Introduction
Gastric cancer (GC) is the second most common cause
of cancer-related mortality worldwide and the third most common
cancer in China (1,2). The primary treatment for operable GC
is surgery. However, recurrence rates are high when using surgery
only (3,4). Compared with surgery only, additional
adjuvant chemotherapy has shown clinical benefits in treating GC
when evaluated by meta-analyses (5,6).
Therefore, it is necessary to develop optimal adjuvant chemotherapy
regimens to decrease recurrence and improve the quality of life for
GC patients following surgical resection.
S-1 is a fourth generation oral fluoropyrimidine,
which contains tegafur/gimeracil/oteracil potassium in a molar
ratio of 1.0:0.4:1.0. Treatment with S-1 subsequent to surgery has
been shown to improve the 5 year overall survival (OS) rate of GC
patients from 61.1% with surgery alone to 71.1%, and the
relapse-free survival rate of GC patients in 5 years from 53.1 to
65.4% (7). However, a subgroup data
analysis showed that use of adjuvant chemotherapy with S-1 alone
following surgery for patients diagnosed with stage III GC did not
result in improved survival (7).
For metastatic or recurrent GC, adjuvant
chemotherapy with S-1 plus cisplatin (SP) showed improved results,
with a longer progression-free survival (PFS) time and a longer OS
time, compared with S-1 alone (8).
Compared with cisplatin, oxaliplatin has a more favorable safety
profile, including less emetogenic and less nephrotoxic potential.
A REAL-2 study revealed that a oxaliplatin-based regimen was just
as effective as a cisplatin-based regimen in patients with
previously untreated advanced GC (AGC) (9). Additionally, a large randomized phase
III study recently reported that S-1 plus oxaliplatin (SOX) showed
non-inferiority to SP in PFS and that the treatment was well
tolerated, with benefits in terms of outpatient-based treatment in
patients with AGC (10).
In our previous study, the SOX regimen with 130
mg/m2 oxaliplatin was found to be effective and safe to
use as a first-line chemotherapy in patients with AGC (11). Therefore, the dose of oxaliplatin
was fixed at 130 mg/m2 in the current study. A dose of
S-1 at 80 mg/m2 (in two seperate half doses) twice daily
on days 1 to 14 every 21 days is widely used for phase II/III
studies (12,13). However, in our previous dose-finding
study on adjuvant chemotherapy with SOX (130 mg/m2
oxaliplatin) for GC, the maximum tolerated dose of S-1 was
initially determined to be 70 mg/m2 (14). Grade 3 vomiting was observed as
dose-limiting toxicity (DLT) during the first treatment cycle in
this study, highlighting the fact that oxaliplatin may play an
important role in DLT. Based on these studies, we hypothesized that
from the two doses (70 vs. 80 mg/m2/day) there should be
an optimal dosage of S-1 when combined with oxaliplatin (130
mg/m2) in adjuvant chemotherapy for GC. The aim of the
present study was therefore to evaluate the feasibility and safety
of adjuvant SOX chemotherapy for GC patients. In addition, the
present study aimed to determine the optimal dosage of S-1 in the
SOX combination for GC patients.
Patients and methods
Patients
A total of 60 eligible patients at the Cancer
Institute and Hospital of the Chinese Academy of Medical Sciences
(Beijing, China) were recruited for this study according to the
following inclusion criteria: Subtotal or total gastrectomy;
histologically proven stage II/III [i.e., pathological stage T2N+,
T3–T4 and/or N+, according to the American Joint Committee on
Cancer tumor-node-metastasis system, 7th edition (15)] GC of the stomach or gastroesophageal
junction; age distribution of 20 to 75 years old; an Eastern
Cooperative Oncology Group performance status (16) of 0–1; an absolute granulocyte count
of >1,500/l; a platelet count of >100,000/l; a hemoglobin
level of >90 g/l; a serum bilirubin level of less than the upper
limit of normal (ULN); a normal creatinine level; an alanine
transaminase and aspartate transaminase level of <1.5×ULN; and
no treatment with chemotherapy prior to the present SOX treatment.
Only patients who could swallow tablets were admitted into the
study group, and all patients were told to practice medically
effective contraception.
