Introduction
Free radicals [reactive oxygen species (ROS) and
reactive nitrogen species] are products of normal cellular
metabolism, and are extremely reactive and potentially damaging
transient chemical species. Numerous endogenous metabolic processes
involving redox enzymes and bioenergetic electron transfer generate
free radicals, which aid in the conversion of normal cells to
cancerous cells (1). ROS are able
to affect a number of significant biological molecules, including
DNA, proteins and lipids, leading to a number of degenerative
diseases, including cancer, Alzheimer’s disease, arthritis and
ischemic reperfusion (2). The
oxidative damage to DNA may be reduced by antioxidant-rich diets,
thus preventing the onset of carcinogenesis (3). In addition, the increasing incidence
of cancer and the lack of anticancer drugs has resulted in the
pharmacological and chemical investigation of anticancer agents
obtained from medicinal plants. At present, >100 novel products
obtained from natural sources are in clinical development,
particularly as anticancer agents and anti-infectives (4).
Betelvine (Piper betle) belongs to the
Piperaceae family, which is regarded as a medicinal plant in
Southeast Asia. The leaves of Piper betle have been found to
exhibit wound healing (5),
hepatoprotective (6), antioxidant
and antifertility effects, as well as antimotility effects on
washed human spermatozoa (7). The
primary constituent of the leaves is a volatile oil that contains
phenols, betel-phenol, chavibetol, chavicol, cadinene and
hydroxychavicol, which have been found to exhibit antioxidant and
anticarcinogenic activities (8–10). In
Bangladesh, the tribal population and aborigines chew these leaves
as a narcotic, which causes fainting, profuse sweating and provides
body warmth during winter (7).
The present study was performed to evaluate the
antioxidant and antitumor activity of the methanolic extract of
Piper betle leaves (MPBL) and its organic soluble fractions
against Ehrlich ascites carcinoma (EAC) in mice.
Material and methods
Plant materials
The leaves of Piper betle L. were collected
from Jahangirnagar University campus, (Dhaka, Bangladesh) in
February 2012. The plant material was taxonomically identified by
the National Herbarium of Bangladesh (Dhaka, Bangladesh) and
recorded as voucher specimen no. JU/3334 for future reference.
Chemicals
Bovine serum albumin and bleomycin were obtained
from Sigma-Aldrich (St. Louis, MO, USA). Trichloroacetic acid was
acquired from Merck (Mumbai, India), and thiobarbituric acid (TBA)
and nitroblue tetrazolium chloride were purchased from Loba Chemie
Pvt. Ltd., (Mumbai, India). 5,5′-Dithiobis(−2-nitro benzoic acid),
phenazonium methosulfate, nicotinamide adenine dinucleotide and
reduced glutathione (GSH) were purchased from Sisco Research
Laboratories Pvt., Ltd., (Mumbai, India). All other chemicals and
reagents used were of the highest analytical grade.
Preparation of plant extract
The plant material was shade-dried with occasional
shifting and then ground to a powder using a mechanical grinder,
passed through a #40 sieve (mesh size, 0.425 μm) and stored in an
air-tight container. A total of 1.0 kg of dried powder material was
refluxed with MeOH for 3 h, then the total filtrate was
concentrated until dry in vacuo at 40°C to render the MeOH
extract (240.0 g). The extract was subsequently suspended in
dH2O and successively partitioned with chloroform
(CHCl3) and ethylacetate (EtOAc) to supply the
CHCl3 (90.0 g) and EtOAc (50.0 g) fractions,
respectively, and the H2O residue (100.0 g).
Animals
A total of 121 female, 6–7 week-old, Swiss albino
mice (weight range, 25–30 g) were used to assess biological
activity. The animals were maintained under standard laboratory
conditions and had access to food and water ad libitum. The
animals were acclimatized to the environment for seven days prior
to the experimental procedures. All animal experiments were
performed in accordance with the guidelines of the Institutional
Animal Ethics Committee of Atish Dipankar University of Science and
Technology, Dhaka, Bangladesh. Animal treatment and maintenance for
acute toxicity and anticancer effects were conducted in accordance
with the Principle of Laboratory Animal Care (NIH publication No.
85-23, revised 1985) and the Animal Care and Use Guidelines of
Atish Dipankar University of Science and Technology.
Acute toxicity study
An acute oral toxicity assay was performed using
healthy, non-pregnant, adult female, Swiss albino mice (weight
range, 25–30 g) divided into six different groups. Increasing oral
doses of MPBL (50, 100, 200, 500 and 1,000 mg/kg body weight) in
distilled water were administered at 20 ml/kg to the different test
groups. The normal group received distilled water only. Following
treatment, the mice were allowed to feed ad libitum and
observed for 48 h for any mortality or behavioral changes (11).
