter gene, pINV, and HPV16 E6/7
The reference pINV gene primers were as follows:
Upstream primer 1, 5′-TTGCGGCCG CAAGCTTCTCCATGTGTCATGT-3′; and
downstream primer 2, 5′-TACTCGAGGAGCTGAGCAGGAGTCAGG-3′. The
designed NotI restriction site was located at the 5′ end of
the upstream primer and the designed XhoI restriction site
was located at the 5′ end of the downstream primer. The E6/7 gene
was designed with reference to the entire genome sequence of HPV16:
Upstream primer 3, 5′-TGCTCGAGATGCACCAAAAGAGAACTG-3; and downstream
primer 4, 5′-ATGGATCCTTATGGTTTCTGAGAA CAGATG-3′. The designed
XhoI restriction sites were located in the 5′ end of the
upstream primer and the designed BamHI restriction sites
were located in the 5′ end of the downstream primer. The tissue
specimens from cervical cancer resection were obtained and the
tissue DNA was extracted as formwork according to the
manufacturer’s instructions for the DNA extraction kit. PCR was
used for the amplification of the pINV gene and HPV 16E6/7 gene
fragments and the PCR products were recovered using a gel
extraction kit subsequent to 0.8% agarose gel electrophoresis.
According to conventional methods, the fragments were ligated and
transformed with the pMD18-T and pGEM-T Easy vectors. The positive
colonies were then selected by blue-white selection and the
positive colonies were added t 5 ml of Luria broth containing 100
μg/ml ampicillin culture medium at 37°C. The bacteria were
cultivated for ~12 h and the alkaline lysis method for extraction
of plasmid DNA was then utilized. The recombinant T-pINV plasmids
were appraised by double restriction enzyme digestion using the
NotI and XhoI restriction enzymes, and the
recombinant pGEM-T-HPV16 E6/7 was assessed by double restriction
enzyme digestion using the XhoI and BamHI restriction
enzymes. Takara Biotechnology Company sequenced the pINV and
HPV16E/7 gene and completed the HPV16 E6/7 gene transformation,
where the E6 gene of leucine codon 50 (TTA), is mutated to glycine
(GGT) and the E7 gene 24 and 26 amino acid codons are modified to
glycine (GGT and GGG). The sequencing of the pINV and HPV16E/7
genes was analyzed using DNA Star software (DNA Star Inc., Madison,
WI, USA).
Transformation of the pCEP4 vector into
cells and construction of the recombinant pCEP4/pINV(-PCMV)
plasmid
The PCMV plasmid was digested by BglII to
remove the original promoter and the digested PCMV then self-linked
to give the pCEP4(-PCMV) plasmid. The pCEP4(-PCMV) and PUC-18
T-pINV plasmids were digested by NotI and XhoI,
respectively, and the pCEP4(-PCMV) vector and the prime pINV gene
were recovered using a gel extraction kit (Horan Bio Technologies
Co., Ltd., Shanghai, China). The recycling products were mixed in a
3:1 molar ratio, the same volume of solution I was added and then
the cells were incubated at 4°C overnight. The cells transformed
with the recombinant plasmid were plated and the positive colonies
were selected to obtain the required pCEP4/pINV(-PCMV) plasmid. The
recombinant pINV-HPV16E6/7 plasmid was then constructed. The
pCEP4(-PCMV) and pGEM-T-HPV16 E6/7 plasmids were digested by
BamHI and XhoI, respectively, mixed with the E6/7
gene and the pCEP4/pINV(-PCMV) vector in a 3:1 ratio, the same
volume of the solution I was added and the cells were incubated at
4°C overnight. The cells transformed with the recombinant plasmid
were plated and the positive colonies were selected to obtain the
required pINV-HPV16E6/7 plasmid. A schematic diagram of the entire
process is shown in Fig. 1.
