Introduction
Prostate cancer (PCa) is the most common type of
cancer in males. This cancer type has a heterogeneous nature and
the characteristics vary during development. Initially, PCa
develops in the prostate gland and is dependent on the androgen,
testosterone for proliferation, growing slowly. At present, no
optimal treatment has been identified due to the difficulties of
predicting the disease progression (1,2).
However, treatment of PCa is not always required, and the most
common course of action is referred to as ‘watchful waiting’. In
total, ~30% of patients on watchful waiting begin an active
treatment within the first five years following diagnosis; ~66% of
these patients undergo a radical prostatectomy and ~20% receive
external irradiation (2). However,
the applied treatment is guided by the tumor characteristics and
the health status and age of the patient. When the tumor is no
longer localized to the prostate gland and begins to invade
surrounding healthy tissue, the applied treatment is more urgent
and aggressive. The preferable treatment at this stage is a
combination of androgen ablation therapy and local irradiation
(3), which results in a clinically
stable state for the patient, which lasts for 1.5–3 years (4).
External irradiation is a common treatment for PCa,
and novel treatment regimens have been developed in order to
increase the doses received by the tumor, whilst sparing the
surrounding tissues. By using image-guided radiation therapy or
intensity modulated therapy, doses of 78–81 Gy may be administered
while the healthy tissue surrounding the tumor is spared.
Combinations with selected radiation boost regimens such as
brachytherapy, may achieve doses of >116 Gy (5). However, 20–40% of patients receiving
external irradiation therapy develop recurrent and more aggressive
PCa within 10 years (6). The
absence of androgen contributes to the clonal selection of androgen
independent cells. This generates a tumor with an altered
phenotype, which is more aggressive, less responsive to existing
therapies and exhibits a higher metastasizing potential (7). Human epidermal growth factor receptor
type 2 (HER2) is a receptor tyrosine kinase (RTK), which has been
identified in 17–22% of analyzed PCa tissues (depending on the
antibody used for detection) in a large retrospective study
(8). The function of HER2 in an
androgen diminished environment is considered to promote cell
division and suppress apoptosis and thus, the protein expression is
significantly associated with a more advanced disease, tumor stage
and recurrence (9). HER2 is an
essential factor in one of the pathways that allows PCa cells to
survive and proliferate, resulting in the development of
androgen-independent metastatic PCa (10). The overexpression of HER2 in PCa has
the capacity to activate androgen receptors in the absence of
androgens, as well as promote the transcription of prostate
specific antigen (11,12). As discussed, PCa may relapse
following external irradiation treatment and HER2-expressing cells
are hypothesized to activate survival mechanisms as a response to
the treatment, which contributes to higher proliferation and
reduced apoptosis rates (13). This
enables the selection of HER2-expressing cell subpopulations,
leading to a progression towards androgen-independence (14). At present, trastuzumab (Herceptin)
is used clinically for the treatment of HER2-expressing breast
cancer (BCa) and studies, including the use of trastuzumab in
bladder (15), endometrial
(16), peritoneal, ovarian,
pancreatic and stomach neoplasms (17) have been previously reported. In the
current study, the suitability of HER2 as a target for treatment of
PCa alone or in combination with external irradiation was
investigated, and the effect of trastuzumab on PCa cell survival
was analyzed. In addition, patient stratification and therapy
outcome were hypothesized to be significantly influenced by
accurate molecular phenotyping, which may indicate suitable
molecular targets, and lead to the development of appropriate
imaging agents. We further hypothesized that in vivo
molecular imaging of HER2 expression in PCa may contribute to an
improved patient selection, as well as improved therapy
outcomes.