Study approval and consent
The present study was approved by the Ethics
Committee of the Cancer Institute and Hospital, (Chinese Academy of
Medical Sciences, Beijing, China). All patients provided written
informed consent.
Treatment schedule
Treatment with SOX therapy was started at 4–8 weeks
post-surgery, and repeated for 8 cycles. All eligible patients were
randomly assigned to either arm A receiving S-1 at a dose of 70
mg/m2/day (in two seperate half doses) or arm B
receiving S-1 at a dose of 80 mg/m2/day (in two seperate
half doses). S-1 was administered orally twice per day, within half
an hour of a meal on days 1 to 14, every 3 weeks (1 cycle).
Oxaliplatin was administered intravenously to all patients on day 1
every 3 weeks at a fixed dose of 130 mg/m2. All patients
received 5-HT3 antagonists as antiemetics following administration
of oxaliplatin. If patients developed grade 4 neutropenia or grade
3/4 thrombocytopenia, or non-hematological toxic effects of above
grade 2, the dose of S-1 was reduced by 10 mg/m2/day,
and at the same time, the dose of oxaliplatin was reduced by 25%.
If recovery from such toxicities was confirmed at a reduced dose,
the administration at the reduced dosage was continued. S-1 and
oxaliplatin could be reduced twice, but treatment was discontinued
if subsequent reduction was indicated. In cases of
oxaliplatin-related neurological adverse events, S-1 could be
continued as monotherapy. Oxaliplatin monotherapy was not allowed
if S-1 was discontinued.
Complete blood count and blood chemistry studies
were performed weekly. Administration of the two agents would be
delayed until adequate hematological recovery (absolute neutrophil
count, ≥1.5×109/l; platelet count, ≥100×109/l) was
achieved. Non-hematological toxicities, excluding alopecia, were
required to be grade 1 or better prior to initiation of each cycle.
If the toxicity failed to recover within 3 weeks after the
scheduled day for starting the next cycle, the patients were
withdrawn from the study. Therapy was discontinued if there was any
evidence of documented recurrence, unacceptable toxicities or
refused treatment.
Evaluation
All eligible patients were considered to be
assessable for feasibility and safety. Adverse events were graded
according to the National Cancer Institute-Common Toxicity Criteria
version 4.0 (17). Feasibility was
evaluated by the completion status of the protocol treatment and
compared between the two arms. The rate of completing ≥6 and 8
cycles of treatment, delayed courses and dose reduction was
evaluated and compared in the two arms. The number of patients was
calculated at the time when the treatment was stopped or delayed,
and when the planned administration dose was reduced. In the two
arms, the relative total administration dose in the 6th treatment
course (R6) and the 8th treatment course (R8) were calculated as
follows: R6 = (D1 + D2 + D3 + D4 + D5 + D6) / (P1 × 6) and R8 = (D1
+ D2 + D3 + D4 + D5 + D6 + D7 + D8) / (P1 × 8), where R is the
relative total administration dose, D is the actual dose in each
cycle and P1 is the planned dose in the first cycle. If no
treatment was offered in this cycle, the actual dose was set to
zero. The cumulative rate of the relative total administration dose
in the 6th and 8th treatment courses were also calculated and
compared in two arms.
Statistics
Patient characteristics, feasibility and adverse
events were analyzed. The differences in the median body surface
area and ages between the two arms were evaluated by the
Mann-Whitney U test, while other characteristics were evaluated by
the χ2 test. The differences in completion status of
protocol treatment and adverse events between the two arms were
evaluated by the χ2 test. The differences in the
relative total administration dose between the two arms were
compared by Student’s t-test. The cumulative rates of the relative
total administration dose of S-1 and oxaliplatin were examined by
the Kaplan-Meier method and differences in the two arms were
calculated by the log-rank test. Two-sided P<0.05 was used to
indicate a statistically significant difference.