Identification of the tissue-specific
pINV
The manufacturer’s instructions for the Fugene 6
transfection reagent were followed in order to transfect the
plasmid into the brain glioma, SP2/0, L929 and HeLa cells (all
obtained from Shanghai Institutes for Biological Sciences of
Chinese Academy of Sciences, Shanghai, China). The primers were
designed according to the sequence of the recombinant
pINV-HPV16E6/7 plasmid. The sequences of the upstream forward and
the downstream reverse primers were 5′-CACAGGAGCGACCCAGAAAGTTA-3′
and 5′-GCTGGG TTTCTCTACGTGTTCTT-3′, respectively. It was estimated
that the amplified DNA was 438 bp in length. The mRNA that was
transfected into the brain glioma, SP2/0, L929 and HeLa cells was
extracted according to the manufacturer’s instructions for the
TRIzol Plus Purification kit (Invitrogen Life Technologies,
Carlsbad, CA, USA). cDNA was amplified using the Super Script kit
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. A total of 10 μl cDNA was mixed with 4 μl 10X PCR
buffer, 2 μl (25 mM) MgCl2, 0.5 μl (100 pmol) of each
primer, 32.7 μl distilled water and 0.3 μl (2.5 U) Taq DNA
polymerase. The PCR reaction conditions were as follows: 35 cycles
of 30 sec at 94°C, 30 sec at 50°C and 2 min at 72°C. The amplified
products were sequenced to confirm pINV-HPV16E6/7 DNA (Shanghai
