Introduction
Renal cell carcinoma (RCC) is a prominent disease,
with ~57,760 new cases and ~12,980 mortalities reported worldwide
in 2009 (1). Although the majority
of patients exhibiting early-stage RCC can be treated surgically,
recurrence with distant metastases was observed upon diagnosis in
~25–50% of RCC patients and the five-year survival rate was <20%
(2,3). Patients that were diagnosed with the
same stage of renal cancer and that received similar treatment to
early-stage RCC patients demonstrated a different prognosis,
indicating that hereditary factors may contribute to the prognosis
of RCC (4,5). Furthermore, previous studies have
reported that vascular endothelial growth factors (VEGFs), and
platelet-derived growth factors and their receptors may have a role
in promoting the pathogenesis of RCC (6,7).
The VEGF gene is a potent endothelial cell mitogen,
which consists of eight exons (8).
It has been hypothesized that 30 types of single nucleotide
polymorphism (SNP) exist in the VEGF gene (8). Three common SNPs, VEGF −2578C/A,
−1154G/A and −634C/G, have been widely investigated and identified
to be associated with VEGF protein production (9). Furthermore, VEGF −2578C/A, −1154G/A
and −634C/G have been associated with the risk or the prognosis of
various diseases, such as breast cancer, pancreatic adenocarcinoma,
oral cancer and Alzheimer’s disease (10–13).
A previous study indicated that VEGF is
overexpressed in RCC tissue when compared with healthy renal tissue
(14). Furthermore, therapeutic
targeting of VEGF has demonstrated clinical efficacy in the
treatment of RCC (15,16); thus, VEGF polymorphisms may be
associated with disease progression and prognosis in RCC patients.
However, three studies reported inconsistent results regarding the
association between VEGF polymorphisms and progression or prognosis
of RCC (17–19). Therefore, the current report
presents a cohort study investigating the association between VEGF
polymorphisms −2578C/A, −1154G/A and −634C/G, and the clinical
outcome of RCC patients, as well as the interaction of the VEGF
polymorphisms with tumor stage, metastasis and size.
Patients and methods
Patients
Patients that were diagnosed with RCC between
January 2006 and November 2007 were enrolled in the present study
from the First Affiliated Hospital of Zhengzhou University.
Patients with a history of pregnancy, malignancy, chemotherapy or
radiotherapy were excluded from the present study. All of the
biopsy samples were obtained via radical or partial nephrectomy.
The RCC tumor stage was determined according to the American Joint
Committee on Cancer tumor-node-metastasis staging system (20). The patients in the study were
followed up until November 2012, however, six patients were lost to
follow-up. Baseline characteristics of all of the patients were
obtained using a self-designed questionnaire and medical records.
Written informed consent was obtained from all patients and this
study was approved by the ethics committee of The First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China).
Polymerase chain reaction (PCR)
All of the patients provided a 5-ml peripheral
venous blood sample after consenting to participate in the study.
Genomic DNA was extracted using a TIANamp Blood DNA kit (Tiangen
Biotech Co., Ltd., Beijing, China) and DNA dissolved in water,
according to the manufacturer’s instructions. The presence of VEGF
−2578C/A, −1154G/A and −634C/G was determined using PCR combined
with a restriction fragment length polymorphism assay (Applied
Biosystems, Foster City, CA, USA). The primers and probes for VEGF
−2578C/A, −1154G/A and −634C/G were designed using Assay Design 3.1
software (Sequenom Inc., San Diego, CA, USA). The PCR reaction was
performed in a 25-μl reaction solution with 25 mM MgCl2,
each primer and 2 mM deoxynucleotide triphosphates, 1 mmol/l
MgCl2, 1.25 units Taq polymerase (Takara Biotechnology
Co., Ltd., Dalian, China) and 0.5 μl 5X PCR buffer (Takara
Biotechnology Co., Ltd.). The DNA was amplified at 95°C for 5 sec,
subjected to 40 cycles at 92°C for 40 sec and elongated at 60°C for
40 sec.