Tumor transplantation
EAC cells were obtained from the Indian Institute of
Chemical Biology (Calcutta, India). The EAC cells were maintained
in vivo in Swiss albino mice by intraperitoneal (i.p.)
transplantation of 2×106 cells per mouse every 10 days.
Ascitic fluid was drawn from the EAC tumor-bearing mice at the log
phase (days 7–8 of tumor-bearing) of the tumor cells and each test
animal received 0.1 ml of i.p. tumor cell suspension containing
2×106 tumor cells.
Treatment schedule
The animals were divided into eight groups (n=12)
and provided with food and water ad libitum. All animals in
each group received EAC cells (2×106 cells/mouse i.p.)
with the exception of group-I, which served as the normal saline
control (5 ml/kg body weight i.p.). Group II served as the EAC
control. At 24 h post-EAC transplantation, groups III, IV and V
received MPBL at doses of 25, 50 and 100 mg/kg i.p., respectively.
Groups VI and VII also received CHCl3 (CPBL) and EtOAc
(EPBL) extract at doses of 100 mg/kg i.p., whereas group VIII,
serving as a positive control, received bleomycin (0.3 mg/kg i.p)
for nine consecutive days (12). At
24 h post-administration of the last dose, the animals were fasted
for 18 h, at which point, six animals in each group were sacrificed
by cardiac puncture for the estimation of hematological and serum
biochemical parameters, and to measure antitumor and liver
biochemical parameters. The remainder were provided with food and
water ad libitum and observed to determine if there were any
changes in lifespan. The antitumor activity of the extract was
measured in the EAC animals as described next.
Determination of tumor and packed cell
volume
The mice were dissected and ascitic fluid was
collected from the peritoneal cavity. The tumor volume was measured
using a graduated centrifuge tube and the packed cell volume was
determined by centrifuging the fluid at 1,000 × g for 5 min.
Viable and non-viable tumor cell
count
The ascitic fluid was collected in a white blood
cell (WBC) pipette and diluted 100 times. A drop of the diluted
suspension was then placed on a Neubauer counting chamber
(Celeromics, Cambridge, UK) and the cells were stained with Trypan
blue (0.4% in normal saline). The cells that did not take up the
dye were considered viable, while those that did were considered
non-viable. The viable and non-viable cells were then counted using
the following formula: Cell count = (number of cells × dilution
factor)/(area × thickness of liquid film).
Determination of median survival time
(MST) and percentage increase in life span
The rate of mortality was monitored by recording the
percentage increase in life span (% ILS) and MST according to the
following formula (13): MST in
days = (day of first mortality + day of last mortality)/2.
Estimation of hematological and serum
biochemical parameters
Blood was collected to estimate the hemoglobin (Hb)
content, and red blood cell (RBC) and WBC counts (12). Differential counts of WBCs were
performed from Leishmen-stained blood smears (13). Serum biochemical parameters,
including serum glutamate oxaloacetate transaminase (SGOT), serum
glutamate pyruvate transaminase (SGPT) (14), serum alkaline phosphatase (SALP),
serum bilirubin (15) and total
protein (16) levels were also
estimated.
Estimation of lipid peroxidation
thiobarbituric acid reactive substances (TBARS)
The TBARS in the liver tissue were measured as
described by Ohkawa et al (17) and expressed as μmols of
malondialdehyde (MDA)/g of liver tissues.
Estimation of reduced GSH level
The GSH level of liver tissue was determined as
described by Ellman (18) and
expressed as μg/g of liver tissues.
Estimation of superoxide dismutase (SOD)
and catalase (CAT) levels
The SOD and CAT activity in the liver tissue was
analyzed according to the methods described by Pari and Latha
(19). The SOD activity was
expressed as U/mg of liver tissue and CAT was expressed in terms of
μmol of hydrogen peroxide decomposed/min/mg of liver tissue.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean of three replicate experiments. Statistical
analysis was performed using the SPSS version 16.0 software (SPSS
Inc., Chicago, IL, USA). All in vivo data were assessed
using analysis of variance followed by Dunnett’s test and P<0.05
was considered to indicate a statistically significant
difference.
Results
Acute toxicity studies
Acute toxicity studies are primarily designed to
develop therapeutic indices, for example, the ratio between the
pharmacologically effective dose and lethal dose against the same
strain and species. MPBL was safe at doses as high as 1,000 mg/kg
[per os (p.o.)] body weight, causing no mortality,
behavioral changes, locomotor ataxia, diarrhea or weight loss in
mice during 48 h of observation. Additionally, food and water
intake did not differ among the groups studied (data not
shown).