Results
Study population
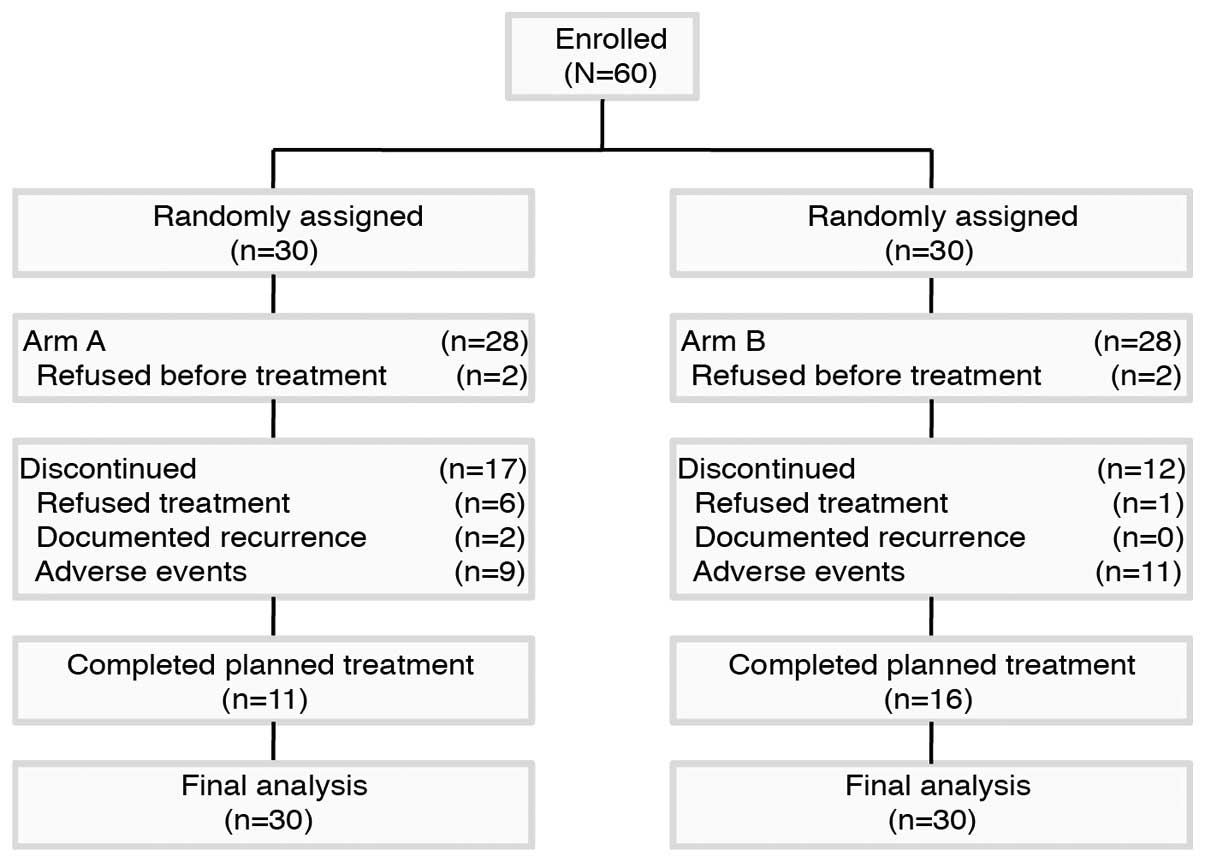
Between June 2011 and June 2013, 60 patients were
recruited to the study. Among these, two patients in arm A and two
patients in arm B refused treatment and were excluded from all
analyses. Patient disposition throughout the study is shown in
Fig. 1. The major reasons for
discontinuation of treatment in arm A were documented recurrence
(6.7%), adverse events (30%) and refused treatment (20%). However,
notably, four patients refused treatment subsequent to finishing 6
cycles of therapy in arm A. Patient baseline characteristics were
well balanced between the two arms (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Arm A (n=30) | Arm B (n=30) | Total (n=60) |
|---|
|
|
|
|
|---|
| Characteristics | No. | % | No. | % | No. | % |
|---|
| Age, years |
| Median | 53.5 | | 52.5 | | 53 | |
| Range | 28–72 | | 27–67 | | 27–72 | |
| BSA,
m2 |
| Median | 1.705 | | 1.705 | | 1.705 | |
| Range | 1.35–2.01 | | 1.48–1.98 | | 1.35–2.01 | |
| Gender |
| Male | 24 | 80.0 | 23 | 76.7 | 47 | 78.3 |
| Female | 6 | 20.0 | 7 | 23.3 | 13 | 21.7 |
| ECOS PS |
| 0 | 13 | 43.3 | 13 | 43.3 | 26 | 43.3 |
| 1 | 17 | 56.7 | 17 | 56.7 | 34 | 56.7 |
| Type of
gastrectomy |
| Total | 6 | 20.0 | 5 | 16.7 | 11 | 18.3 |
| Partial | 24 | 80.0 | 25 | 83.3 | 49 | 81.7 |
| TNM stage |
| IA | 2 | 6.7 | 2 | 6.7 | 4 | 6.7 |
| II | 9 | 30.0 | 8 | 26.7 | 17 | 28.3 |
| III | 18 | 60.0 | 19 | 63.3 | 37 | 61.7 |
| IV | 1 | 3.3 | 1 | 3.3 | 2 | 3.3 |
Feasibility
In total, 48.2% of patients completed the planned 8
cycles of treatment and 75% of patients completed 6 cycles of
therapy. A total of 39.3% of patients in arm A received the full 8
cycles of SOX compared with 57.1% in arm B [not significant (NS),
P=0.181] (Table II). However,
82.1% of patients in arm A received ≥6 cycles of therapy, which was
higher the percentage of 67.9% in arm B (NS, P=0.217). In total,