Statistical analysis
All analyses were conducted using SPSS version 16.0
software (SPSS Inc., Chicago, IL, USA). Continuous variables are
expressed as the mean ± standard deviation and categorical
variables are expressed as frequencies (percentages). The
χ2 test was used to compare the genotype frequencies
between the patients and the controls. The primary end point was
five-year survival, which was calculated as the time period from
diagnosis to mortality (from any cause), or the last known date
that the patient was alive. Survival differences were compared
using the log-rank test and multivariate analysis of survival was
conducted using Cox’s proportional hazard regression analysis (with
hazard ratios [HR] and 95% confidence intervals [CI]) to identify
independent prognostic variables. Survival distributions were
estimated using the Kaplan-Meier method and assessed using the
log-rank test. Two-tailed P-values of <0.05 were considered to
indicate a statistically significant difference.
Results
Patient characteristics and outcomes
A total of 336 patients with RCC were invited to
participate in the present study; 310 patients consented, resulting
in a participation rate of 92.26%. The demographic and clinical
characteristics of the patients are presented in Table I. The cohort consisted of 206 males
(66.45%) and 104 females (33.55%) with a median age of 61.5 years
(range, 27.2–81.4 years) upon initial diagnosis. Of the 310
patients, 91.61% exhibited clear cell RCC, 60.32% exhibited stage
I–II cancer, 61.29% had a small tumor (longest diameter, ≤4 cm),
and 78.39% presented with lymph node or distant metastases.
Patients exhibiting clear cell RCC, stage I–II cancer, a small
tumor (longest diameter, ≤4 cm) and no metastasis were associated
with a longer OS period.
 | Table IUnivariate analysis of the demographic
and clinic characteristics of 310 renal cell carcinoma patients
(age, 61.5 years; range, 27.2–81.4 years). |
Table I
Univariate analysis of the demographic
and clinic characteristics of 310 renal cell carcinoma patients
(age, 61.5 years; range, 27.2–81.4 years).
| Variable | Patients, n (%) | Overall survival
period, months |
|---|
| Gender |
| Male | 206 (66.45) | 33.5 |
| Female | 104 (33.55) | 34.6 |
| Tumor histology |
| Non-clear cell | 26 (8.39) | 32.5 |
| Clear cell | 284 (91.61) | 35.7 |
| Tumor stage |
| I–II | 187 (60.32) | 38.6 |
| III–IV | 123 (39.68) | 27.4 |
| Tumor size, cm |
| >4 | 120 (38.71) | 28.7 |
| ≤4 | 190 (61.29) | 36.4 |
| Metastasis |
| No | 67 (21.61) | 35.5 |
| Yes | 243 (78.39) | 29.2 |
VEGF genotypes and tumor
characteristics
The genotype distributions of VEGF −2578C/A,
−1154G/A and −634C/G demonstrated Hardy-Weinberg equilibrium.
Conditional regression analysis identified that the VEGF −2578 AA
genotype was significantly more frequent in stage III–IV patients
(odds ratio [OR], 0.47; 95% CI, 0.24–0.95) and patients with larger
tumors (longest diameter, >4 cm; OR=0.44; 95% CI, 0.22–0.89)
when compared with the −2578 CC genotype (Table II). Furthermore, the VEGF −634 GG
genotype was significantly more frequent in patients with a large
tumor (longest diameter, >4 cm) when compared with the −634 CC
genotype (OR, 0.68; 95% CI, 0.48–0.97).
 | Table IIAssociation of VEGF polymorphism with
tumor stage, size and metastasis. |
Table II
Association of VEGF polymorphism with
tumor stage, size and metastasis.