Tumor growth and survival parameters
MPBL and EPBL at a dose of 100 mg/kg body weight
significantly reduced the body weight, tumor volume, packed cell
volume and viable tumor cell count (Fig. 1), however, the non-viable tumor cell
count was increased compared with the EAC control group (data not
shown). However, CPBL exerted no significant effects at a dose of
100 mg/kg. The MST increased to 22.31±0.11 (% ILS, 10.99),
29.01±0.17 (% ILS, 44.25) and 33.23±0.21 days (% ILS, 65.25)
following the administration of MPBL at doses of 25, 50 and 100
mg/kg body weight, respectively, while the EPBL and the reference
drug, bleomycin, exhibited survival times of 36.23±0.31 (% ILS,
80.15) and 37.60±0.11 days (% ILS, 86.97), respectively (Fig. 1). Finally, the change in body weight
(data not shown) of the animals indicated that Piper betle
extracts had the potential to inhibit tumor growth.
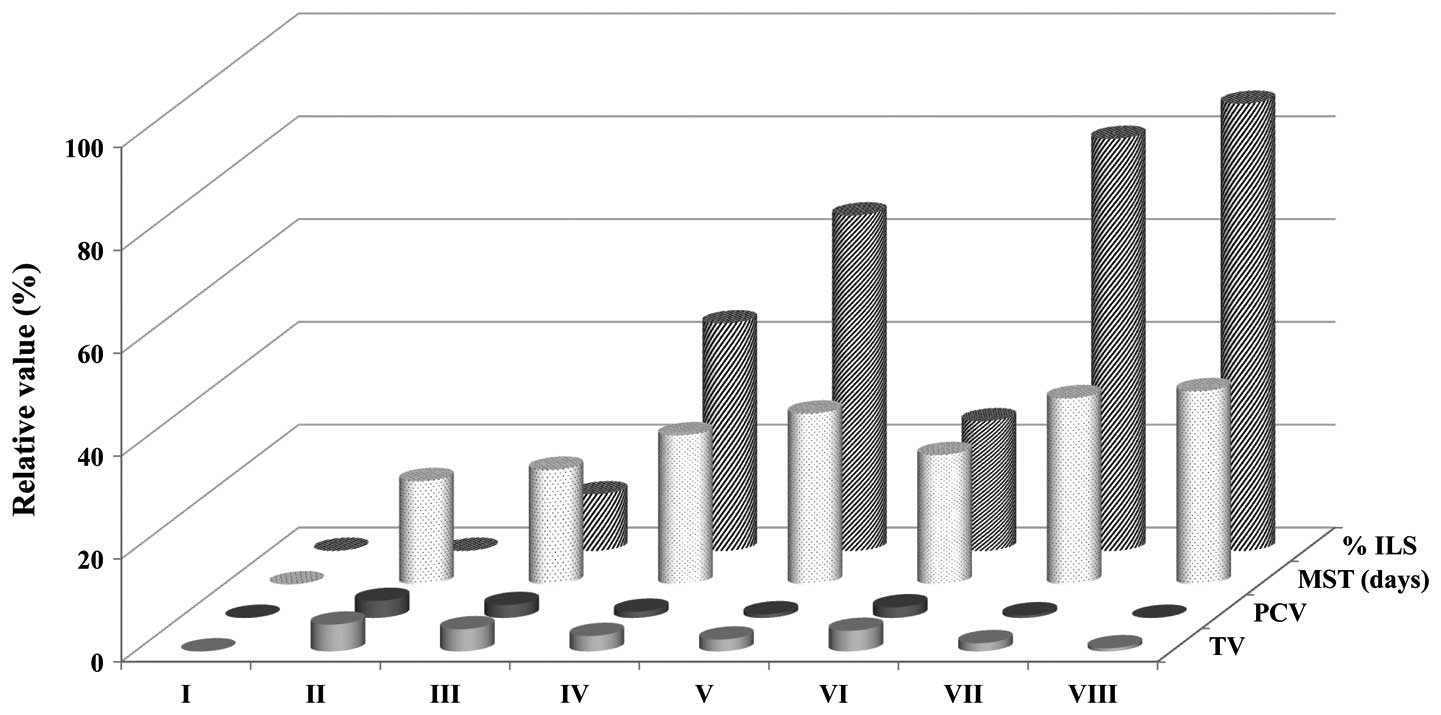 | Figure 1Effects of Piper betle extract
on tumor volume, packed cell volume, MST and % ILS in EAC-bearing
mice. The data are presented as the mean ± standard error of the
mean (n=12 mice per group). *P<0.05 vs. EAC control
group. Group I animals received normal saline (5 ml/kg), whereas
group II animals received EAC control (2×106
cell/mouse), group VIII received bleomycin, 0.3 mg/kg body weight,
and groups III, IV and V, were treated with 25. 50 and 100 mg/kg
body weight (p.o.) of the MPBL, respectively. Groups VI and VII
were treated with 100 mg/kg body weight (p.o.) of the CPBL and
EPBL, respectively. EAC, Ehrlich ascites carcinoma; TV, total
volume; PCV, packed cell volume; MST, mean survival time; % ILS,
percentage increase in life span; p.o., per os; MPBL, methanolic
extract of Piper betle leaves; CPBL, chloroform; EPBL,.