91.1% of patients were observed with delayed courses. Among them,
82.1% of patients underwent delayed courses in arm A, while 100%
were observed in arm B (P=0.019). In total, the rate of patients
with a dose-reduction of S-1 and oxaliplatin was 33.9% and 42.9%,
respectively. The rate of patients with a dose-reduction of S-1 was
14.3% in arm A and 53.6% in arm B (P=0.002). In the patients with a
dose-reduction of oxaliplatin, a rate of 39.3% was observed in arm
A and 46.4% was observed in arm B (NS, P=0.558).
 | Table IICompletion status of protocol
treatment. |
Table II
Completion status of protocol
treatment.
| Total (n=56) | Arm A (n=28) | Arm B (n= 28) | |
|---|
|
|
|
| |
|---|
| No. | % | No. | % | No. | % | P-value |
|---|
| Patients received
≥6 cycles of therapy | 42 | 75.0 | 23 | 82.1 | 19 | 67.9 | 0.217 |
| Patients received 8
cycles of therapy | 27 | 48.2 | 11 | 39.3 | 16 | 57.1 | 0.181 |
| Patients with
delayed courses | 51 | 91.1 | 23 | 82.1 | 28 | 100.0 | 0.019 |
| Patients with
dose-reduction |
| S-1 | 19 | 33.9 | 4 | 14.3 | 15 | 53.6 | 0.002 |
| Oxaliplatin | 24 | 42.9 | 11 | 39.3 | 13 | 46.4 | 0.558 |
The mean of the relative total administration dose
of S-1 in the 6th treatment course was 89.43% in arm A and 81.36%
in arm B (NS, P=0.213), and the mean of the relative total
administration dose of S-1 in the 8th treatment course in arm A was
higher than that in arm B (77.18 vs. 73.07%; NS, P=0.551) (Table III). The mean of the relative
total administration dose of oxaliplatin in the 6th treatment
course was 83.57% in arm A and 81.89% in arm B (NS, P=0.810), and
the mean of the relative total administration dose of oxaliplatin
in the 8th treatment course in arm A was higher that in arm B
(66.57 vs. 70.68%; NS, P=0.540) (Table III).
 | Table IIIRelative administration dose analysis
of S-1 and oxaliplatin. |
Table III
Relative administration dose analysis
of S-1 and oxaliplatin.