The specific aims of this study were to analyze and
evaluate PCa cell survival, as well as the HER2-expression as an
acute response to external irradiation and anti-HER2 drug
treatment, such as trastuzumab. In total, three cell lines, LNCap
(lymph node metastasis of PCa, androgen and estrogen receptor
positive), PC3 (bone metastasis of PCa, androgen sensitive) and
DU-145 (brain metastasis of PCa, hormone insensitive) were selected
for this study. Together, these cell lines may represent the tumor
heterogeneity due to differences in androgen sensitivity and
aggressiveness. The cell panel was treated with external
irradiation, modeling one of the current therapy modalities for a
localized disease, alone or in combination with a HER2-targeting
drug. Cell survival, as well as membranous expression of HER2 in
response to therapy, was investigated. Trastuzumab, a clinically
approved therapeutic monoclonal antibody (Herceptin), which binds
to the extracellular domain of HER2 and downregulates its
expression (18), was selected for
this study.
Materials and methods
Cell lines and treatment
The cell lines LNCap, PC3 and DU-145 originally from
the American Type Culture Collection (Manassas, VA, USA) were
provided by LGC Standards (Borås, Sweden). The HER2-receptor
expression of the cell lines was evaluated in a previous study
(19). The cells were cultured in
complete RPMI-media, supplemented with 10% fetal bovine serum, 2 mM
L-glutamate, 100 IU/ml penicillin and 100 μg/ml streptomycin. For
LNCap cells, the medium was supplemented with sodium-pyruvate
(Lonza, Verviers, Belgium) and HEPES. All other reagents including
trypsin-EDTA were obtained from Biochrom AG Biotechnologie (Berlin,
Germany). All plastics for cell culturing were obtained from
Corning, Inc. (Corning, NY, USA) for cell cultivation. Cell culture
was performed in a humidified atmosphere of 5% CO2 at
37°C.
Trastuzumab (infusion, 21 mg/ml) was used for in
vitro treatment. The drug was diluted in cell cultivation
medium to 0.05 mg/ml. External irradiation was performed using a
Gammacell 40 Exactor (137Cs γ-ray photon radiation;
Nordion, Ottawa, ON, Canada).
For HER2 quantification the affibody molecule,
Z2395 (Affibody AB, Solna, Sweden), was used.
Radiolabeling of Z2395 with technetium-99m was performed
as described by Ahlgren et al (20). Radioactivity was measured using an
automated γ-counter with a 3-inch NaI (Tl) detector (1480 WIZARD;
PerkinElmer Life Sciences, Waltham, MA, USA). Cells were counted
using an electronic Scepter™ cell counter (Millipore, Billerica,
MA, USA).
Statistical analysis
Student’s t-test was used to evaluate the
significance of changes in proliferation and receptor expression.
*P<0.05 was considered to indicate a statistically
significant difference.
External irradiation and drug
treatment
Cells were treated according to protocol A (Fig. 1). Cells were seeded at a density of
106 cells/well in six-well plates one day prior to the
experiments. The cells were subjected to a 6 Gy dose of external
irradiation (group II), treatment with trastuzumab (group III), or
a combination of the two (group IV). One group of cells was used as
a control (group I) and treated in the same manner as all other
cells, without exposure to any drug or irradiation. All experiments
were performed in triplicate.
Receptor quantification
Quantification of HER2 expression was conducted 24
and 48 h post irradiation exposure in all groups (I-IV). To
evaluate the changes in receptor expression as response to external
irradiation alone, cells were seeded as described, one day prior to
the first irradiation exposure and treated according to protocol B
(Fig. 1). For the experiment, 100
nM of unlabeled Z2395 was added to half of the dishes
and the cells were subsequently incubated for 1 h at room
temperature. This was followed by the addition of 10 nM
99mTc-labeled Z2395 to all dishes and
incubation for 1 h. The cells were subsequently trypsinized,
resuspended, collected and counted according to protocols described
previously (19,21). The radioactivity in cell samples was
measured using an automated γ-counter. All experiments were
performed in triplicate.