Sangon Biological Engineering Technology & Services Co.,
Ltd.).
Results
Gene cloning and identification of the
tissue-specific pINV
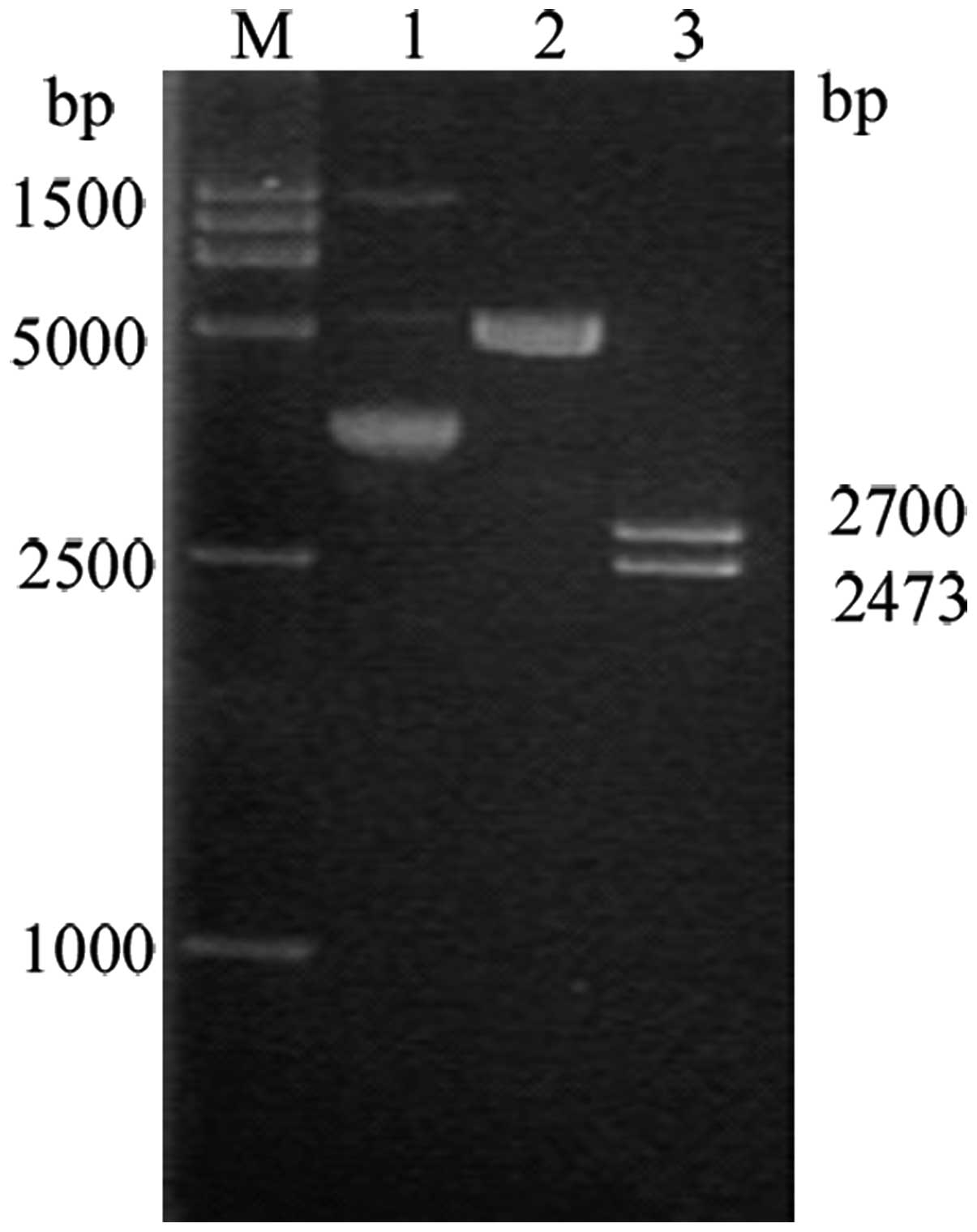
pINV fragments were amplified using PCR. A distinct
band at 2,474 bp could be observed on 1% agarose gel
electrophoresis. The fragments were recovered and ligated with
PGEM-T. Cells were transformed with the resulting plasmid and the
cells positive for the pMD18-T-pINV plasmid were selected using the
blue-white screening method. Bands containing fragments 2474 and
2692 bp in size were obtained during double enzyme digestion using
NotI and XhoI (Fig.
2).
Cloning and identification of the
pINV-HPV16E6/7 plasmid
The E6/7 fragment was amplified using PCR and 1%
agarose gel electrophoresis revealed a clear band at 780 bp. The
fragments were recovered and ligated using pGEM-T Easy vector
(Promega, Madison, WI, USA), introduced to cells and the cells
positive for the pGEM-T-E6/7 plasmid were selected using the
blue-white method. Double enzyme digestion by BamHI and
XhoI yielded visible bands at 3,015 and 780 bp on 1% agarose
gel electrophoresis (Fig 3).
Replacement of the promoter within the
vector with pINV
Following the removal of the original promoter of
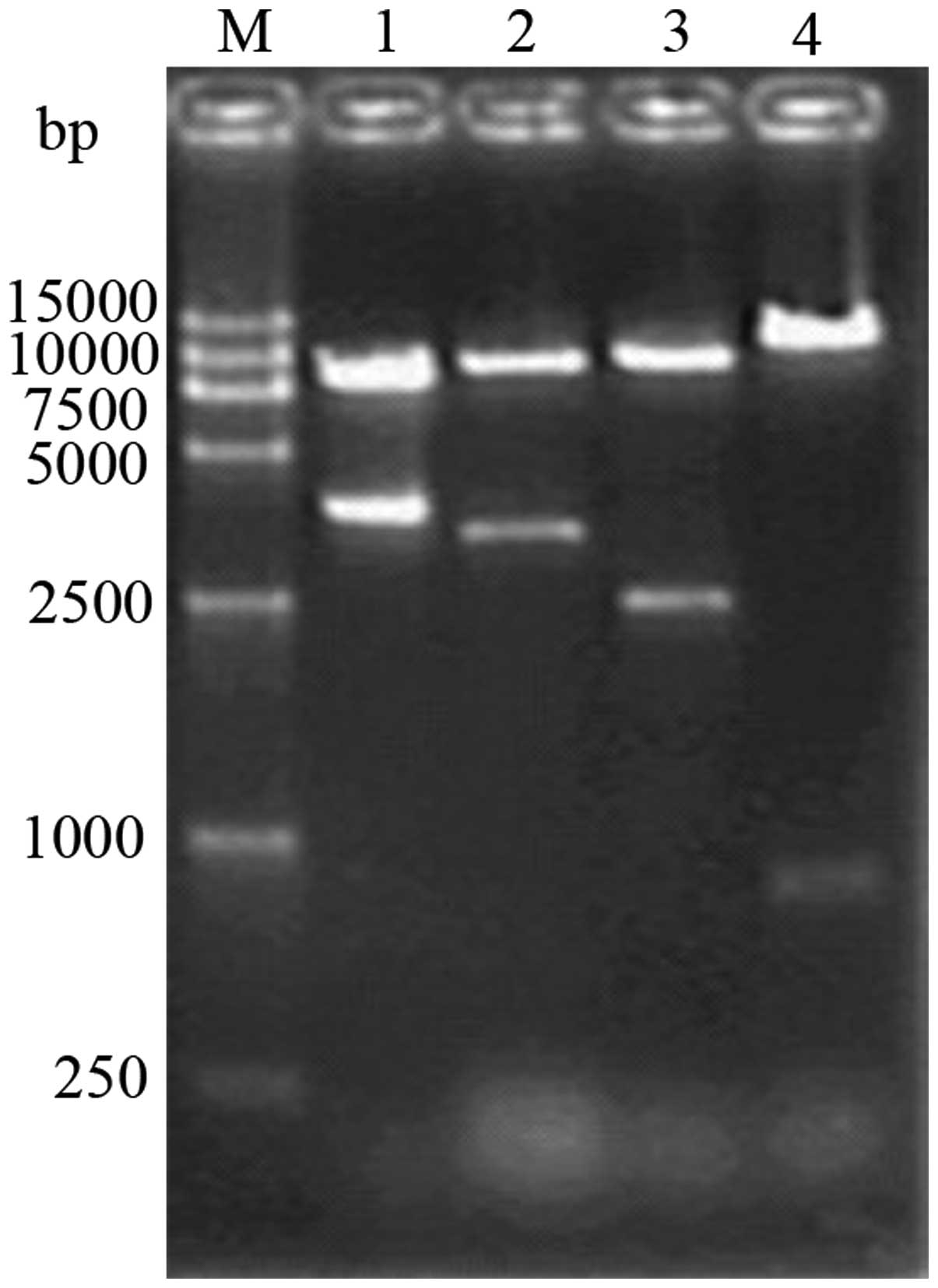
the pCEP4 vector by enzyme digestion using BglII, 1% agarose