ethylacetate fraction. |
Hematological parameters
The hematological parameters of the tumor-bearing
mice were found to be significantly different compared with the
normal group. The total WBC count increased (P<0.05) and the Hb
content and RBC count decreased in the EAC control animals compared
with the normal saline group. Treatment with MPBL and EPBL at a
dose of 100 mg/kg body weight significantly increased the Hb
content and RBC count towards normal levels (data not shown).
Additionally, the number of neutrophils (Fig. 2A) was increased, while the numbers
of lymphocytes (Fig. 2B) and
monocytes (Fig. 2C) were found to
decrease in the EAC control group compared with the normal group.
These results indicated that treatment with varying doses of
Piper betle extract could significantly change these altered
parameters to near normal values (Fig.
2).
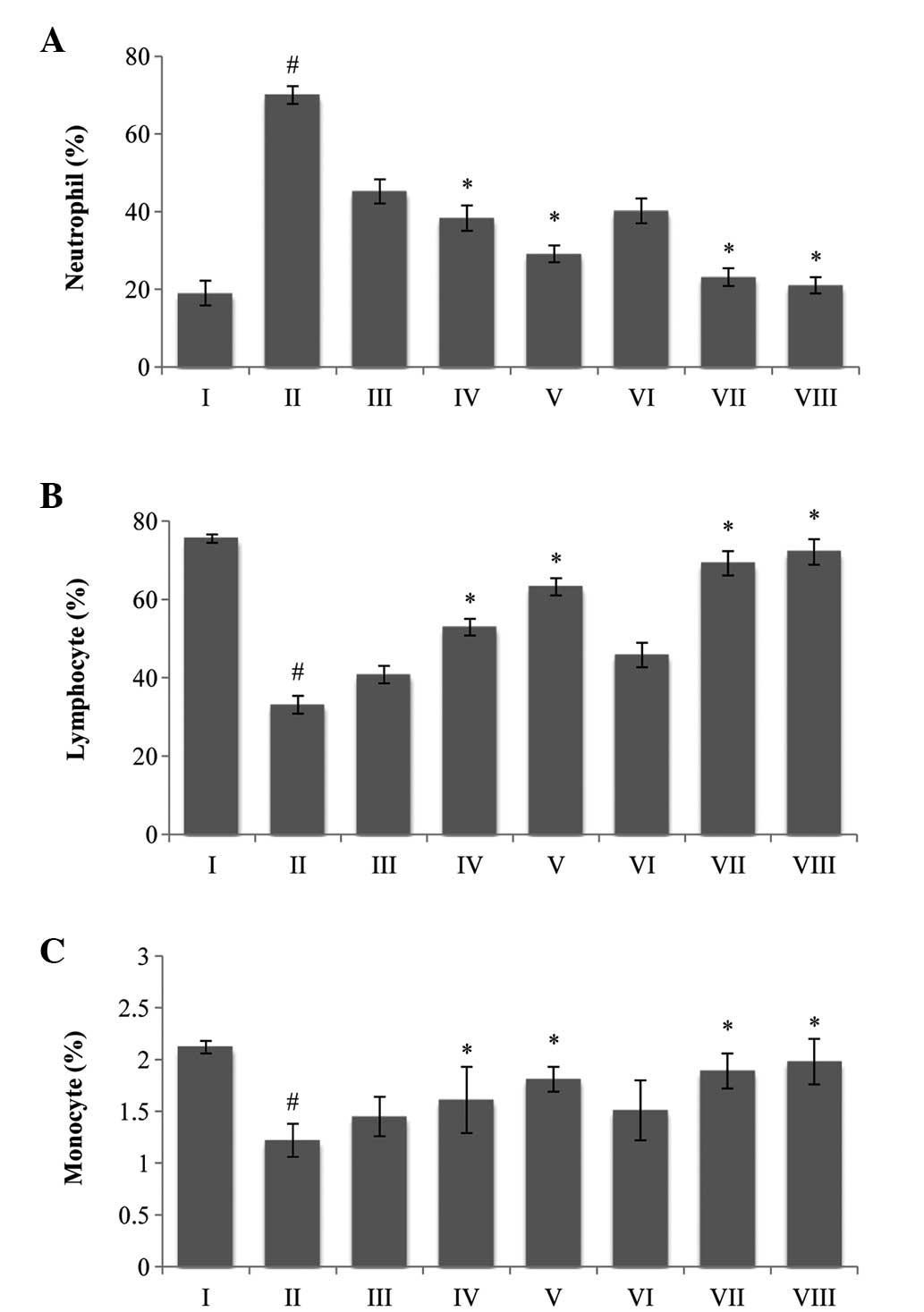 | Figure 2A classical feature of Piper
betle extracts on differential count of WBCs; (A) neutrophils,
(B) lymphocytes and (C) monocytes. The data are presented as the
mean ± standard error of the mean. (n=6 mice per group),
#P<0.05 vs. normal saline group.*P<0.05
vs. EAC control group. Group I animals received normal saline (5
ml/kg), whereas group II received EAC control (2×106
cell/mouse), group VIII received bleomycin, 0.3 mg/kg body weight,
and groups III, IV and V were treated with 25, 50 and 100 mg/kg
body weight (p.o.) of MPBL, respectively. Groups VI and VII were
treated with 100 mg/kg body weight (p.o.) of CPBL and EPBL,
respectively. EAC, Ehrlich ascites carcinoma; p.o., per os;
MPBL, methanolic extract; CPBL, chloroform; EPBL, ethylacetate
fraction. |
Effect on biochemical parameters
As shown in Table I,
the biochemical parameters, including SGOT, SGPT, SALP and
bilirubin levels, in the EAC control group were significantly
upregulated compared with the normal group. Treatment with MPBL and