| VEGF
polymorphism | Cases, n | Tumor stage, n | OR (95% CI)a | P-value | Metastasis, n | OR (95% CI)a | P-value | Tumor size, n | OR (95% CI)a | P-value |
|---|
|
|
|
|---|
| I–II | III–IV | No | Yes | ≤4 cm | >4 cm |
|---|
| −2578C/A |
| CC | 145 | 96 | 49 | 1.0 (−) | | 36 | 109 | 1.0 (−) | | 98 | 47 | 1.0 (−) | |
| CA | 113 | 66 | 47 | 0.72 (0.42–1.23) | 0.20 | 23 | 90 | 0.77 (0.41–1.45) | 0.40 | 67 | 46 | 0.70 (0.41–1.20) | 0.17 |
| AA | 52 | 25 | 27 | 0.47 (0.24–0.95) | 0.02 | 8 | 44 | 0.55 (0.21–1.33) | 0.16 | 25 | 27 | 0.44 (0.22–0.89) | 0.01 |
| C allele | 403 | 258 | 145 | 1.0 (−) | | 95 | 308 | 1.0 (−) | | 263 | 140 | 1.0 (−) | |
| A allele | 217 | 116 | 101 | 0.63
(0.45–0.90) | 0.01 | 39 | 178 | 0.71
(0.45–1.08) | 0.10 | 117 | 100 | 0.61
(0.43–0.87) | 0.00 |
| −1154G/A |
| GG | 191 | 117 | 74 | 1.0 (−) | | 44 | 147 | 1.0 (−) | | 120 | 71 | 1.0 (−) | |
| GA | 97 | 57 | 40 | 0.90
(0.53–1.53) | 0.68 | 20 | 77 | 0.87
(0.45–1.63) | 0.64 | 58 | 39 | 0.88
(0.52–1.50) | 0.62 |
| AA | 22 | 13 | 9 | 0.91
(0.34–2.55) | 0.84 | 3 | 19 | 0.53
(0.10–1.92) | 0.31 | 12 | 10 | 0.71
(0.27–1.94) | 0.45 |
| G allele | 479 | 301 | 178 | 1.0 (−) | | 108 | 371 | 1.0 (Ref.) | | 298 | 181 | 1.0 (−) | |
| A allele | 141 | 73 | 68 | 0.63
(0.43–0.94) | 0.02 | 26 | 115 | 0.78
(0.46–1.27) | 0.30 | 82 | 59 | 0.84
(0.57–1.26) | 0.38 |
| −634C/G |
