Introduction
After lung cancer, gastric cancer is the second most
common cause of cancer-associated mortalities worldwide (1). Despite an overall decline in the
incidence of gastric cancer, the disease remains prevalent in Asian
countries (1,2). At present, the majority of patients
with gastric cancer are diagnosed with late-stage disease, which
unlike the curable early stages, has limited therapeutic strategies
(3). Currently, surgery and
combination chemotherapies confer an overall five-year survival
rate of <24% for patients with advanced gastric cancer (4,5).
Therefore, an understanding of the molecular and genetic factors
that underlie the progression of gastric cancer may enable the
identification of novel gastric biomarkers and potential targeted
therapies.
Prior to metastasizing, tumor cells must complete a
multi-step progression, which includes detachment, local invasion
and motility. Throughout these stages, causative molecules,
including matrix degradation enzymes, can be used as prognostic
factors (6). The matrix
metalloproteinases (MMPs) are a family of enzymes located in the
extracellular milieu of various tissues, and with important roles
in extracellular matrix degradation and angiogenesis during tumor
invasion and metastasis. The overexpression of MMPs can promote
tumor cell detachment and metastasis, which have been associated
with malignancy and a poor clinical outcome for patients (7,8). At
present, there are 26 known MMPs, which share a number of common
structural and functional similarities, but differ in their
substrate specificity (9).
MMP-17 (also known as MT4-MMP) and MMP25 (also known
as MT6-MMP) are held in the plasma membrane by a
glycosyl-phosphatidyl inositol (GPI) anchor, which equips the
enzymes with a set of regulatory and functional mechanisms that
differentiates these subtypes from other members of the MMP family.
Recent studies have demonstrated that GPI-membrane type (MT)-MMPs
are highly expressed in human cancers (10), where they have a role in disease
progression. Furthermore, biochemical and functional evidence also
highlights the distinct properties of the enzymes. The present
study investigated the expression and clinicopathological features
of GPI-MT-MMPs in gastric cancer.
Materials and methods
Tissue samples
In total, 42 tissue samples were obtained from
patients with gastric cancer who had undergone surgery, with no
radiotherapy or chemotherapy, between January 2011 and December
2013, in the Renmin Hospital of Wuhan University (Wuhan, Hubei,
China). The study was approved by the ethics committee of Renmin
Hospital of Wuhan University and written informed consent was
obtained from the patients or the family of the patient. Subsequent
to a physical examination, 42 subjects with normal gastric mucosa
and 40 cases of atrophic gastritis were also enrolled in the study.
Of all the tissue samples taken, one sample from each subject was
immediately fixed in 4% paraformaldehyde solution and embedded in
paraffin for immunohistochemical staining, while another was stored
at −80°C for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) testing.
Immunohistological analysis
In total, 4-μm thick sections of the tissue arrays
were deparaffinized, and antigen retrieval was performed by
microwaving the slides in 7.5 mM sodium citrate buffer (pH 6.0;
Beyotime Institute of Biotechnology, Shanghai, China). Subsequent
to rinsing in Tris-buffered saline (TBS; pH 8.0; Beyotime Institute
of Biotechnology), endogenous peroxidase activity and non-specific
background staining were blocked by incubating the samples for 30
min in 3% hydrogen peroxide (Beyotime Institute of Biotechnology)
in methanol, followed by 30 min in 0.3% bovine serum albumin in
TBS. The slides were then rinsed for 2 min each in TBS, TBS
containing 0.01% Triton X-100 (Beyotime Institute of
Biotechnology), and then TBS again. Next, the slides were incubated
for 1 h at room temperature with rabbit antiserum pAb107 to human
MT4-MMP and MT6-MMP at a dilution of 1:400 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The slides were then
rinsed in TBS and incubated for 30 min with goat anti-rabbit
immunoglobulin G conjugated to a horseradish peroxidase-labeled
polymer (EnVision+ System; Dako, Glostrup, Denmark). Incubation was
followed by additional TBS rinses, visualization with
diaminobenzidine chromogen and counterstaining with hematoxylin.
