Introduction
Thrombocytosis is commonly observed in patients
exhibiting malignant tumors. Retrospective studies have
demonstrated that 10–60% of patients with untreated malignancies,
such as pulmonary, gastrointestinal and hepatic cancers (1–3),
exhibit increased platelet (PLT) counts. The frequency of
associated thrombocytosis in primary lung cancer patients is
~16–32% and PLT counts range from 350×109/l to
1,000×109/l, or >1,000×109/l in rare cases
(4–6). Previous investigations have proposed
that thrombocytosis is an independent predictor of poor prognosis
in patients exhibiting malignancies, including primary lung cancer
(7,8).
The mechanism(s) underlying the association between
thrombocytosis and malignancy remains unknown. As demonstrated in
previous studies, increased concentrations of humoral factors, such
as thrombopoietin, interleukin-6 and interleukin-11, stimulate PLT
production in patients exhibiting malignancies (9–11). In
addition, the bone marrow microenvironment (12), PLT granule protein (13) and coagulation system activation may
be important in the development of reactive thrombocytosis
(14).
The mechanism(s) responsible for the poor prognosis
of patients exhibiting malignant tumors and concomitant
thrombocytosis requires elucidation. As demonstrated by a previous
study, PLT aggregation induced by tumor cells contributes to the
adhesion and encapsulation of PLTs with circulating tumor cells
(15). This enhances the ability of
tumor cells to escape the destructive effects of immune
surveillance cells, such as natural killer cells. Furthermore,
tumor cell-induced PLT aggregation may promote microcirculatory
adhesion and colonization of tumor cells; thus, PLTs are involved
in the development of hematogenous metastases (16). In addition, activated PLTs release
vascular endothelial growth factor, epidermal growth factor,
PLT-derived growth factor and a number other cytokines, which
stimulate the growth of malignant cells and promote angiogenesis
(17). PLTs are also involved in
the development of Trousseau syndrome (18); however, it is not known whether
there is a statistically significant difference in PLT activation
and its effects on prognosis between patients with and without
thrombocytosis, and whether any such differences are clinically
relevant. Previous clinical studies have provided no consistent
evidence to support a correlation between the incidence of
thrombocytosis, and tumor node metastasis (TNM) stage,
differentiation or tumor size. Furthermore, according to the
majority of studies (1,3), the incidence of thrombocytosis is
independent of the tumor pathology. Thus, the mechanism(s)
responsible for the poor prognosis of patients exhibiting
malignancies and concomitant thrombocytosis requires further
investigation.
In the present study, the clinical data of 308
pulmonary adenocarcinoma patients were retrospectively analyzed and
correlations between thrombocytosis and clinicopathological
features, prognosis and distant metastases (particularly to the
bone) were investigated.
Patients and methods
Patients
The records of 758 patients with histopathologically
confirmed pulmonary adenocarcinoma, admitted to Changhai Hospital
(Shanghai, China) from 1 July 2006 to 30 April 2009, were assessed
in the present study. Patients exhibiting other tumors (previous
and current), and blood, rheumatic, acute and chronic infectious or
chronic inflammatory diseases, were excluded. Thus, 523 patients
were selected for follow-up by telephone to obtain information
regarding survival periods and distant metastasis. The median
survival period was 93.9 weeks (range, 3.6–299.0 weeks) for 308/523
patients; the remaining 215 patients were lost to follow-up as they
could not be contacted. The following data was collected from the
study patients: i) General data, including gender, age and smoking
index; and b) post-diagnostic data, including Eastern Cooperative
Oncology Group performance status (ECOG PS) score (19), TNM stage (TNM, 6th edition)
(20), white blood cell (WBC)
count, hemoglobin, albumin, carcinoembryonic antigen (CEA) and
alkaline phosphatase (AKP) levels, PLT count, erythrocyte
sedimentation rate (ESR), activated partial thromboplastin time,
tumor differentiation and metastasis. The overall survival (OS) was
defined as the time in weeks from definite diagnosis to all-cause
mortality or the termination of follow-up (30 June, 2012). Written
informed consent was obtained from all patients.
Measurements and PLT counts
The date of definite diagnosis was defined as the
date the Department of Pathology, Changhai Hospital received
samples of the tumor. Survival was defined as the time (in weeks)
from the date of definite diagnosis to all-cause mortality or the
cut-off date of 30 June, 2012. Peripheral blood PLT counts were
measured using an ADVIA® 120 (Siemens AG, Erlangen,
Germany) hematology analyzer.