| CC | 147 | 91 | 54 | 1.0 (−) | | 34 | 113 | 1.0 (−) | | 98 | 49 | 1.0 (−) | |
| CG | 109 | 63 | 46 | 0.81
(0.47–1.39) | 0.42 | 24 | 85 | 0.94
(0.49–1.77) | 0.83 | 64 | 45 | 0.71
(0.41–1.23) | 0.19 |
| GG | 54 | 34 | 20 | 1.0
(0.51–2.05) | 0.98 | 9 | 45 | 0.66
(0.26–1.56) | 0.32 | 28 | 26 | 0.54
(0.27–1.07) | 0.06 |
| C allele | 403 | 245 | 158 | 1.0 (−) | | 92 | 311 | 1.0 (−) | | 260 | 143 | 1.0 (−) | |
| G allele | 217 | 131 | 86 | 1.01
(0.76–1.33) | 0.96 | 42 | 175 | 0.81
(0.52–1.24) | 0.32 | 120 | 97 | 0.68
(0.48–0.97) | 0.02 |
Multivariate analysis
The association between the VEGF genotype and
survival with RCC is demonstrated in Table III. The median duration of the
follow-up was 33.8 months (range, 2–69 months). Among the 310
patients, 174 patients succumbed due to cancer during the follow-up
period, providing a five-year survival rate of 43.87%. The VEGF
−2578 AA genotype was significantly associated with poor OS
(adjusted HR, 2.96; 95% CI, 1.40–6.53), and the five-year survival
rate of patients exhibiting the AA genotype was 25.0% (Table III). However, the VEGF −1154G/A
and −634C/G polymorphisms were not significantly associated with
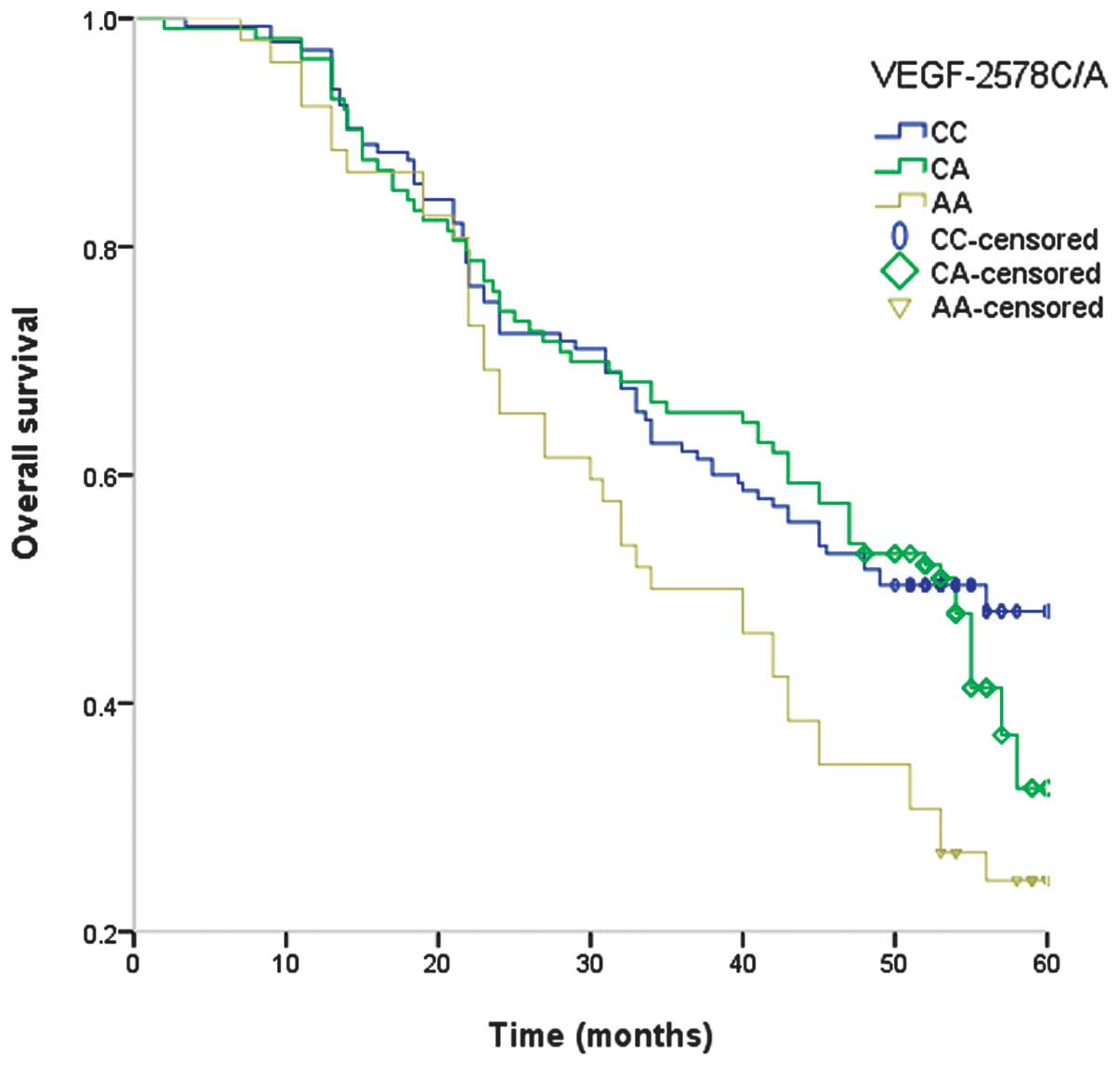
the OS of RCC patients. Examination of Kaplan-Meier curves for the
VEGF −2578A/C genotypes indicated that the VEGF −2578A/C
polymorphism is associated with the overall survival of RCC
patients (Fig. 1).