The negative controls were treated with pre-immune rabbit serum
instead of the primary antibody. The sections were analyzed for
histopathological features by a pathologist blinded to the patient
data. The cell count was obtained using a microscope
(magnification, ×400), and five fields were randomly selected for
each slice, with each specimen represented by three slices. The
expression of MMP-17 and -25 was identified by the percentage of
positive cells and the staining intensity scores. The percentage of
positive cells was ranked according to four grades: i) ≤5%, 0
points; ii) 6–25%, 1 point; iii) 26–50%, 2 points; and iv) >50%,
3 points. The staining intensity scoring criteria was as follows:
i) no staining, 0 points; ii) weak staining (pale-yellow), 1 point;
iii) moderate staining (brown), 2 points and; iv) strong staining
(brown), 3 points. The sum of the two ratings was scored
semi-quantitatively from zero to six as follows: 0, negative; 1–2,
weak staining; 3–4, moderate staining; and 5–6, strong staining.
For the negative control group, phosphate-buffered saline was used
as an alternative to the primary antibody.
RT-qPCR
The total RNA was extracted from the gastric
carcinoma, atrophic gastritis and normal gastric tissues with
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
and cDNA synthesis was performed using the Transcriptor First Stand
cDNA Synthesis kit (Roche, Basel, Switzerland) using 2 μg total
RNA. The RT-qPCR was performed with the LightCycler 480 SYBR Green
I Master (Roche) using the LightCycler 480 Real-Time PCR System,
according to the manufacturer’s instructions (Roche). Using the
published cDNA sequence (GenBank Accession no. AF219624), primers
were designed to amplify a product of human MMP17 [forward, 5′-GGT
GCG TGC ACT CAT GTA CT-3′; and antisense, 5′-TCA TCG TCA AAG TGG
GTG TC-3′ (product length, 216 bp)], MMP-25 [forward, 5′-CCC AAA
CCC CAT ATG ACA AG-3′; and antisense 5′-GGG GCC TTT GAA GAAGAA
AG-3′ (product length, 164 bp)] and β-actin [forward, 5′-CAC GAT
GGA GGG GCC GGA CTC ATC-3′; and antisense, 5′-TAA AGA CCT CTA TGC
CAA CAC AGT-3′ (product length, 240 bp)]. The following PCR
conditions were used: Initial denaturation at 95°C for 10 sec,
denaturation for 40 cycles at 95°C for 10 sec, annealing at 60°C
for 10 sec and extension at 72°C for 20 sec. The relative
expression levels of mRNA were calculated as ratios to the
reference gene, β-actin.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. χ2 test was used to analyze the clinical
and pathological characterstics of the patients. The statistical
significance between groups was determined using a two-tailed
Student’s t-test or one-way analysis of variance. χ2 and
t: the comparation of normal gastric tissue and atrophic gastritis;
χ12 and t1: the comparation of
normal gastric tissue and gastric cancer; χ22
and t2: the comparation of gastric cancer and atrophic
gastritis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Stage, grade and location of gastric
cancer
In total, 124 patients with gastric cancer, atrophic
gastritis or normal gastric tissues were included in the present
study. The mean age of the patients was 54 years, and 67% of the
participants were male. According to the TNM classification of
malignant tumors developed by the American Joint Committee on
Cancer Classification, the Japanese Gastric Cancer Research and the
International Union Against Cancer (11), the stages, histological grade and
location of the 42 cases of gastric cancer were as follows: i) T1,
18 cases; T2–T4, 24 cases; N0, 22 cases; and Nl–N3, 20 cases; ii)
grade I, 13 cases; and grade II–III, 29 cases; and iii) antrum, 16
cases; the gastric body, 19 cases; and the gastric cardia, 19
cases.