Statistical analysis
Data were analyzed using SPSS software (version
17.0; SPSS Inc., Chicago, IL, USA). The normality of the PLT count
distribution was determined by performing a Kolmogorov-Smirnov test
and the association between thrombocytosis and tumor pathology was
analyzed using univariate and multivariate analyses. The Wald test
was also performed. The Cox proportional-hazards regression model,
the last observation carried forward and the Kaplan-Meier method
were employed for survival analysis. In addition, inter-group
comparisons of survival were based on the log-rank test and
inter-group comparisons of remote metastasis were performed using
the χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data
The present study included 308 pulmonary
adenocarcinoma patients, aged 27–83 years (mean age ± standard
deviation, 59.6±10.3). The gender ratio was 2.2:1 (213 male
patients; 95 female patients) and TNM stage varied as follows:
Stage I, 71 cases; Stage II, 32 cases; Stage IIIa, 44 cases; Stage
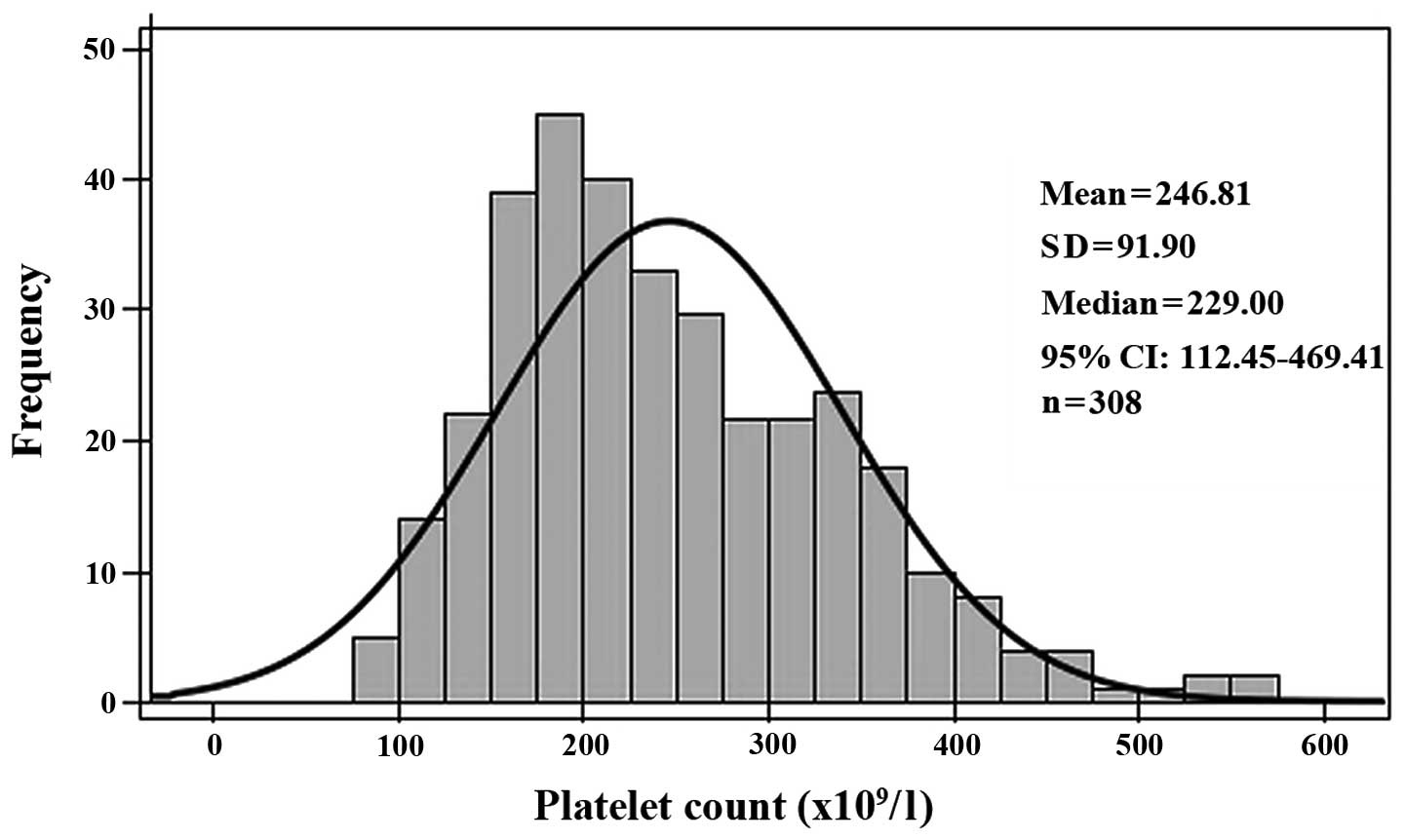
IIIb, 49 cases; and Stage IV, 112 cases. Upon diagnosis, the mean
PLT count was 246.8±91.9×109/l; this included four
(1.3%), 222 (72.1%) and 82 (26.6%) patients with PLT counts below,
within and above the normal range (100–300×109/l),
respectively. A right-skewed distribution of PLT counts was
observed (Fig. 1).