Results
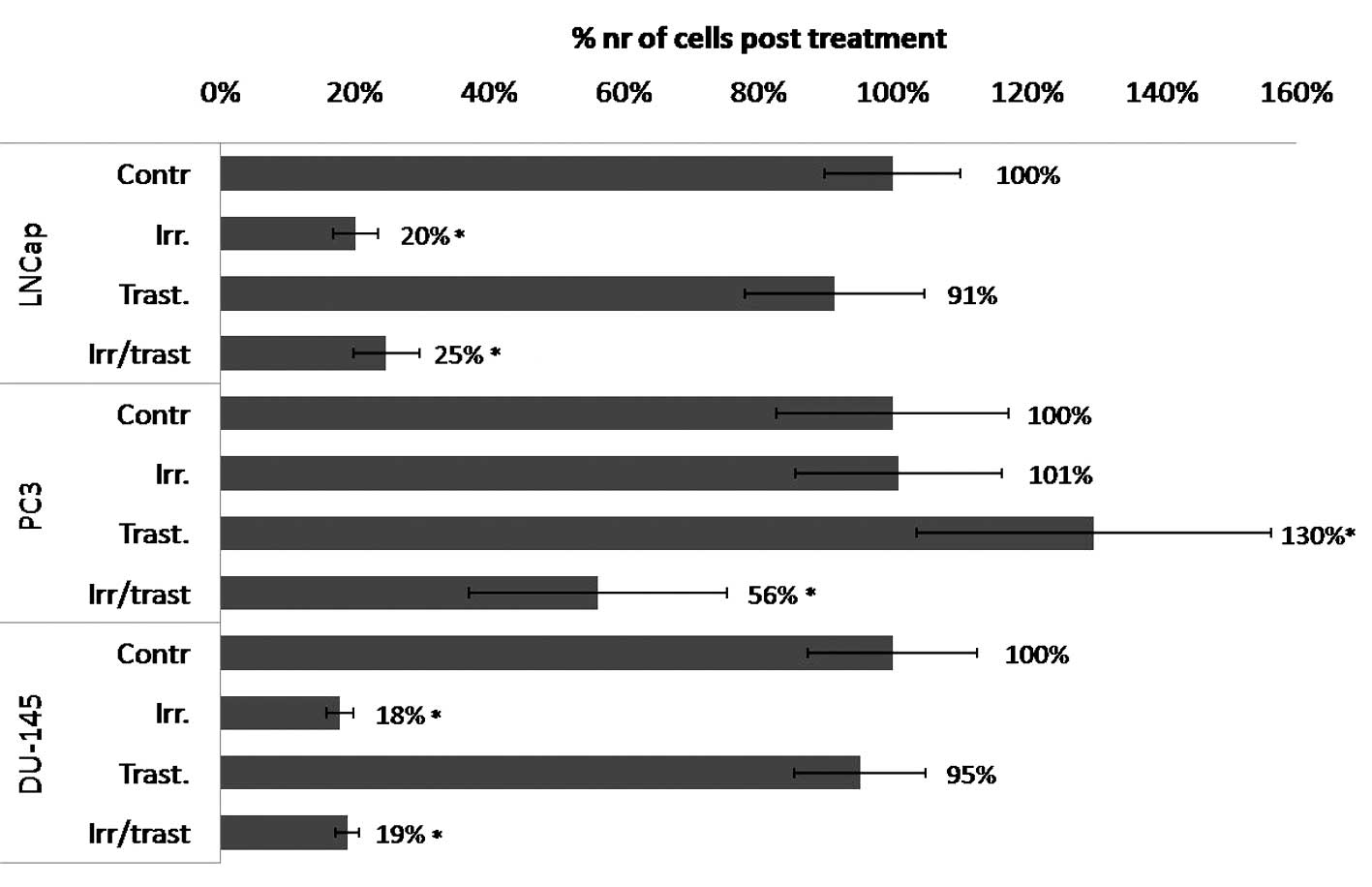
PCa cell lines treated with a 6 Gy dose of external
irradiation exhibited a varied response with regards to cell
survival and HER2 expression. Irradiation treatment alone was not
observed to exert any effect on PC3 cells, whereas a 5-fold
decrease in cell survival was detected in the LNCap (P<0.00001)
and DU-145 (P<0.0001) cells (Fig.