| Parameter | Arm A (n=28) | Arm B (n=28) | P-value |
|---|
| Cumulative rate of
relative total administration dose of S-1 at 100% in the 6th
treatment course, % (95% CI) | 71.40
(56.50–90.30) | 21.40
(10.50–43.60) | 0.001 |
| Relative total
administration dose of S-1 (6th treatment course), % | | | 0.213 |
| Mean | 89.43 | 81.36 | |
| Standard
deviation | 22.08 | 25.70 | |
| Cumulative rate of
relative total administration dose of S-1 at 100% in the 8th
treatment course, % (95% CI) | 32.10
(18.80–55.10) | 14.30
(5.77–35.40) | 0.276 |
| Relative total
administration dose of S-1 (8th treatment course), % | | | 0.551 |
| Mean | 77.18 | 73.07 | |
| Standard
deviation | 23.74 | 27.36 | |
| Cumulative rate of
relative total administration dose of OXA at 100% in the 6th
treatment course, % (95% CI) | 46.40
(31.20–69.10) | 32.10
(18.80–55.10) | 0.464 |
| Relative total
administration dose of OXA (6th treatment course), % | | | 0.810 |
| Mean | 83.57 | 81.89 | |
| Standard
deviation | 24.74 | 27.09 | |
| Cumulative rate of
relative total administration dose of OXA at 100% in the 8th
treatment course, % (95% CI) | 7.14
(1.88–27.20) | 14.30
(5.77–35.40) | 0.230 |
| Relative total
administration dose of OXA (8th treatment course), % | | | 0.540 |
| Mean | 66.57 | 70.68 | |
| Standard
deviation | 22.13 | 27.38 | |
The cumulative rates of the relative total
administration dose of S-1 in the 6th treatment course at 100% was
71.4% (95% CI, 56.5–90.3%) in arm A, which was significantly higher
than the 21.4% (95% CI, 10.5–43.6%) in arm B (P=0.001) (Fig. 2A; Table III). However, when calculated in
the 8th treatment course, the rates were 32.1% (95% CI, 18.8–55.1%)
in arm A and 14.3% (95% CI, 5.77–35.4%) in arm B (NS, P=0.276)
(Fig. 2B; Table III). The cumulative rates of the
relative total administration dose of oxaliplatin in the 6th
treatment course at 100% were 46.4% (95% CI, 31.2–69.1%) in arm A
and 32.1% (95% CI, 18.8–55.1%) in arm B (NS, P=0.464) (Fig. 2C; Table III). Additionally, when calculated
in the 8th treatment course, the rates were 7.14% (95% CI,
1.88–27.2%) in arm A and 14.3% (95% CI, 5.77–35.4%) in arm B (NS,
P=0.23) (Fig. 2D; Table III).
Adverse events
Drug-related adverse events are listed in Table IV. In total, the most common grade
3/4 hematological toxicities were neutropenia (19.6%) and
thrombocytopenia (19.6%). A total of 10.7% of patients in arm A and
28.6% in arm B experienced grade 3/4 neutropenia (NS, P=0.093),
while grade 3/4 thrombocytopenia was observed in 7.1% of patients
in arm A and in 32.1% of patients in arm B (P=0.019). Grade 1/2
thrombocytopenia was observed at different frequencies in each arm,
with 15 patients (53.6%) in arm A and 8 patients (28.6%) in arm B
(NS, P=0.057). Only one patient in arm B and no patients in arm A
developed grade 3 anemia (NS, P=0.313).
 | Table IVDrug-related adverse events. |
Table IV
Drug-related adverse events.