 | Table IIICox’s proportional hazard regression
analysis of VEGF polymorphisms on the survival of renal cell
carcinoma patients. |
Table III
Cox’s proportional hazard regression
analysis of VEGF polymorphisms on the survival of renal cell
carcinoma patients.
| VEGF
polymorphism | Cases, n | Mortalities, n | Five-year survival
rate, % | Hazard ratio (95%
CI)a | P-value |
|---|
| −2578C/A |
| CC | 145 | 73 | 49.7 | 1.0 (−) | |
| CA | 113 | 62 | 45.1 | 1.20
(0.71–2.02) | 0.47 |
| AA | 52 | 39 | 25.0 | 2.96
(1.40–6.53) | 0.00 |
| C allele | 403 | 208 | 48.5 | 1.0 (−) | |
| A allele | 217 | 140 | 35.3 | 1.73
(1.21–2.46) | 0.00 |
| −1154G/A |
| GG | 191 | 104 | 45.5 | 1.0 (−) | |
| GA | 97 | 55 | 43.3 | 1.10
(0.65–1.85) | 0.72 |
| AA | 22 | 15 | 31.8 | 1.79
(0.65–5.43) | 0.22 |
| G allele | 479 | 263 | 45.1 | 1.0 (−) | |
| A allele | 141 | 85 | 39.7 | 1.25
(0.84–1.87) | 0.12 |
| −634C/G |
| CC | 147 | 76 | 48.3 | 1.0 (−) | |
| CG | 109 | 63 | 42.2 | 1.28
(0.75–2.18) | 0.45 |
| GG | 54 | 35 | 35.2 | 1.72
(0.86–3.49) | 0.10 |
| C allele | 403 | 219 | 46.7 | 1.0 (−) | |
| G allele | 217 | 129 | 38.7 | 1.38
(0.98–1.97) | 0.06 |
Discussion
VEGF is involved in the regulation of angiogenesis
and is considered to be a potent stimulatory cytokine in tumor
angiogenesis, which appears to be key in influencing tumor
metastasis and prognosis (21).
Previous studies have indicated that polymorphisms in the VEGF gene
are associated with various types of cancer, including breast,
prostate and gastric cancer. VEGF gene polymorphisms are correlated
with various characteristics of cancer, such as susceptibility,
tumor grade and OS of cancer (22–24).
Three gene polymorphisms, VEGF −2578C/A, −1154G/A and −634C/G,
located at the promoter region of VEGF, are involved in altering
the gene transcription and expression of VEGF (25). Few previous studies have
investigated the association between VEGF −2578C/A, −1154G/A and
−634C/G polymorphisms and the prognosis of RCC patients (17–19),
thus, the present study is, to the best of our knowledge, the first
to identify that the VEGF −2578C/A polymorphism is associated with
the prognosis of RCC patients, as well as demonstrating an
interaction with the tumor stage and size.
The present study identified that the VEGF −2578 AA
genotype is associated with shorter OS period in RCC patients,
indicating that the VEGF −2578 AA genotype increases the expression
of VEGF and promotes tumor angiogenesis, thus resulting in a higher
tumor stage and decreased OS in RCC patients. Therefore, VEGF gene
polymorphisms may be critical in altering VEGF expression and
influencing the progression of RCC patients. Furthermore, two
previous studies reported results that were consistent with the
present study, which demonstrated an association between VEGF gene
polymorphisms and the prognosis of various diseases (17,26).
Hefler et al (27) reported
that VEGF −634G/C, −1154G/A, and −2578C/A polymorphisms were
associated with increased VEGF expression and a shortened OS period
in ovarian cancer patients (26).
An additional study identified that the −2578 C allele was
correlated with increased VEGF production in vitro (25). However, reports of an association
between −2578C/A polymorphisms and tumor prognosis are inconsistent
(21,28,29).