Clinicopathological characteristics
The study patients were classified as having
thrombocytosis (PLT≥300×109/l; 82 cases) or not
(PLT<300×109/l; 226 cases). This factor, together
with various other clinicopathological factors, was subjected to
univariate analysis, which revealed that an ECOG PS score of ≥2
points, advanced TNM stage and leukocytosis were risk factors for
thrombocytosis (Table I). According
to multivariate analysis, leukocytosis, anemia and increased ESR
were correlated with thrombocytosis (Table II).
 | Table IUnivariate analysis of relevant
clinicopathological risk factors for thrombocytosis. |
Table I
Univariate analysis of relevant
clinicopathological risk factors for thrombocytosis.
| Frequency, n (%) | | | | |
|---|
|
| | | | |
|---|
| Clinicopathological
factor | Thrombocytosis | No
thrombocytosis | OR | 95% CI | χ2
value | P-value |
|---|
| ECOG PS score |
| ≤1 | 72 (87.8) | 217 (96.0) | | | | |
| ≥2 | 10 (12.2) | 9 (4.0) | 3.213 | 1.262–8.180 | 7.011 | 0.008 |
| TMN stage |
| IA–IIIA | 29 (35.4) | 118 (52.2) | | | | |
| IIIA–IV | 53 (64.6) | 108 (47.8) | 1.231 | 1.012–2.094 | 6.845 | 0.009 |
| Hematological
factors |
| WBC,
×109/l |
| <10.0 | 61 (74.4) | 217 (96.0) | | | | |
| ≥10.0 | 21 (25.6) | 9 (4.0) | 7.406 | 3.351–16.367 | 32.012 | <0.001 |
| Hgb, g/l |
| <120 | 26 (31.7) | 42 (18.6) | | | | |
| ≥120 | 56 (68.3) | 184 (81.4) | 0.420 | 0.246–0.719 | 6.023 | 0.014 |
| Albumin, g/l |
| <30 | 48 (58.5) | 177 (78.3) | | | | |
| ≥30 | 34 (41.5) | 49 (21.7) | 2.393 | 1.427–4.013 | 11.961 | 0.001 |
| ESR, mm/H
(n=242) |
| <20 | 18 (29.5) | 88 (48.6) | | | | |
| ≥20 | 43 (70.5) | 93 (51.4) | 2.741 | 1.609–4.670 | 10.835 | 0.001 |
| APTT, sec
(n=258) |
| 23–43 | 64 (91.4) | 184 (97.9) | | | | |
| >43 | 6 (8.6) | 4 (2.1) | 4.985 | 1.424–17.450 | 5.894 | 0.015 |
| AKP, U/l |
| <92 | 53 (64.6) | 174 (77.0) | | | | |
| ≥92 | 29 (35.4) | 52 (23.0) | 1.896 | 1.129–3.184 | 4.740 | 0.029 |
 | Table IIMultivariate analysis of relevant
clinicopathological risk factors for thrombocytosis (n=228). |
Table II
Multivariate analysis of relevant
clinicopathological risk factors for thrombocytosis (n=228).