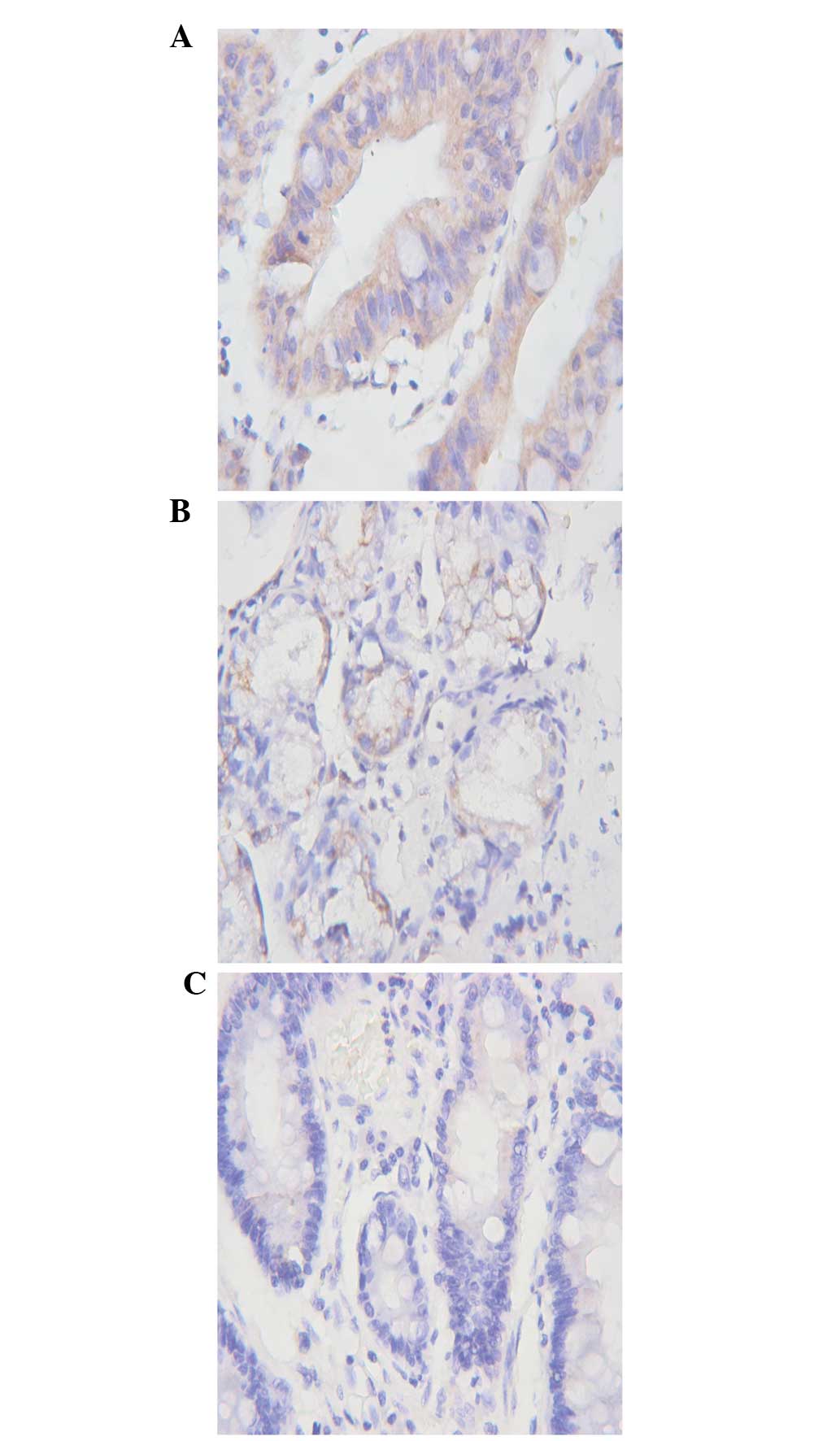
Expression of MMP17 in gastric cancer,
atrophic gastritis and normal gastric tissues
The expression of MMP17 protein in the cytoplasm was
identified by pale-yellow, brown or tan staining. The
MMP17-positive cells had a scattered or nest-like distribution in
the gastric cancer tissues, and were markedly expressed on the edge
of the cancer nest (Fig. 1A). In
addition to the cancer cells, MMP17-positive staining was also
observed in nearby cancer stromal cells, which suggested that
stromal cells have an important role in the process of tumor
invasion and metastasis. The expression of MMP17 in atrophic
gastritis and normal gastric tissues is presented in Fig. 1B and C. No significant difference
was identified between the expression of MMP17 in the normal tissue
and atrophic gastritis specimens (3/42 and 4/40 cases,
respectively; χ2=0.21; P>0.05). However, the
expression of MMP17 in the gastric cancer specimens was
significantly higher than that in the normal and atrophic gastritis
tissues (31/42 cases; χ12=38.74;
χ22=34.10; P<0.05). No significant
difference was identified between the mRNA expression of MMP17 in
the normal gastric and atrophic gastritis tissues (0.754±0.074 and
1.226±0.082, respectively; t=0.602; P>0.05), however, an evident
difference was observed in the gastric cancer tissues
(12.126±0.743; t1 8.079; t2=4.493; all
P<0.05) (Fig. 2).