gel electrophoresis revealed clear bands at 753 and 9,435 bp
(Fig. 4) The fragments obtained by
double digestion of the pCEP4(-PCMV) plasmid using NotI and
XhoI were recovered and linked with the recovered fragments
from pINV. The resulting plasmid was then introduced into cells and
the cells positive for the recombinant pCEP4/pINV(-PCMV) plasmid
were selected. The cells were identified as positive for the
plasmid by single digestion using BglII, yielding a fragment
of 11.9 kb and double digestion using NotI and XhoI,
and NotI and BamHI, respectively, yielding fragments
of 2,474 bp and 9,435 bp in size, respectively, on 1% agarose gel
electrophoresis, which was consistent with the expected fragment
size (Fig. 5).
Construction and identification of the
recombinant pINV-HPV16E6/7 plasmid
Following the double digestion of the pGEM-T-E6/7
plasmid, the E6/7 fragments were recovered and linked with the
recovered pCEP4/pINV(-PCMV). The product was then introduced into
cells and the cells positive for the plasmids were selected. The
plasmids were identified by single and double digestion using
SalI, NotI and BamHI, and NotI and
XhoI, respectively. On 1% agarose gel electrophoresis, the
XhoI and BamHI double digestion yielded visible bands
at 8.9, 3.8, 9.5, 3.2, 10.2, 2.5 and 11.9 kb, and 780 bp (Fig 6), which were consistent with the
expected fragment sizes.