| Risk factor | OR | 95% confidence
interval | Wald value | P-value |
|---|
| Fever |
| No |
| Yes | 2.575 | 1.098–6.039 | 4.365 | 0.030 |
| WBC,
×109/l |
| <10.0 |
| ≥10.0 | 7.596 | 2.997–19.255 | 9.941 | 0.002 |
| Hgb, g/l |
| ≥120 |
| <120 | 3.360 | 1.376–4.735 | 6.417 | 0.011 |
| Albumin, g/l |
| <30 |
| ≥30 | 2.543 | 1.262–5.124 | 5.662 | 0.017 |
| ESR, mm/H |
| <20 |
| ≥20 | 2.323 | 1.194–4.517 | 6.215 | 0.013 |
| APTT, sec |
| ≤43 |
| >43 | 7.869 | 1.917–32.301 | 8.273 | 0.004 |
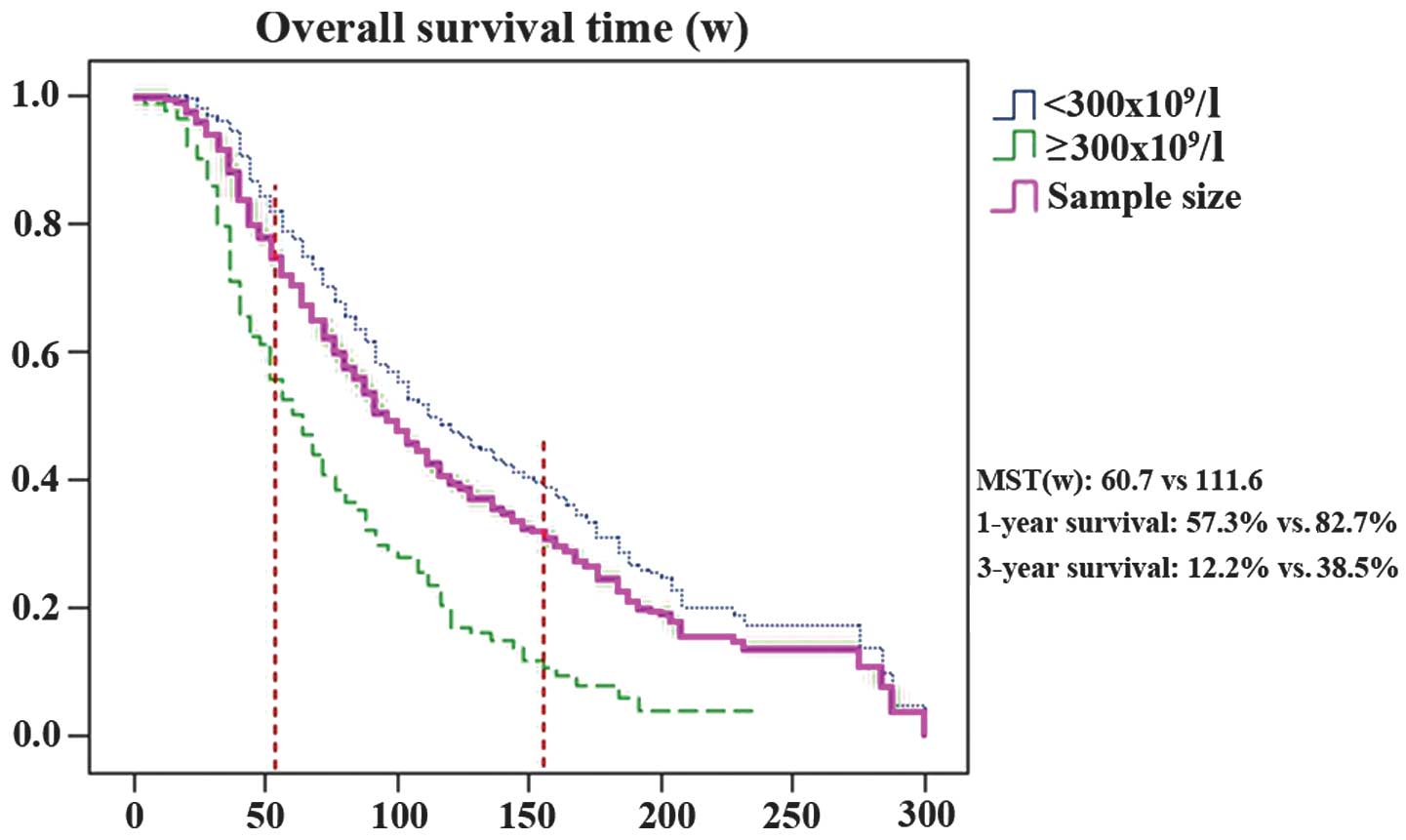
Overall survival and survival rate
By final follow-up, 244/308 study patients had died
and 64 study patients had survived. The one- and three-year
survival rates were 76.0 and 31.5%, respectively. The 82
thrombocytosis patients had a mean survival time (MST) of 60.7
weeks (range, 3.6–235.9); however, the 226 non-thrombocytosis
patients had an MST of 111.6 weeks (range, 16.6–299.0). Inter-group
differences between one- and three-year survival rates (P<0.001;
Table III) and within overall
survival (log rank, χ2=43.095; P<0.001; Fig. 2) were statistically significant.
 | Table IIIComparison of survival between
thrombocytosis and non-thrombocytosis patients. |
Table III
Comparison of survival between
thrombocytosis and non-thrombocytosis patients.