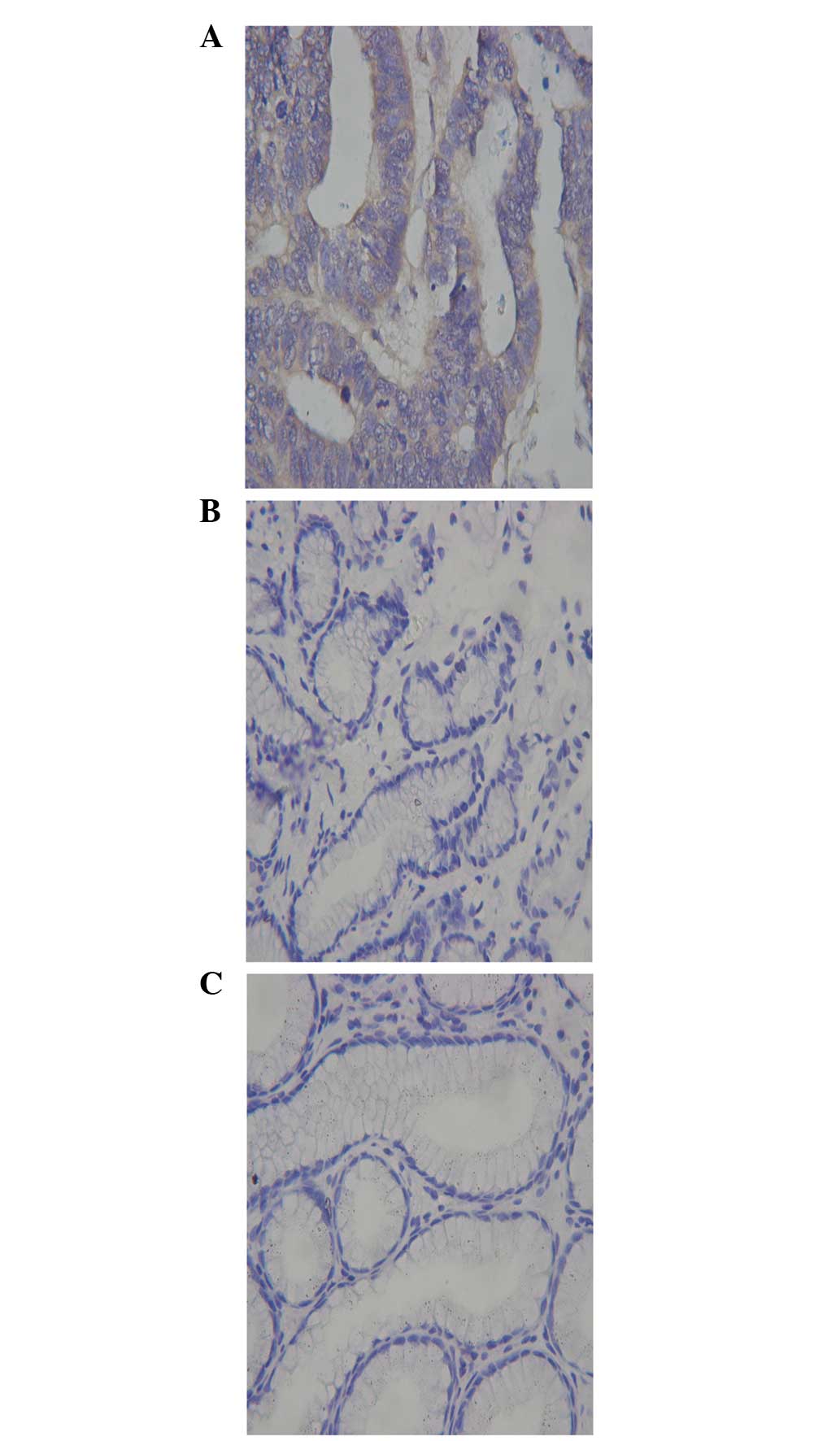
Expression of MMP25 in gastric cancer,
atrophic gastritis and normal gastric tissues
MMP25 was expressed in the normal gastric, atrophic
gastritis and gastric carcinoma tissues. However, MMP25-positive
staining was significantly higher in the gastric cancer and
atrophic gastritis tissues (40/42 and 33/40 cases, respectively),
than in the normal gastric tissues (9/42 cases;
χ12=44.08; χ2=28.19; P<0.05).