2). After 24 h, PC3 cells exhibited a significant 30% increase
in HER2-expression and 48 h post-irradiation, the receptor
expression was 50% higher than that of the untreated control cells
(Fig. 3). No similar response was
detected in the other cell lines, which maintained stable receptor
expression.
The HER2-targeting drug, trastuzumab, was
administered following irradiation treatment to evaluate its
potential additive effect (Fig. 2).
The PC3 cells that received trastuzumab treatment only, exhibited a
significant increase in cell number, however, the post-irradiation
administration of trastuzumab resulted in a 2-fold decrease in cell
number when compared with the untreated control cells and a
2.5-fold decrease when compared with that of trastuzumab alone. No
additive effect was identified in LNCap and DU-145 cells, when
compared with irradiation treatment alone.
Discussion
The most common first line treatment for
disseminated PCa is external irradiation, which is often combined
with removal or blockage of androgen. However, 20–40% of patients
relapse within 10 years following treatment. The occurrence of
relapsing and more aggressive PCa with androgen independent clones
is considered to be caused by an activation of androgen-independent
pathways, required for cancer progression (11). HER2 is a RTK, which is known to be
upregulated in numerous types of cancer, promoting cell motility,
division and suppressing apoptosis. Previous studies have
consistently reported HER2 expression in PCa (8,22) as
well as its clear involvement in the androgen independent PCa
(23). The pathway that allows
continued PCa cell proliferation is dependent on HER2, and
overexpression of the protein may activate androgen receptors in
the absence of androgen (9). A
number of arguments have been presented for the utilization of HER2
as a potential target for the treatment of PCa. We hypothesize that
HER2 may be involved in radioresistance and measuring of
HER2-expression as a response to external irradiation may be used
to monitor treatment response in PCa for the stratification of
patients and their response to additional therapy. We further
hypothesized that anti-HER2 therapy shortly following irradiation
treatment may overcome radioresistance.
In the current study, a panel of PCa cell lines was
used to represent the heterogeneity of the disease. As the tumor is
not homogenous, the use of one cell line would not adequately
represent the various characteristics of PCa. Therefore, three PCa
cell lines, LNCap, PC3 and DU-145, were selected, which exhibit
different metastatic potentials and proliferation rates, as well as
different degrees of androgen independence. Together the cell lines
constitute a broad panel for preclinical PCa studies.
The level of HER2 expression in the selected cell
lines is considered to be 20,000–50,000 receptors/cell (19) which correlates with clinical data
regarding HER2 expression in PCa and corresponds to the very low
expression in BCa (+1 according to a HercepTest), where tumor
biopsies with a score of 1+ (a faint/barely perceptible membrane
staining in >10% of tumor cells) are considered to present
negative HER2-expression, however, a score of 3+ (strong complete
membrane staining is observed in >10% of tumor cells) is
considered to present strong positive HER2-expression (8). However, the detection and
visualization of such low HER2 expression levels is now possible
with highly sensitive small protein-based imaging probes, such as
affibody molecules (19,24,25).
In this study, PCa cells were exposed to one dose of
external radiation (6 Gy), which corresponds to one dose of
fractionated therapy, and the short-term response in cell survival
and HER2 expression was analyzed as a model for early therapy
modeling. The rapid response to this treatment was observed as a
5-fold reduction in cell number in LNCap and DU-145 cells when
compared with untreated controls (Fig.
2), whereas PC3 cells demonstrated radioresistance. The HER2
expression in LNCap and DU-145 cells remained stable 48 h following
irradiation, whereas the receptor expression in PC3 cells
significantly increased by 50% after 48 h (Fig. 3). As the HER-family is involved in
cell survival and proliferation, the increase in receptor
expression observed is consistent with the activation of this
cell-survival mechanism. This protective behavior is typical for
HER2 expressing cells and has been demonstrated previously
(26). Therefore, we hypothesize
that these results, which showed an increase in the membranous
expression of HER2 as an acute response to external irradiation,
indicate cell radioresistance.