EPBL at doses of 100 mg/kg significantly decreased the SGOT, SGPT,
SALP and bilirubin levels to approximately normal levels, whereas
CPBL at this dose did not produce the optimum results. The total
protein level was significantly lower in the EAC control group
compared with the normal group (P<0.05). The administration of
MPBL and EPBL at a dose of 100 mg/kg body weight in the EAC-bearing
mice led to a significant increase in total protein level compared
with the EAC control group.
 | Table IEffects of Piper betle on serum
biochemical parameters in EAC-bearing mice. |
Table I
Effects of Piper betle on serum
biochemical parameters in EAC-bearing mice.
| Group, n | SGOT, IU/l | SGP, IU/l | SALP, IU/l | Total protein,
mg/dl | Bilirubin, mg/dl |
|---|
| I | 38.32±1.41 | 28.02±4.32 | 77.91±2.24 | 9.67±0.24 | 0.91±0.19 |
| II | 74.12±1.11a | 66.32±5.32a | 120.11±3.24a | 5.78±0.14a | 3.75±0.12a |
| III | 65.52±3.51b | 52.12±5.12b | 108.31±1.24b | 6.08±0.19b | 2.85±0.75b |
| VI | 42.52±5.11b | 43.32±2.32b | 97.19±5.24b | 6.18±0.34b | 2.15±0.18b |
| V | 38.12±1.01b | 39.39±1.12b | 88.10±1.27b | 6.98±1.34b | 1.75±1.18b |
| VI | 55.52±3.51b | 48.12±5.12b | 101.31±1.24b | 6.78±0.19b | 2.35±0.75b |
| VII | 34.12±1.01b | 36.39±1.12b | 83.10±1.27b | 7.78±1.34b | 1.25±1.18b |
| VIII | 35.13±1.91b | 33.01±1.31b | 73.90±1.92b | 8.38±1.14b | 0.98±1.18b |
Effect on lipid peroxidation and reduced
GSH
Following administration of 100 mg/kg MPBL or EPBL,
or 0.3 mg/kg bleomycin to the EAC-bearing mice, the level of lipid
peroxidation decreased by 91.34±1.10, 82.34±1.10 and 167.21±1.04
μM/g, respectively, compared with the EAC control group
(166.19±3.06 μM/g of wet liver tissue; P<0.05; Fig. 3A). Reduced GSH levels (39 μg/g of
wet liver tissue) were found to be significantly elevated towards
the normal level upon administration of MPBL and EPBL at 100 mg/kg
compared with the EAC control group (P<0.05; Fig. 3B).