For example, Supic et al (30) conducted a study of 114 oral squamous
cell carcinoma (OSCC) patients and 126 control subjects, and
reported a non-significant association between VEGF −2578C/A
polymorphisms and the prognosis of OSCC patients (29). The discrepancies between the
above-mentioned studies may be due to differences in genetic
variant frequencies between individuals with different ethnicities
or carcinoma types.
Notably, the VEGF −634 GG genotype is associated
with tumor size, although, no association was identified between
the VEGF −634C/G polymorphism and the prognosis of RCC patients.
One study indicated that the VEGF-634 GG genotype is associated
with high serum VEGF levels and reduced OS periods in advanced
gastric cancer patients when compared with the CC genotype
(21). An additional study
identified that the −634 CC genotype was significantly correlated
with larger tumor size and higher histological grade (31).
The present study has two limitations. First, the
study was conducted in a single hospital in China; thus, it may not
be representative of the general population. However, the allele
frequencies demonstrated Hardy-Weinberg equilibrium and were
similar to the minor allele frequencies obtained from the The
National Center for Biotechnology Information database (www.ncbi.nlm.nih.gov/SNP), which indicates that the
population of the present study may be representative of the
general population. Second, the number of cases analyzed in the
present study was relatively small, which may reduce the
statistical power to detect differences between the various VEGF
allele groups. Therefore, further studies using a large multicenter
cohort are required to investigate the association between VEGF
gene polymorphisms and the prognosis of RCC patients.
In conclusion, the present study identified that the
VEGF −2578C/A polymorphism may be associated with the prognosis of
RCC patients, and may interact with the tumor stage and size.
Therefore, the present study may aid with predicting the clinical
outcome of RCC patients. Further large cohort studies are required
to demonstrate the clinical significance of the VEGF −2578C/A
polymorphism.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao Q, Wang J, Zhang M, et al: Genetic
Variants in RKIP Are Associated with Clear Cell Renal Cell
Carcinoma Risk in a Chinese Population. PLoS One. 9:e1092852014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mu HJ, Zou J, Xie P, Xu ZQ, Ruan J, Yang
SD and Yin Y: Association of leptin receptor Lys109Arg and
Gln223Arg polymorphisms with increased risk of clear cell renal
cell carcinoma. Asian Pac J Cancer Prev. 15:4211–4215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patard JJ, Pouessel D, Bensalah K and
Culine S: Targeted therapy in renal cell carcinoma. World J Urol.
26:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vincenti V, Cassano C, Rocchi M and
Persico G: Assignment of the vascular endothelial growth factor
gene to human chromosome 6p21.3. Circulation. 93:1493–1495. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watson CJ, Webb NJ, Bottomley MJ and
Brenchley PE: Identification of polymorphisms within the vascular
endothelial growth factor (VEGF) gene: correlation with variation
in VEGF protein production. Cytokine. 12:1232–1235. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koukourakis MI, Papazoglou D,
Giatromanolaki A, et al: VEGF gene sequence variation defines VEGF
gene expression status and angiogenic activity in non-small cell
lung cancer. Lung Cancer. 46:293–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo T, Chen L, He P, et al: Vascular
endothelial growth factor (VEGF) gene polymorphisms and breast
cancer risk in a Chinese population. Asian Pac J Cancer Prev.
14:2433–2437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sivaprasad S, Govardhan B, Harithakrishna
R, et al: Association of vascular endothelial growth factor (VEGF)
gene polymorphism and increased serum VEGF concentration with
pancreatic adenocarcinoma. Pancreatology. 13:267–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandal RK, Yadav SS, Panda AK and Khattri
S: Vascular endothelial growth factor 936 c>T polymorphism
increased oral cancer risk: evidence from a meta-analysis. Genet
Test Mol Biomarkers. 17:543–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He D, Lu W, Chang K, et al: Vascular
endothelial growth factor polymorphisms and risk of Alzheimer’s
disease: a meta-analysis. Gene. 518:296–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garcia-Donas J, Rodriguez-Antona C and
Jonasch E: Molecular markers to predict response to therapy. Semin
Oncol. 40:444–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vrdoljak E, Rini B, Schmidinger M, et al:
Bisphosphonates and vascular endothelial growth factor-targeted
drugs in the treatment of patients with renal cell carcinoma
metastatic to bone. Anticancer Drugs. 24:431–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jäkel CE, Hauser S, Rogenhofer S, et al:
Clinical studies applying cytokine-induced killer cells for the
treatment of renal cell carcinoma. Clin Dev Immunol.