Identification of the tissue-specific
pINV
The recombinant pINV-HPV16E6/7 plasmid was
transfected into cells derived from various tissues. RT-PCR and 1%
agarose gel electrophoresis identified that the HPV16E6 mRNA was
only expressed in the transfected HeLa cells, but was not expressed
in the other cells (Fig. 7).
Discussion
pINV is a tissue-specific promoter that is ~2474 bp
in length and possesses two important regulatory regions, a distal
regulatory region (DRR) and a proximal regulatory region (PRR).
There are five binding sites (from AP1-1 to AP1-5) in these
regions, and the existence of these sites is closely associated
with the activity of the promoter and the subsequent gene
transcription levels (15). The DRR
(-2474/-1953 bp) mainly regulates downstream gene expression in
keratinocyte cells near the surface and the PRR (-986/-41 bp)
mainly regulates downstream gene expression in the inner layer of
keratinocyte cells. The -1953/-986 bp region is mainly associated
with the different expression model of downstream genes (5–13). HPV
is a type of DNA virus and its rate varies according to ethnicity
(16). The virus is also
tissue-specific and is associated with human skin and mucous
membrane tumors. In recent years, sexually transmitted diseases and
cervical cancer caused by HPV infection have exhibited an ascendant
trend, and diseases associated with HPV infection are difficult to
cure (17). Currently, gene therapy
has become an important method for cancer treatment and targeted
gene therapy is the hot spot, linking the therapeutic gene with a
tissue-specific promoter (18). A
previous study has revealed that tissue-specific promoters play an
important role in gene therapy for prostate cancer (19), and the specific expression of the
therapeutic gene in the target tissue can reduce the side effects
and improve the treatment effects. Establishing a skin-specific
promoter would have a role in the treatment of HPV infection
diseases by directing the therapeutic gene to the target skin and
mucous membrane. In the present study, the eukaryotic vector pCEP4
was used as a skeleton and the tissue-specific pINV was inserted
following the removal of the CMV promoter. Subsequently, the HPV16
E6/7 gene was inserted downstream of the pINV, following the
removal of the cancer-causing section, resulting in the
pINV-HPV16E6/7 recombinant plasmid. The tissue-specificity of the
pINV was judged through the detection of HPV16 E6/7 expression in
various types of cells. The recombinant pINV-HPV16E6/7 plasmid was
transfected into brain glioma, SP2/0, L929 and HeLa cells. RT-PCR
and 1% agarose gel electrophoresis revealed that mRNA was only
expressed in the transfected HeLa cells and that there was no
expression in the other cells. In conclusion, pINV in the
recombinant pINV-HPV16E6/7 plasmid is expressed in a skin-specific
manner. At present, no effective method for the prevention and
tratement of HPV infection has been identified. Thus, the
development of an effective vaccine against HPV may lead to a
decrease in the morbidity and mortality rates of cervical cancer.
However, it is difficult to obtain a large number of HPV viral
particles in vitro to produce the traditional dead or
attenuated viral vaccines, which has subsequently limited the
development of HPV vaccines. The E6 and 7 proteins are considered
ideal target antigens for therapeutic HPV vaccines, however, they
exhibit carcinogenicity. In the present study, the carcinogenic
fraction of the PV16E6/7 gene was removed and a pINV-HPV16E6/7
recombinant plasmid with skin tissue specificity was constructed.
Future in vivo studies, which investigate whether this
recombinant plasmid induces humoral and cellular immune responses
are required.