Furthermore, no significant difference was identified between the
expression of MMP25 in the atrophic gastritis and gastric cancer
tissues (χ22=2.223; P>0.05) (Fig. 3). The expression of MMP25 mRNA in
the normal gastric tissues was significantly lower than that in the
atrophic gastritis and gastric carcinoma tissues (0.703±0.014,
6.175±0.702 and 7.328±1.235, respectively; t=7.149,
t1=6.123; P>0.05). In addition, no significant
difference was identified between the atrophic gastritis and
gastric carcinoma tissues (t2=0.602; P>0.05)
(Fig. 4).
MMP17 and clinicopathological
features
The present study demonstrated that MMP17 protein
and mRNA expression was associated with the depth of tumor
invasion, lymph node metastasis and serosal involvement of the
gastric cancer patients (P<0.05), but not with age, gender,
lesion length or histological grade (P>0.05; Table I). The expression of MMP17 in
advanced gastric carcinoma was revealed to be higher than that in
early-stage disease (21/24 and 10/18 cases; t=2.437; P<0.05).
Furthermore, it was identified that MMP17 expression was elevated
in patients with lymph node metastasis and serosal involvement.
 | Table IAssociation between matrix
metalloproteinase-17 and -25 protein and mRNA expression and
clinical pathological characteristics. |
Table I
Association between matrix
metalloproteinase-17 and -25 protein and mRNA expression and
clinical pathological characteristics.
| Clinical pathological
features | n | MMP17 protein | P-value | MMP17 mRNA | P-value | MMP25 protein | P-value | MMP25 mRNA | P-value |
|---|
|
|
|---|
| − | + | % | − | + | % |
|---|
| Age, years | | | | | | | | | | | | | |
| ≤60 | 23 | 6 | 17 | 73.91 | | 0.483±0.031 | | 5 | 18 | 78.26 | | 0.304±0.003 | |
| >60 | 19 | 5 | 14 | 73.78 | 0.987 | 0.469±0.029 | 0.209 | 1 | 18 | 94.74 | 0.205 | 0.311±0.007 | 0.288 |
| Gender | | | | | | | | | | | | | |
| Male | 25 | 6 | 19 | 76.00 | | 0.484±0.030 | | 5 | 20 | 80.00 | | 0.346±0.005 | |
| Female | 17 | 5 | 12 | 70.59 | 0.699 | 0.472±0.028 | 0.527 | 1 | 16 | 94.12 | 0.263 | 0.370±0.001 | 0.312 |
| Size, cm | | | | | | | | | | | | | |
| ≤2 | 20 | 6 | 14 | 70.00 | | 0.482±0.034 | | 3 | 17 | 85.00 | | 0.315±0.003 | |
| >2 | 22 | 5 | 17 | 77.27 | 0.597 | 0.470±0.037 | 0.312 | 3 | 19 | 86.36 | 0.256 | 0.308±0.002 | 0.114 |
| Depth of
invasion | | | | | | | | | | | | | |
| T1 | 18 | 8 | 10 | 55.56 | | 0.398±0.029 | | 1 | 17 | 94.44 | | 0.309±0.009 | |
| T2–T4 | 24 | 3 | 21 | 87.50 | 0.021 | 0.513±0.043 | 0.009 | 5 | 19 | 79.17 | 0.986 | 0.536±0.002 | 0.006 |
| Histological
grading | | | | | | | | | | | | | |
| I | 20 | 6 | 14 | 70.00 | | 0.477±0.028 | | 3 | 17 | 85.00 | | 0.426±0.006 | |
| II–III | 22 | 5 | 17 | 77.27 | 0.597 | 0.529±0.038 | 0.195 | 3 | 19 | 86.36 | 0.256 | 0.418±0.005 | 0.102 |
| Lymph node
metastasis | | | | | | | | | | | | | |
| N0 | 22 | 9 | 13 | 59.09 | | 0.403±0.035 | | 5 | 17 | 77.27 | | 0.337±0.002 | |
| N1–N3 | 20 | 2 | 18 | 90.00 | 0.025 | 0.523±0.022 | 0.010 | 1 | 19 | 95.00 | 0.011 | 0.552±0.004 | 0.003 |
| Location | | | | | | | | | | | | | |
| Antral | 16 | 4 | 12 | 75.00 | | 0.480±0.041 | | 2 | 14 | 87.50 | | 0.478±0.014 | |
| Body | 19 | 6 | 13 | 68.42 | | 0.469±0.032 | | 3 | 16 | 84.21 | | 0.435±0.005 | |