In order to investigate whether the HER2 targeting
drug, trastuzumab, exhibits an effect post irradiation, PCa cells
were exposed to trastuzumab treatment following external
irradiation, or to trastuzumab treatment alone. Treatment with
trastuzumab alone did not exhibit an inhibitory effect on cell
survival, and notably demonstrated a pro-proliferative effect on
PC3 cells (Fig. 2). Although PC3
cells were not affected by irradiation or anti-HER2 treatment, the
trastuzumab administration post-irradiation was efficacious, and
resulted in a significant 44% decrease in cell number. These
results are also consistent with those presented in another study,
investigating the effect of irradiation on HER2 positive and
negative BCa cell lines, which received HER2 targeting therapy
(27). The results of the present
study may indicate a suppressive action of trastuzumab on the
survival mechanism mediated by HER2 expressing cells. DU-145 and
LNCap cells exhibited radiosensitivity, however, no additive
effects were observed following the post-irradiation administration
of trastuzumab.
This study preclinically evaluated a combination of
clinically available therapies and drugs, and their effect on cell
survival and HER2 expression. External irradiation therapy in
combination with HER2-targeting drugs was demonstrated to alter the
receptor expression profile in radioresistant PCa cell lines. The
observed changes in HER2 expression as a response to this treatment
regime may be used to monitor the response to therapy.
In conclusion, measurement of HER2 expression prior
to and following therapy may present a step towards patient
stratification and more personalized treatment, as well as
emphasizing the potential requirement for additional therapy.
Additional studies investigating how analysis of HER2 in a
xenograft model may serve as a marker to identify non-responders
that may benefit from other treatments are required.
Acknowledgements
The authors would like thank Apoteket Farmaci AB
(Uppsala, Sweden) for providing trastuzumab, and Affibody (Solna,
Sweden) for providing Z2395 affibody molecules.
References
|
1
|
Chen W, Mao K, Liu Z and Dinh-Xuan AT: The
role of the RhoA/Rho kinase pathway in angiogenesis and its
potential value in prostate cancer (Review). Oncol Lett.
8:1907–1911. 2014.PubMed/NCBI
|
|
2
|
Sieh W, Lichtensztajn DY, Nelson DO, et
al: Treatment and mortality in men with localized prostate cancer:
a population-based study in California. The Open Prost Cancer J.
6:1–9. 2013. View Article : Google Scholar
|
|
3
|
Widmark A, Klepp O, Solberg A, et al;
Scandinavian Prostate Cancer Group Study 7; Swedish Association for
Urological Oncology. Endocrine treatment, with or without
radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3):
an open randomised phase III trial. Lancet. 373:301–308. 2009.
View Article : Google Scholar
|
|
4
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harmenberg U, Hamdy FC, Widmark A,
Lennernäs B and Nilsson S: Curative radiation therapy in prostate
cancer. Acta Oncol. 50-1:98–103. 2011. View Article : Google Scholar
|
|
6
|
Kelloff GJ, Choyke P and Coffey DS:
Prostate Cancer Imaging Working Group: Challenges in clinical
prostate cancer: role of imaging. Am J Roentgenol. 192:1455–1470.
2009. View Article : Google Scholar
|
|
7
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minner S, Jessen B, Stiedenroth L, et al:
Low level HER2 overexpression is associated with rapid tumor cell
proliferation and poor prognosis in prostate cancer. Clin Cancer
Res. 16:1553–1560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Signoretti S, Montironi R, Manola J, et
al: Her-2-neu expression and progression toward androgen
independence in human prostate cancer. J Natl Cancer Inst.