References
|
1
|
Al-Hendy A, Magliocco AM, Al-Tweigeri T,
Braileanu G, et al: Ovarian cancer gene therapy: repeated treatment
with thymidine kinase in an adenovirus vector and ganciclovir
improves survival in a novel immunocompetent murine model. Am J
Obstet Gynecol. 182:553–559. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoff-Khalili MA, Dall P and Curiel DT:
Gene therapy for carcinoma of the breast. Cancer Gene Ther.
13:633–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das S, E1-Deiry WS and Somasundaram K:
Efficient growth inhibition of HPV 16 E6-expressing cells by an
adenovirus-expressing p53 homologue p73beta. Oncogene.
22:8394–8402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu W, Putral L, Hengst K, et al:
Inhibition of cervical cancer cell growth in vitro and in vivo with
lentiviral-vector delivered short hairpin RNA targeting human
papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 13:1023–1032.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alencar TR, Cerqueira DM, da Cruz MR, et
al: New HPV-16 European and non-European variants in central
Brazil. Virus Genes. 35:1–4. 2007. View Article : Google Scholar
|
|
6
|
Kim DH, Kim EM, Lee EH, et al: Human
papillomavirus 16E6 suppresses major histocompatibility complex
class I by upregulating lymphotoxin expression in human cervical
cancer cells. Biochem Biophys Res Commun. 409:792–798. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scheffner M and Whitaker NJ: Human
papillomavirus-induced carcinogenesis and the ubiquitin-proteasome
system. Semin Cancer Biol. 13:59–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song S, Liem A, Miller JA and Lambert PF:
Human papillomavirus types 16 E6 and E7 contribute differently to
carcinogenesis. Virology. 267:141–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, McKalip A and Herman B: Human
papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human
keratinocytes to apoptosis induced by chemotherapeutic agents:
roles of p53 and caspase activation. J Cell Biochem. 78:334–349.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tran NQ and Crowe DL: Regulation of the
human involucrin gene promoter by co-activator proteins. Biochem J.
381:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tzvetkov MV, Meineke C, Oetijen E,
Hirsch-Ernst K and Brockmöller J: Tissue-specific alternative
promoters of the serotonin receptor gene HTR3B in human brain and
intestine. Gene. 386:52–62. 2007. View Article : Google Scholar
|
|
12
|
Crish JF and Eckert RL: Synergistic
activation of human involucrin gene expression by Fra-1 and p300 -
evidence for the presence of a multiprotein complex. J Invest
Dermatol. 128:530–541. 2008.
|
|
13
|
Adhikary G, Crish J, Lass J and Eckert RL:
Regulation of involucrin expression in normal human corneal
epithelial cells: a role for activator protein one. Invest
Ophthalmol Vis Sci. 45:1080–1087. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kasparek P, Krenek P, Buryova H, et al:
Transgenic mouse model expressing tdTomato under involucrin
promoter as a tool for analysis of epidermal differentiation and
wound healing. Transgenic Res. 21:683–689. 2012. View Article : Google Scholar
|
|
15
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: a meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porto CR, De Oliveira Kleine JP, Longatto
Filho A and Da Silva ID: Polymorphism of Interleukin-6 is not
associated with the presence or absence of high HPV E6/E7.
Anticancer Res. 34:3501–3504. 2014.PubMed/NCBI
|
|
17
|
Miller J, Dakic A, Chen R, Palechor-Ceron
N, et al: HPV16 E7 protein and hTERT proteins defective for
telomere maintenance cooperate to immortalize human keratinocytes.
PLoS Pathog. 9:e10032842013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Li B, Peng C, et al: Inhibition of
cervical cancer cell growth in vitro and in vivo by
lentiviral-vector mediated shRNA targeting the common promoter of
HPV16 E6 and E7 oncogenes. Antiviral Res. 98:305–313. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiming Z, Yong X, Deling K, et al:
Tissue-selective RNA interference in prostate cancer cell using
prostate specific membrane antigen promoter/enhancer. Urol Oncol.
27:539–547. 2009. View Article : Google Scholar
|