| Cardia | 7 | 1 | 6 | 85.71 | 0.756 | 0.486±0.034 | 0.691 | 1 | 6 | 85.71 | 0.454 | 0.426±0.006 | 0.214 |
| Serosal
involvement | | | | | | | | | | | | | |
| No | 19 | 8 | 11 | 57.89 | | 0.432±0.034 | | 5 | 14 | 73.68 | | 0.326±0.017 | |
| Yes | 23 | 3 | 20 | 86.96 | 0.035 | 0.512±0.038 | 0.029 | 1 | 22 | 95.65 | 0.001 | 0.488±0.015 | 0.043 |
| Survival time,
years | | | | | | | | | | | | | |
| <2 | 10 | 7 | 3 | 30.00 | | 0.490±0.031 | | 5 | 5 | 50.00 | | 0.326±0.075 | |
| ≥2 | 32 | 4 | 28 | 87.50 | 0.005 | 0.522±0.028 | 0.018 | 1 | 31 | 96.88 | 0.001 | 0.518±0.023 | 0.001 |
MMP25 and clinicopathological
features
No significant difference in the expression of MMP25
between advanced gastric carcinoma and early-stage disease (17/20
and 19/22 cases, t=0.101; P>0.05) was identified. The MMP25
protein and mRNA expression was associated with the depth of tumor
invasion, lymph node metastasis and serosal involvement of the
gastric cancer patients (P<0.05), but not with age, gender,
lesion length or histological grade (P>0.05; Table I).
Discussion
The present study compared the expression of MMP17
and MMP25 in gastric carcinoma, atrophic gastritis and normal
gastric tissues. The expression of MMP17 in the normal gastric and
atrophic gastritis tissues was significantly lower than that
observed in the gastric cancer tissues. MT-MMPs are efficient,
pericellular, proteolytic enzymes that are presented at the cell
surface by membrane anchoring domains. MMP-17 and MMP-25 are
attached to the plasma membrane via a GPI anchor. This equips the
enzymes with distinct functional and regulatory properties that
distinguish MMP-17 and MMP-25 from other members of the MT-MMP
subfamily. Despite their discovery almost a decade ago, studies
conducted on GPI-MT-MMPs are limited compared with other MT-MMPs.
However, recent evidence (12–14)
has revealed that GPI-MT-MMP expression is elevated in human
cancers. The data from the present study demonstrated that the
GPI-MT-MMPs MMP17 and MMT25, similar to other MT-MMPs, are highly
expressed in gastric carcinoma. In addition, the fact that MMP25 is
highly expressed in atrophic gastritis suggests that it may be
involved in the early stage of tumor development. The variety of
physical properties of GPI-MT-MMPs encourages further study to
determine their involvement in the development of tumors.
The clinicopathological features were closely
associated with the prognosis of cancer (15,16).
The association between the expression of MMP17 and MMP25 and
disease clinicopathological features was investigated in the
present study. It was identified that the expression of MMP17 and
MMP25 was significantly associated with the depth of tumor
invasion, lymph node metastasis and serous membrane involvement,
but not with patient age and gender, or lesion length, site and
histological grade. This observation was in accordance with other
MMPs (17–20). Since the depth of tumor invasion,
lymph node metastasis and serous membrane involvement were closely
associated with tumor progression, MMP17 and MMP25 were associated
with tumor progression. Furthermore, it was demonstrated that
GPI-MT-MMPs, in addition to other MT-MMPs, play a significant role
in tumor progression. However, their contribution to the
development of gastric carcinoma is unclear, and requires further
investigation.