 | Figure 3Effect of Piper betle on (A)
lipid peroxide, (B) reduced glutathione, (C) superoxide dismutase
and (D) catalase levels in EAC-bearing mice. The data are presented
as the mean ± standard error of the mean (n=6 mice per group),
#P<0.05 vs. normal saline group.*P<0.05
vs. EAC control group. Group I animals received normal saline (5
ml/kg), whereas group II received EAC control (2×106
cell/mouse), group VIII received bleomycin, 0.3 mg/kg body weight,
and groups III, IV and V were treated with 25, 50 and 100 mg/kg
body weight (p.o.) of MPBL, respectively. Groups VI and VII were
treated with 100 mg/kg body weight (p.o.) of CPBL and EPBL,
respectively. EAC, Ehrlich ascites carcinoma; p.o., per os;
MPBL, methanolic extract; CPBL, chloroform; EPBL, ethylacetate
fraction. |
Effect on SOD and CAT
The administration of MPBL at a dose of 25, 50 and
100 mg/kg markedly increased the levels of SOD and CAT in a
dose-dependent manner (P<0.05; Fig.
3C and D) compared with the EAC control group. By contrast,
EPBL exhibited almost the same activity as standard bleomycin for
the two parameters (Fig. 3).
Discussion
Initially reported as a spontaneous murine mammary
adenocarcinoma, the Ehrlich tumor can be grown in the majority of
mouse strains and is accepted as a transplantable tumor model to
examine the antitumor effects of a number of substances (20).
In the present study, a rapid increase in ascitic
tumor volume was observed in EAC tumor-bearing mice and the
treatment with Piper betle extracts reduced the
intraperitoneal tumor burden, thereby reducing the tumor volume,
tumor weight and viable tumor cell count, while increasing the life
span of the tumor-bearing mice. Therefore, it may be hypothesized
that the increase in lifespan of EAC-bearing mice in response to
MPBL and EPBL at 100 mg/kg may be due to a decrease in nutritional
fluid volume and a delay in cell division (12). Reductions in viable cell count and
increased non-viable cell count towards normal in tumor hosts
indicate antitumor effects against EAC cells in mice. These results
indicate that MPBL and EPBL have a direct association with tumor
cells at higher doses as they absorb the anticancer drug by direct
absorption in the peritoneal cavity, resulting in lysis of the
cells via a direct and cytotoxic mechanism. Anemia and
myelosuppression have frequently been observed in ascites carcinoma
due to a deficiency in iron, in hemolytic or myelopathic
conditions, resulting in a reduced number of RBCs (21). In the present study, treatment with
Piper betle extracts returned the hemoglobin content and RBC
and WBC counts to almost normal levels (data not shown), indicating
that the extracts exhibit hematopoietic protecting activity without
myelotoxicity, the most common side-effect of cancer
chemotherapy.
A preliminary phytochemical study indicated the
presence of alkaloids, steroids, tannins, phenolic and flavonoid
compounds and glycosides in crude extracts of Piper betle
(7). A number of studies have
indicated that the presence of steroids, terpenoids and phenolic
compounds, including coumarins, tannins and flavonoids, exert a
chemopreventive role in the progression of cancer by affecting
signal transduction in cell proliferation and angiogenesis
(22). Incorporation of
phytosterols into the cell membrane can alter the fluidity of
membranes and the activity of membrane-bound enzymes. In addition,
phytosterols cause alterations in pathway signal transduction,
resulting in the growth of tumors and the stimulation of apoptosis
in tumor cell lines. Phytosterols have been demonstrated to enhance
the in vitro proliferation of human peripheral blood
lymphocytes and T cells, indicating the possible stimulation of the
immune system (23). The marked
anticancer activities of MPBL and EPBL are possibly due to the
presence of alkaloids, phenolic compounds, flavonoids and
terpenoids, and their synergistic effects.
ROS exhibit multiple functions and are involved in
tumor initiation and progression (24). MDA, a free oxygen radical product
formed during oxidative degeneration of cancerous tissues (25) and as the end product of lipid
peroxidation, is a biomarker of oxidative stress that has been
reported to be exhibited at higher levels in cancer tissues than in
non-diseased organs (26). The
results of the present study indicated that the TBARS levels in the
cancerous tissues were higher than those in the normal tissues
(Fig. 3). Treatment with EPBL
inhibited hepatic lipid peroxidation, as indicated by the reduction
of MDA levels toward normal levels, emphasizing the reduction in
free radical production and the subsequent decrease in damage to
the cell membrane and MDA production in the tumor-bearing mice.
Depleted endogenous antioxidant enzyme levels with
enhanced free radical generation have been well documented in
carcinogenesis (27). Numerous
tumor cells with pro-oxidant status promote oxidative stress, which
increases the surviving potential of the cancer cells by inducing
mutations, activating redox signaling and stimulating pro-survival
factors, such as nuclear factor-κB and activator protein-1
(28). GSH, which strongly inhibits
the neoplastic process, is important in the endogenous antioxidant
system. This compound acts mainly as a reducing agent and
detoxifies hydrogen peroxide when GSH peroxidase is present
(29). In the current study, the
GSH levels in the experimental mice were found to be significantly
lower than those in the EAC control mice (Fig. 3). These results revealed that the
antitumor activity of MPBL and EPBL was accompanied by the
enhancement of non-enzymatic antioxidant protection.