2012:4732452012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai Y, Sakano S, Korenaga Y, Eguchi S
and Naito K: Associations of single nucleotide polymorphisms in the
vascular endothelial growth factor gene with the characteristics
and prognosis of renal cell carcinomas. Eur Urol. 52:1147–1155.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sáenz-López P, Vazquez F, Cozar JM, et al:
VEGF polymorphisms are not associated with an increased risk of
developing renal cell carcinoma in Spanish population. Hum Immunol.
74:98–103. 2013. View Article : Google Scholar
|
|
20
|
Edge SB, Byrd DR, Compton CC, et al:
Kidney. AJCC Cancer Staging Manual. 7th edition. Springer; New
York, NY: pp. 2010479–2010489. 2010
|
|
21
|
Abe A, Sato K, Habuchi T, et al: Single
nucleotide polymorphisms in the 3′ untranslated region of vascular
endothelial growth factor gene in Japanese population with or
without renal cell carcinoma. Tohoku J Exp Med. 198:181–190. 2002.
View Article : Google Scholar
|
|
22
|
Salven P, Teerenhovi L and Joensuu H: A
high pretreatment serum vascular endothelial growth factor
concentration is associated with poor outcome in non-Hodgkin’s
lymphoma. Blood. 90:3167–3172. 1997.PubMed/NCBI
|
|
23
|
Martinez-Fierro ML, Garza-Veloz I,
Rojas-Martinez A, et al: Positive association between vascular
endothelial growth factor (VEGF) −2578 C/A variant and prostate
cancer. Cancer Biomark. 13:235–241. 2013.
|
|
24
|
Chen P, Zhu J, Liu DY, et al:
Over-expression of survivin and VEGF in small-cell lung cancer may
predict the poorer prognosis. Med Oncol. 31:7752014. View Article : Google Scholar
|
|
25
|
Oh SY, Kwon HC, Kim SH, et al: The
relationship of vascular endothelial growth factor gene
polymorphisms and clinical outcome in advanced gastric cancer
patients treated with FOLFOX: VEGF polymorphism in gastric cancer.
BMC Cancer. 13:432013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Absenger G, Szkandera J, Stotz M, et al: A
common and functional gene variant in the vascular endothelial
growth factor a predicts clinical outcome in early-stage breast
cancer. Mol Carcinog. 52(Suppl 1): E96–E102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hefler LA, Mustea A, Könsgen D, et al:
Vascular endothelial growth factor gene polymorphisms are
associated with prognosis in ovarian cancer. Clin Cancer Res.
13:898–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shahbazi M, Fryer AA, Pravica V, et al:
Vascular endothelial growth factor gene polymorphisms are
associated with acute renal allograft rejection. J Am Soc Nephrol.
13:260–264. 2002.
|
|
29
|
Sa-Nguanraksa D, Chuangsuwanich T,
Pongpruttipan T, et al: Vascular endothelial growth factor −634G/C
polymorphism is associated with increased breast cancer risk and
aggressiveness. Mol Med Rep. 8:1242–1250. 2013.PubMed/NCBI
|
|
30
|
Supic G, Jovic N, Zeljic K, Kozomara R and
Magic Z: Association of VEGF-A genetic polymorphisms with cancer
risk and survival in advanced-stage oral squamous cell carcinoma
patients. Oral Oncol. 48:1171–1177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin Q, Hemminki K, Enquist K, et al:
Vascular endothelial growth factor polymorphisms in relation to
breast cancer development and prognosis. Clin Cancer Res.
11:3647–3653. 2005. View Article : Google Scholar : PubMed/NCBI
|