In conclusion, the expression of MMP17 and MMP25 was
increased in the gastric cancer tissues in the present study.
Furthermore, the detection of MMP17 may be of clinical value for
the prognosis of patients with gastric cancer. The present study
included a limited number of cases and was a single-center study.
Therefore, further analysis is required to determine whether MMP17
expression in gastric cancer exhibits regional differences. In
addition, as gastric cancer is a multi-factorial and multi-linkage
disease, the specific role of MMP17 in disease progression warrants
further investigation.
Acknowledgments
The authors would like to thank Dr He-sheng Luo for
providing support by proofreading the manuscript. Some of the study
results were previously presented at Asian Pacific Digestive Week
2012 and published as poster P20-19 in J Gastroenterol Hepatol 27
(Suppl 5): 408, 2012.
References
|
1
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.PubMed/NCBI
|
|
2
|
Naylor GM, Gotoda T, Dixon M, Shimoda T,
Gatta L, Owen R, Tompkins D and Axon A: Why does Japan have a high
incidence of gastric cancer? Comparison of gastritis between UK and
Japanese patients. Gut. 55:1545–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun W and Haller DG: Recent advances in
the treatment of gastric cancer. Drugs. 61:1545–1551. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasui W, Oue N, Aung PP, et al:
Molecular-pathological prognostic factors of gastric cancer: a
review. Gastric Cancer. 8:86–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng CW, Liu XL, Liu X and Li Y:
Co-evolution of cancer microenvironment reveals distinctive
patterns of gastric cancer invasion: laboratory evidence and
clinical significance. J Transl Med. 8:1012010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sohail A, Sun Q, Zhao H, Bernado MM, Cho
JA and Fridman R: MT4-(MMP17) and MT6-MMP (MMP25), A unique set of
membrane-anchored matrix metalloproteinases: properties and
expression in cancer. Cancer Metastasis Rev. 27:289–302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung H, Lee HH, Song KY, et al: Validation
of the seventh edition of the American Joint Committee on Cancer
TNM staging system for gastric cancer. Cancer. 117:2371–2378. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nuttall RK, Pennington CJ, Taplin J, et
al: Elevated membrane-type matrix metalloproteinases in gliomas
revealed by profiling proteases and inhibitors in human cancer
cells. Mol Cancer Res. 1:333–345. 2003.PubMed/NCBI
|
|
13
|
Wallard MJ, Pennington CJ,
Veerakumarasivam A, et al: Comprehensive profiling and localisation
of the matrix metalloproteinases in urothelial carcinoma. Br J
Cancer. 94:569–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riddick AC, Shukla CJ, Pennington CJ, et
al: Identification of degradome components associated with prostate
cancer progression by expression analysis of human prostatic
tissues. Br J Cancer. 92:2171–2180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saeki H, Oki E, Tsuda Y, et al: Relevance
of totally laparoscopic gastrectomy for patients with advanced
gastric cancer. Fukuoka Igaku Zasshi. 104:405–412. 2013.
|
|
16
|
Seo JH, Jeong ES and Choi YK: Therapeutic
effects of lentivirus-mediated shRNA targeting of cyclin D1 in
human gastric cancer. BMC Cancer. 14:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Merdad A, Karim S, Schulten HJ, et al:
Expression of matrix metalloproteinases (MMPs) in primary human
breast cancer: MMP-9 as a potential biomarker for cancer invasion
and metastasis. Anticancer Res. 34:1355–1366. 2014.PubMed/NCBI
|
|
18
|
Dey S, Ghosh N, Saha D, et al: Matrix
metalloproteinase-1 (MMP-1) promoter polymorphisms are well linked
with lower stomach tumor formation in eastern Indian population.
PLoS One. 9:e880402014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fabre B, Filipiak K, Díaz N, et al: An
integrated computational and experimental approach to gaining
selectivity for MMP-2 within the gelatinase subfamily. Chembiochem.
15:399–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Albrechtsen R, Kveiborg M, Stautz D, et
al: ADAM12 redistributes and activates MMP-14, resulting in gelatin
degradation, reduced apoptosis and increased tumor growth. J Cell
Sci. 126:4707–4720. 2013. View Article : Google Scholar : PubMed/NCBI
|