| Prognosis | Total, n=308 | Thrombocytosis,
n=82 | No thrombocytosis,
n=226 | χ2 | P-value |
|---|
| Outcome, n (%) |
| Survival | 64 (2.8) | 6 (7.3) | 58 (25.7) | | |
| Mortality | 244 (79.2) | 76 (92.7) | 168 (74.3) | 13.536 | <0.001 |
| Prognosis |
| MST, weeks | 94.0 | 60.7 | 111.6 | | |
| One-year survival,
n (%) | 234 (76.0) | 47 (57.3) | 187 (82.7) | 21.310 | <0.001 |
| Three-year
survival, n (%) | 97 (31.5) | 10 (12.2) | 87 (38.5) | 19.291 | <0.001 |
Bone metastasis and thrombocytosis
Bone metastases were identified upon diagnosis in 29
(35.4%) and 51 (22.6%) patients with and without thrombocytosis,
respectively. This inter-group difference was statistically
significant (χ2=5.127; P=0.024). Of the thrombocytosis
patients, 50 (61.0%) developed bone metastases during the course of
disease progression, in comparison with 88 (38.9%)
non-thrombocytosis patients. This difference was also statistically
significant (χ2=11.816; P=0.001). However, differences
in metastasis to sites other than bone were not statistically
significant between thrombocytosis and non-thrombocytosis patients
(Table IV). According to
univariate analysis, thrombocytosis, weight loss, an ECOG PS score
of ≥2 points, anemia, increased ESR, and increased AKP and CEA
levels were risk factors for bone metastasis (Table V). According to multivariate
analysis, thrombocytosis, weight loss, and increased AKP and CEA
levels were correlated with bone metastasis (Table VI).
 | Table IVComparison of frequency of distant
metastasis between thrombocytosis and non-thrombocytosis
patients. |
Table IV
Comparison of frequency of distant
metastasis between thrombocytosis and non-thrombocytosis
patients.
| Distant metastasis
site | Sample size, n (%)
(n=308) | Thrombocytosis, n
(%) (n=82) | No thrombocytosis,
n (%) (n=226) | χ2 | P-value |
|---|
| Lymph node |
| Upon
diagnosis | 218 (70.8) | 61 (74.4) | 157 (69.5) | 0.705 | 0.401 |
| During the disease
course | 209 (67.9) | 59 (72.0) | 150 (66.4) | 0.859 | 0.354 |
| Bone |
| Upon
diagnosis | 80 (26.0) | 29 (35.4) | 51 (22.6) | 5.127 | 0.024 |
| During the disease
course | 138 (44.8) | 50 (61.0) | 88 (38.9) | 11.816 | 0.001 |
| Lung |
| Upon
diagnosis | 54 (17.5) | 17 (20.7) | 37 (16.4) | 0.791 | 0.374 |
| During the disease
course | 93 (30.2) | 27 (32.9) | 66 (29.2) | 0.396 | 0.529 |
| Brain | | | | | |
| Upon
diagnosis | 24 (7.8) | 7 (8.5) | 17 (7.5) | 0.086 | 0.769 |
| During the disease
course | 67 (21.8) | 17 (20.7) | 50 (22.1) | 0.069 | 0.794 |
| Liver |
| Upon
diagnosis | 24 (7.8) | 7 (8.5) | 17 (7.5) | 0.086 | 0.769 |
| During the disease
course | 46 (14.9) | 13 (15.9) | 33 (14.6) | 0.074 | 0.785 |
| Adrenal gland |
| Upon
diagnosis | 6 (1.9) | 1 (1.2) | 5 (2.2) | 0.311 | 0.577 |
| During the disease
course | 11 (3.6) | 3 (3.7) | 8 (3.7) | 0.002 | 0.960 |
| Kidney |
| Upon
diagnosis | 2 (0.6) | 1 (1.2) | 1 (0.4) | 0.563 | 0.453 |
| During the disease
course | 5 (1.6) | 3 (3.7) | 2 (0.9) | 2.898 | 0.089 |
 | Table VUnivariate analysis of relevant
clinicopathological risk factors for bone metastasis (n=308). |
Table V
Univariate analysis of relevant
clinicopathological risk factors for bone metastasis (n=308).