It is well-known that cells exhibit enzymatic
antioxidant mechanisms, such as the generation of SOD and CAT,
which are involved in the elimination of free radicals (30). SOD and CAT are involved in the
scavenging of superoxide and hydrogen peroxide. In a previous
study, decreased levels of SOD activity were detected in
EAC-bearing mice in response to the loss of Mn2+-SOD
activity and mitochondria in EAC cells, resulting in a decrease in
the amount of total SOD activity in the liver (31). The inhibition of SOD and CAT
activity as a consequence of tumor growth has also been reported
(32). Similar findings were
observed in the present study on EAC-bearing mice. The
administration of MPBL and EPBL at higher doses increased the SOD
and CAT levels towards normal levels.
Plant-derived extracts with antioxidant potential
have demonstrated cytotoxicity against tumor cells and antitumor
activity in experimental animals (33). The cytotoxic and antitumor activity
of plant-derived products occurs either through the induction of
apoptosis or the inhibition of neovascularization (34). In the present study, higher doses of
MPBL and EPBL dramatically reduced tumor growth and the viability
of the tumor cells, and normalized the hematological and serum
biochemical profiles, increasing the life span compared with the
EAC control mice. MPBL and EPBL treatment improved the endogenous
non-enzymatic and enzymatic antioxidant systems (Fig. 3). The decrease in lipid peroxidation
and the elevation of GSH, SOD and CAT levels in the MPBL- and
EPBL-treated mice indicated the potential of Piper betle
extract as an inhibitor of EAC-induced intracellular oxidative
stress.
In conclusion, Piper betle leaf extracts
exhibited marked antitumor activity against EAC in the mice of the
present study, possibly by modulating lipid peroxidation and
augmenting endogenous antioxidant defense systems. Future studies
are required to investigate the isolation and characterization of
lead compounds responsible for the aforementioned activity of this
plant.
Acknowledgements
The authors would like to thank Professor M. Ekramul
Haque (Department of Pharmacy, Rajshahi University, Rajshahi,
Bangladesh) for providing the EAC cells.
References
|
1
|
Rajkumar V, Guha G and Kumar RA:
Antioxidant and anti-neoplastic activities of Picrorhiza kurroa
extracts. Food Chem Toxicol. 49:363–369. 2011. View Article : Google Scholar
|
|
2
|
Suja KP, Jayalekshmy A and Arumughan C:
Free radical scavenging behavior of antioxidant compounds of sesame
(Sesamum indicum L.) in DPPH* system. J Agric Food Chem.
52:912–915. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyskens FL Jr and Szabo E: Diet and
cancer: the disconnect between epidemiology and randomized clinical
trials. Cancer Epidemiol Biomarkers Prev. 14:1366–1369. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murukami A, Ali AM, Mat-Salleh K,
Koshimizu K and Ohigashi H: Screening for the in vitro
anti-tumor-promoting activities of edible plants from Malaysia.
Biosci Biotechnol Biochem. 64:9–16. 2000. View Article : Google Scholar
|
|
5
|
Santhanam G and Nagarajan S: Wound healing
activity of Curcuma aromatica and Piper betle. Fitoterapia.
61:458–459. 1990.
|
|
6
|
Saravanan R, Prakasam A, Ramesh B and
Pugalendi KV: Influence of Piper betle on hepatic marker enzymes
and tissue antioxidant status in ethanol-treated Wistar rats. J Med
Food. 5:197–204. 2002. View Article : Google Scholar
|
|
7
|
Choudhary D and Kale RK: Antioxidant and
non-toxic properties of Piper betle leaf extract: in vitro and in
vivo studies. Phytother Res. 16:461–466. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhide SV, Zariwala MB, Amonlar AJ and
Azuine MA: Chemo-preventive efficacy of betel leaf extract against
benzo[a]pyrene induced fore-stomach tumors in mice. J
Ethnopharmacol. 34:207–213. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garg SC and Jain R: Biological activity of
the essential oil of Piper betle L. J Essent oil Res. 23:601–606.
1992. View Article : Google Scholar
|
|
10
|
Singh M, Shakya S, Soni VK, et al: The
n-hexane and chloroform fractions of Piper betle L. trigger
different arms of immune responses in BALB/c mice and exhibit
antifilarial activity against human lymphatic filarid Brugia
malayi. Int Immunopharmacol. 9:716–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zahan R, Alam MB, Islam MS, et al:
Anticancer Activity of Alangium salviifolium flower in Ehrlich
ascites carcinoma bearing mice. Int J Cancer Res. 7:254–262. 2011.