92:1918–1925. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Craft N, Shostak Y, Carey M and Sawyers
CL: A mechanism for hormone-independent prostate cancer through
modulation of androgen receptor signaling by the HER-2/neu tyrosine
kinase. Nat Med. 5:280–285. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Culig Z, Hobisch A, Cronauer MV, et al:
Androgen receptor activation in prostatic tumor cell lines by
insulin-like growth factor-I, keratinocyte growth factor, and
epidermal growth factor. Cancer Res. 54:5474–5478. 1994.PubMed/NCBI
|
|
13
|
Valerie K, Yacoub A, Hagan MP, Curiel DT,
Fisher PB, Grant S and Dent P: Radiation induced cell signaling:
inside-out and outside-in. Mol Cancer Ther. 6:789–801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
So A, Gleave M, Hurtado-Col A and Nelson
C: Mechanisms of the development of androgen independence in
prostate cancer. World J Urol. 23:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
The US National Institutes of Health.
Paclitaxel and radiation therapy with or without trastuzumab in
treating patients who have undergone surgery for bladder cancer.
NCT00238420. 2014, ClinicalTrials.govurisimpleClinicalTrials.gov.
|
|
16
|
The U.S National Institutes of Health.
Evaluation of carboplatin/paclitaxel with and without trastuzumab
(Herceptin) in uterine serous cancer. NCT01367002. 2014, ClinicalTrials.govurisimpleClinicalTrials.gov.
|
|
17
|
The U.S National Institutes of Health.
Safety study of 212Pb-TCMC-trastuzumab radio
immunotherapy. NCT01384253. 2014, ClinicalTrials.govurisimpleClinicalTrials.gov.
|
|
18
|
Albanell J, Codony J, Rovira A, Mellado B
and Gascón P: Mechanism of action of anti-HER2 monoclonal
antibodies: scientific update on trastuzumab and 2C4. Adv Exp Med
Biol. 532:253–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malmberg J, Tolmachev V and Orlova A:
Imaging agents for in vivo molecular profiling of disseminated
prostate cancer: Cellular processing of [(111)In]-labeled
CHX-A″DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate
cancer cell lines. Exp Ther Med. 2:523–528. 2011.PubMed/NCBI
|
|
20
|
Ahlgren S, Orlova A, Wållberg H, et al:
Targeting of HER2-expressing tumors using 111In-ABY-025, a
second-generation affibody molecule with a fundamentally
reengineered scaffold. J Nucl Med. 51:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malmberg J, Tolmachev V and Orlova A:
Imaging agents for in vivo molecular profiling of disseminated
prostate cancer targeting EGFR receptors in prostate cancer:
comparison of cellular processing of [111In]-labeled affibody
molecule Z (EGFR:2377) and cetuximab. Int J Oncol. 41:1128–1138.
2011.
|
|
22
|
Baek KH, Hong ME, Jung YY, et al:
Correlation of AR, EGFR, and HER2 Expression Levels in Prostate
Cancer: Immunohistochemical Analysis and Chromogenic In Situ
Hybridization. Cancer Res Treat. 44:50–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrion Salip D, Panosa C, Menendez JA, et
al: Androgen-independent prostate cancer cells circumvent EGFR
inhibition by overexpression of alternative HER receptors and
ligands. Int J Oncol. 41:1128–1138. 2012.PubMed/NCBI
|
|
24
|
Malmberg J, Perols A, Varasteh Z, et al:
Comparative evaluation of synthetic anti-HER2 Affibody molecules
site-specifically labelled with 111In using N-terminal DOTA, NOTA
and NODAGA chelators in mice bearing prostate cancer xenografts.
Eur J Nucl Med Mol Imaging. 39:481–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wallberg H, Orlova A, Altai M, et al:
Molecular design and optimization of 99mTc-labeled recombinant
affibody molecules improves their biodistribution and imaging
properties. J Nucl Med. 52:461–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duru N, Fan M, Candas D, et al:
HER2-associated radioresistance of breast cancer stem cells
isolated from HER2-negative breast cancer cells. Clin Cancer Res.
18:6634–6647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
No M, Cho EJ and Kim IA: Targeting HER2
signaling pathway for radiosensitization: alternative strategy for
therapeutic resistance. Cancer Biol Ther. 8:2351–2361. 2009.
View Article : Google Scholar : PubMed/NCBI
|