| Frequency (%) | | | | |
|---|
|
| | | | |
|---|
| Risk factor | Bone
metastasis | No bone
metastasis | OR | 95% CI | χ2 | P-value |
|---|
| Weight loss
(n=288) |
| Yes | 33 (41.3) | 39 (17.1) | | | | |
| No | 47 (58.5) | 189 (82.9) | 3.403 | 1.938–5.975 | 19.274 | <0.001 |
| ECOG PS score |
| ≥2 | 11 (13.8) | 8 (3.5) | | | | |
| ≤1 | 69 (86.3) | 220 (96.5) | 4.384 | 1.695–11.336 | 10.731 | 0.001 |
| PLT,
×109/l |
| ≥300 | 29 (36.3) | 53 (23.2) | | | | |
| <300 | 51 (63.8) | 175 (76.8) | 1.878 | 1.084–3.253 | 8.127 | 0.008 |
| Hgb, g/l |
| <120 | 25 (31.3) | 43 (18.9) | | | | |
| ≥120 | 55 (68.8) | 185 (81.1) | 0.511 | 0.287–0.911 | 5.285 | 0.022 |
| ESR, mm/h
(n=242) |
| ≥20 | 38 (68.7) | 98 (53.9) | | | | |
| <20 | 20 (31.3) | 86 (46.1) | 1.169 | 1.027–1.330 | 5.320 | 0.021 |
| AKP, U/l |
| ≥92 | 39 (48.8) | 42 (18.4) | | | | |
| <92 | 41 (51.3) | 186 (81.6) | 1.580 | 1.270–1.966 | 28.152 | <0.001 |
| CEA, U/l
(n=287) |
| ≥10 | 49 (63.8) | 63 (30.7) | | | | |
| <10 | 27 (36.3) | 148 (69.3) | 3.969 | 2.323–6.782 | 27.117 | <0.001 |
 | Table VIMultivariate analysis of relevant
clinicopathological risk factors for bone metastasis (n=237). |
Table VI
Multivariate analysis of relevant
clinicopathological risk factors for bone metastasis (n=237).
| Risk factor | OR | 95% CI | Wald value | P-value |
|---|
| Weight loss |
| No |
| Yes | 3.002 | 1.603–5.623 | 11.790 | 0.001 |
| PLT,
×109/l |
| <300 |
| ≥300 | 1.436 | 1.043–2.871 | 4.013 | 0.048 |
| AKP, U/l |
| <92 |
| ≥92 | 3.466 | 1.887–6.364 | 16.068 | <0.001 |
| CEA, U/l |
| <10 |
| ≥10 | 2.916 | 1.621–5.247 | 12.751 | <0.001 |
Discussion
Thrombocytosis can be divided into two major
categories: Clonal thrombocytosis and reactive thrombocytosis.
Clonal thrombocytosis is induced by clonal myeloproliferative
diseases, including idiopathic thrombocythemia and polycythemia
vera (11). By contrast, reactive
thrombocytosis is a secondary response to various factors,
including infection, cancer and tissue injury, and is the most
common type of thrombocytosis. Reactive thrombocytosis has been
observed in various malignancies, including lung, gastrointestinal
tract and liver cancer, at a reported incidence rate of 10–60%
(1–3). In the present study, the clinical data
of 308 pulmonary adenocarcinoma patients was retrospectively
analyzed. The clinical characteristics of patients exhibiting
thrombocytosis and pulmonary adenocarcinoma, as well as
correlations between thrombocytosis and various clinicopathological
factors, were investigated and are discussed from a clinical
perspective.
In the current study, the incidence of
thrombocytosis was 26.6%, which is consistent with the 16–32%
reported in studies from other countries (1–3). A PLT
count of ≥300×109/l was chosen as the criterion for the
diagnosis of thrombocytosis. Peripheral blood PLT counts exhibited
a right-skewed distribution, indicating that untreated pulmonary
adenocarcinoma patients have higher PLT counts than healthy
subjects. Thus, the present study identified the phenomenon of
pulmonary adenocarcinoma-associated thrombocytosis in Chinese
patients. A PLT count of ≥300×109/l was selected as the
criterion for diagnosing thrombocytosis, as it defines the upper
limit of the 95% CI for PLT counts in healthy Chinese subjects. By
contrast, the majority of reports from other countries use a PLT
count of ≥400×109/l or ≥350×109/l as the
criterion for thrombocytosis. This difference in the normal range
of PLT counts between Europe and the USA, and China, must be
addressed; it may be associated with differences in ethnicity
and/or measurement instruments. Using pairwise comparison, Zeng
et al (21) identified that
the frequency of the thrombopoietin receptor (TPOR) C allele at
position 550 is significantly higher in subjects with high PLT
counts, and that thrombocytosis is associated with a C to A
transversion at position 550 in the 5′-promoter region of TPOR
(21). Therefore, the present study
postulates that genetic factors may be involved in the mechanism
that determines the differences in the normal range of PLT in
counts in Chinese subjects versus European and American
subjects.