View Article : Google Scholar
|
|
12
|
Sur P, Bag SP and Khanam JA:
Choroacetohydroxamic acid as antitumor agent against Ehrlich
ascites carcinoma in mice. Neoplasma. 44:197–201. 1997.
|
|
13
|
Gupta M, Mazumder UK, Kumar RS, et al:
Antitumor activity and antioxidant status of Caesalpinia bonducella
against Ehrlich ascites carcinoma in Swiss albino mice. J Pharmacol
Sci. 94:177–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bergmeyer HU, Scelibe P and Wahlefeld AW:
Optimization of methods for aspirate aminotransferase and alanine
aminotransferase. Clin Chem. 24:58–73. 1978.PubMed/NCBI
|
|
15
|
Malloy HT and Evelyn KA: The determination
of bilirubin with the photometric colorimeter. J Biol Chem.
119:481–490. 1937.
|
|
16
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin-phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
17
|
Ohkawa H, Onishi N and Yagi K: Assay for
lipid peroxidation in animal tissue by thiobarbituric acid
reaction. Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellman GL: Tissue sulfhydryl groups. Arch
Biochem Biophys. 82:70–77. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pari L and Latha M: Protective role of
Scoparia dulcis plant extract on brain antioxidant status and lipid
peroxidation in STZ diabetic male Wistar rats. BMC Compliment
Alternat Med. 4:162004. View Article : Google Scholar
|
|
20
|
Segura JA, Barbero LG and Márquez J:
Ehrlich ascites tumor unbalances splenic cell populations and
reduced responsiveness of T cells to Staphylococcus aureus
enterotoxin B stimulation. Immunomol Lett. 74:111–115. 2000.
View Article : Google Scholar
|
|
21
|
Opare Kennedy D, Kojima A, Hasuma T, et
al: Growth inhibitory effects of green tea extract and
(−)-epigallocatechin in Ehrlich ascites tumor cells involves a
cellular thiol-dependent activation of mitogenic-activated protein
kinases. Chem Biol Interac. 134:113–133. 2001. View Article : Google Scholar
|
|
22
|
Blois MS: Antioxidant determination by the
use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
23
|
Jones PJ and AbuMweis SS: Phytosterols as
functional food ingredients: linkages to cardiovascular disease and
cancer. Curr Opin Clin Nutr Metabol Care. 12:147–151. 2009.
View Article : Google Scholar
|
|
24
|
Valko M, Leibfritz D, Moncol J, et al:
Free radicals and antioxidants in normal physiological functions
and human disease. Int J Biochem Cell Biol. 39:44–84. 2007.
View Article : Google Scholar
|
|
25
|
Valenzuela A: The biological significance
of malondialdehyde determination in the assessment of tissue
oxidative stress. Life Sci. 48:301–309. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yagi K: Lipid peroxides and human
diseases. Chem Phys Lipids. 45:337–351. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
28
|
Seeram NP, Adams LS, Henning SM, et al: In
vitro antiproliferative, apoptotic and antioxidant activities of
punicalagin, ellagic acid and a total pomegranate tannin extract
are enhanced in combination with other polyphenols as found in
pomegranate juice. J Nutr Biochem. 16:360–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haldar PK, Kar B, Bala A, et al: Antitumor
activity of Sansevieria roxburghiana rhizome against Ehrlich
ascites carcinoma in mice. Pharm Biol. 48:1337–1343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rushmore TH and Picket CB: Glutathione
S-transferases, structure, regulation and therapeutic implication.
J Biol Chem. 268:11475–11478. 1993.PubMed/NCBI
|
|
31
|
Marklund SL, Westman NG, Lundgren E and
Roos G: Copper- and zinc-containing superoxide dismutase, manganese
containing superoxide dismutase, catalase, and glutathione
peroxidase in normal and neoplastic human cell lines and normal
human tissues. Cancer Res. 42:1955–1961. 1982.PubMed/NCBI
|
|
32
|
Sun Y, Oberley LW, Elwell JH and
Sierra-Rivera E: Antioxidant enzyme activities in normal and
transformed mice liver cells. Int J Cancer. 44:1028–1033. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumour and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rakshit S, Mandal L, Pal BC, et al:
Involvement of ROS in chlorogenic acid-induced apoptosis of
Bcr-Abl+ CML cells. Biochem Pharmacol. 80:1662–1675.
2010. View Article : Google Scholar : PubMed/NCBI
|

