Previous studies have found that advanced TNM stage,
a high ECOG PS score and poorly differentiated carcinomas are
associated with poor prognosis in lung carcinoma (22). In the present study, univariate
analysis demonstrated that an ECOG PS score of ≥2 points, advanced
TNM staging and leukocytosis were risk factors for decreased
overall survival. Multivariate analysis demonstrated that
thrombocytosis is an independent risk factor for poor prognosis in
pulmonary adenocarcinoma, with a relative risk of 2.103–3.814,
indicating that the mortality of pulmonary adenocarcinoma with
thrombocytosis is 2.103–3.814-fold greater than non-thrombocytosis
patients. Furthermore, thrombocytosis patients exhibit a
significantly shorter MST compared with non-thrombocytosis patients
(difference, 50.9 weeks; 60.7 vs.111.6 weeks). In addition, the
one- and three-year survival rates were significantly lower
compared with patients not exhibiting thrombocytosis (difference,
25.4%; 57.3 vs. 82.7% and difference, 26.3%, 12.2 vs. 38.5%,
respectively). Therefore, pulmonary adenocarcinoma patients
exhibiting thrombocytosis have a worse prognosis than patients not
exhibiting thrombocytosis, which is consistent with the findings
reported for other malignancies.
Numerous reports from other countries support the
significant effects of thrombocytosis on the survival of patients
exhibiting malignancies (23–25).
Thrombocytosis is a common presentation of paraneoplastic syndrome
and has been recognized to accompany the development,
proliferation, differentiation, invasion and metastasis of specific
tumors. Furthermore, thrombocytosis may effect the survival of
patients exhibiting various malignancies. Besides the stimulatory
effects of activated and increased PLTs on hematogenous metastasis,
tumor growth, and angiogenesis, the presence of thrombocytosis may
correlate with the biological characteristics and behavior of
malignant cells. Malignancies with associated thrombocytosis may
possess different characteristics with regard to differentiation,
invasion and metastasis compared with the same types of malignancy
not associated with thrombocytosis. Furthermore, interaction of
these factors may affect the patients’ prognoses (26–28).
Distant metastasis is an important biological
characteristic of malignant tumors and an important prognostic
factor (29). In the present study,
pulmonary adenocarcinoma most frequently metastasized, in
descending order, to the lymph nodes, bone, lung, brain, liver,
adrenal glands and kidney. Thus, the most common site of
hematogenous metastases was the bone. As PLTs are produced by bone
marrow, the present study assessed the correlation between PLT
count and bone metastasis and identified a statistically
significant correlation. The risk of bone metastasis in patients
exhibiting pulmonary adenocarcinoma and thrombocytosis was
1.436-fold higher than in patients not exhibiting thrombocytosis.
However, the correlation coefficient for thrombocytosis versus bone
metastasis was weak. Possible explanations for the weakness of this
correlation include the following: i) Reactive thrombocytosis may
have been induced by a number of other factors, such as infection,
rheumatic autoimmune disease and coronary heart disease (30,31);
b) relevant data may not have been recorded during follow-up; c)
thrombocytosis may only correlate with one type of bone metastasis
(osteoblastic, osteolytic or mixed) (32); and d) the small sample size may have
effected the sampling error and therefore the overall findings of
the study.
The present report was a retrospective study, thus,
perspective and randomized sampling design were not considered and
substantial relevant data was unavailable due to various factors,
such as incomplete records, incomprehensible data and non-uniform
reporting of data. These factors may have compromised the findings
of the present study. Therefore, larger studies of tumor-induced
thrombocytosis should be conducted to clarify the findings of the
present report.
In conclusion, the present retrospective study of
308 pulmonary adenocarcinoma patients identified that
thrombocytosis correlates with the development of bone
metastases.
References
|
1
|
Pedersen LM and Milman N: Prognostic
significance of thrombocytosis in patients with primary lung
cancer. Eur Respir J. 9:1826–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aoe K, Hiraki A, Ueoka H, et al:
Thrombocytosis as a useful prognostic indicator in patients with
lung cancer. Respiration. 71:170–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehmann J, Retz M, Nürnberg N, et al: The
superior prognostic value of humoral factors compared with
molecular proliferation markers in renal cell carcinoma. Cancer.
101:1552–1562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada H, Oohira G, Okazumi S, et al:
Thrombocytosis associated with poor prognosis in patients with
esophageal carcinoma. J Am Coll Surg. 198:737–741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki K, Aiura K, Kitagou M, et al:
Platelets counts closely correlate with the disease-free survival
interval of pancreatic cancer patients. Hepatogastroenterology.
51:847–853. 2004.PubMed/NCBI
|
|
6
|
Brockmann MA, Giese A, Mueller K, et al:
Preoperative thrombocytosis predicts poor survival in patients with
glioblastoma. Neuro Oncol. 9:335–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwasaki A, Hamanaka W, Harnada T, et al:
Significance of platelet counts in patients who underwent surgical
treatment for lung metastasis. Int Surg. 92:103–109.
2007.PubMed/NCBI
|
|
8
|
Tomita M, Shimizu T, Hara M, et al:
Prognostic impact of thrombocytosis in resectable non-small cell
lung cancer. Interact Cardiovasc Thorac Surg. 7:613–615. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaushansky K: Thrombopoietin: the primary
regulator of platelet production. Blood. 86:419–431.
1995.PubMed/NCBI
|
|
10
|
Kaushansky K: Thrombopoietin the primary
regulator of platelet production. Trends Endocrinol Metab. 8:45–50.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schafer AI: Thrombocytosis. New Engl J
Med. 350:1211–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slayton WB, Wainman DA, Li XM, et al:
Developmental differences in megakaryocyte maturation are
determined by the microenvironment. Stem cells. 23:1400–1408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sungaran R, Chisholm OT, Markovic B, et
al: The role of platelet alpha-granular proteins in the regulation
of thrombopoietin messenger RNA expression in human bone marrow
stromal cells. Blood. 95:3094–3101. 2000.PubMed/NCBI
|
|
14
|
Canonico S, Sciaudone G, Santoriello A, et
al: Blood coagulation changes in patients with post-splenectomy
persistent thrombocytosis. Chir Ital. 53:537–542. 2001.(In
Italian). PubMed/NCBI
|
|
15
|
Jurasz P, Alonso-Escolano D and Radomski
MW: Platelet - cancer interactions: mechanisms and pharmacology of
tumour cell-induced platelet aggregation. Br J Pharmacol.
143:819–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morimoto K, Satoh-Yamaguchi K, Hamaguchi
A, et al: Interaction of cancer cells with platelets mediated by
Necl-5/poliovirus receptor enhances cancer cell metastasis to the
lungs. Oncogene. 27:264–273. 2008. View Article : Google Scholar
|
|
17
|
Sierko E and Wojtukiewicz MZ: Platelets
and angiogenesis in malignancy. Semin Thromb Hemost. 30:95–108.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bick RL: Cancer-associated thrombosis. New
Engl J Med. 349:109–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH and Wittekind C: Rules for
Classification. International Union Against Cancer (UICC) TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss; New
York, NY: pp. 99–103. 2002
|
|
21
|
Zeng SM, Murray JC, Widness JA, Strauss RG
and Yankowitz J: Association of single nucleotide polymorphisms in
the thrombopoietin-receptor gene, but not the thrombopoietin gene,
with differences in platelet count. Am J Hematol. 77:12–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawabata N, Maeda H, Yokota S, et al:
Postoperative serum carcinoembryonic antigen levels in patients
with pathologic stage IA nonsmall cell lung carcinoma: subnormal
levels as an indicator of favorable prognosis. Cancer. 101:803–809.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Göğüş C, Baltaci S, Filiz E, Elhan A and
Bedük Y: Significance of thrombocytosis for determining prognosis
in patients with localized renal cell carcinoma. Urology.
63:447–450. 2004. View Article : Google Scholar
|
|
24
|
Ikeda M, Furukawa H, Imamura H, et al:
Poor prognosis associated with thrombocytosis in patients with
gastric cancer. Ann Surg Oncol. 9:287–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang SG, Kim KM, Cheong JH, et al: Impact
of pretreatment thrombocytosis on blood-borne metastasis and
prognosis of gastric cancer. Eur J Surg Oncol. 38:562–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Connor DE and Joseph JE: Cyclic
thrombocytopenia associated with marked rebound thrombocytosis and
fluctuating levels of endogenous thrombopoietin and reticulated
platelets: A case report. Am J Hematol. 87:120–122. 2012.
View Article : Google Scholar
|
|
27
|
Onal H, Adal E, Ersen A, Onal Z and
Keskindemirci G: Miliaria rubra and thrombocytosis in
pseudohypoaldosteronism: case report. Platelets. 23:645–647. 2012.
View Article : Google Scholar
|
|
28
|
Li AJ and Karlan BY: Androgen mediation of
thrombocytosis in epithelial ovarian cancer biology. Clin Cancer
Res. 11:8015–8018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SC, Chang HK, Lin YC, et al:
Prognosis of breast cancer after supraclavicular lymph node
metastasis: not a distant metastasis. Ann Surg Oncol. 13:1457–1465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen HC, Wang CY and Wang CS: Marked
thrombocytosis during treatment with ceftazidime for pulmonary
infection. Pharm World Sci. 30:70–72. 2008. View Article : Google Scholar
|
|
31
|
Nanavati A, Patel N and Burke J:
Thrombocytosis and coronary occlusion. JACC Cardiovasc Interv.
5:e18–e19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roodman GD: Mechanisms of bone metastasis.
New Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|