| Total (n=56) | Arm A (n=28) | Arm B (n=28) | |
|---|
|
|
|
| |
|---|
| G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | P-value |
|---|
|
|
|
|
|
|---|
| Toxicitya | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | G1/2 | G3/4 |
|---|
| Anemia | 20 (35.7) | 1 (1.8) | 10 (35.7) | 0 (0.0) | 10 (35.7) | 1 (3.6) | 1.000 | 0.313 |
| Leukopenia | 39 (69.6) | 4 (7.1) | 20 (71.4) | 1 (3.6) | 19 (67.9) | 3 (10.7) | 0.771 | 0.299 |
| Neutropenia | 32 (57.1) | 11 (19.6) | 18 (64.3) | 3 (10.7) | 14 (50.0) | 8 (28.6) | 0.280 | 0.093 |
|
Thrombocytopenia | 23 (41.1) | 11 (19.6) | 15 (53.6) | 2 (7.1) | 8 (28.6) | 9 (32.1) | 0.057 | 0.019 |
| TBIL | 2 (3.6) | 0 (0.0) | 2 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.150 | - |
|
Hyperpigmentation | 33 (58.9) | 0 (0.0) | 18 (64.3) | 0 (0.0) | 15 (53.6) | 0 (0.0) | 0.415 | - |
| Asthenia | 37 (66.1) | 0 (0.0) | 17 (60.7) | 0 (0.0) | 20 (71.4) | 0 (0.0) | 0.397 | - |
| Nausea | 45 (80.4) | 4 (7.1) | 20 (71.4) | 2 (7.1) | 25 (89.3) | 2 (7.1) | 0.093 | 1.000 |
| Vomiting | 25 (44.6) | 9 (16.1) | 12 (42.9) | 4 (14.3) | 13 (46.4) | 5 (17.9) | 0.788 | 0.716 |
| Stomatitis | 4 (7.1) | 0 (0.0) | 2 (7.1) | 0 (0.0) | 2 (7.1) | 0 (0.0) | 1.000 | - |
| Diarrhea | 13 (23.2) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 12 (42.9) | 0 (0.0) | <0.001 | - |
| Neurotoxicity | 42 (75.0) | 0 (0.0) | 22 (78.6) | 0 (0.0) | 20 (71.4) | 0 (0.0) | 0.537 | - |
| Hand-foot
syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - |
| ALT elevation | 13 (23.2) | 0 (0.0) | 6 (21.4) | 0 (0.0) | 7 (25.0) | 0 (0.0) | 0.752 | - |
In total, the most common grade 3/4
non-hematological toxicity was vomiting (16.1%). With regard to the
overall incidence of adverse events, hyperpigmentation, asthenia,
nausea and neurotoxicity were the most frequent non-hematological
toxicities in each arm. Nausea, with the highest rates of grade 1/2
non-hematological toxicity, was observed in 71.4% of patients in
arm A and 89.3% in arm B (NS, P=0.093). With regard to the adverse
events induced by S-1 administration, the incidence of diarrhea
(3.6 vs. 42.9%; P<0.001) was significantly higher in arm B
compared with arm A, as anticipated.
Thrombocytopenia was the most frequent reason for
dose reduction and cycle delay in each arm. Among the
non-hematological toxicities, vomiting in arm A and diarrhea in arm
B were the most common reasons for dose reduction and cycle
delay.
Discussion
The role of post-operative adjuvant chemotherapy
following curative D2 gastrectomy has long been debated. Multiple
randomized, controlled studies have evaluated the role of
post-operative adjuvant chemotherapy for GC (19–21),
however, as a result of population and regimen heterogeneity, no
consensus has been reached with regard to the chemotherapeutic
regimen, schedule or duration of adjuvant chemotherapy. The results
of the G-SOX study reflected the efficacy and safety of SOX in
patients with AGC (12). Therefore,
SOX is considered to be a candidate for an experimental arm in the
next adjuvant chemotherapy trial.
In the present study, 48.2% of patients completed 8
cycles as planned, however, 75% of patients received >6 cycles
of treatment. SOX has shown more advantages compared with the SP
regimen in adjuvant chemotherapy for GC patients, since 22.6%
completed 5 cycles as planned in the CCOG0703 study (22) and 60.8% completed 6 cycles as
planned in the study by Kang et al (23). In the present study, the treatments
were generally well tolerated. The most frequently observed grade
3/4 toxicities were neutropenia (19.6%), thrombocytopenia (19.6%)
and vomiting (16.1%).
To the best of our knowledge, this is the first
randomized feasibility study comparing two common doses of S-1 in
combination with oxaliplatin in GC patients following curative
surgery. From the viewpoint of the completion status of the
protocol treatment, the completion rate was not significantly
different, however, significantly less delay to the course was
observed in arm A (91.1 vs. 82.1%; P=0.019). Furthermore, less
grade 3/4 neutropenia (19.6 vs. 10.7%; P=0.093) and
thrombocytopenia (grade 1/2, 41.1 vs. 53.6%; NS, P=0.057; and grade
3/4, 19.6 vs. 7.1%; P=0.019) occurred in arm A, which may have
contributed to the less delay to the course. With regard to the
adverse events induced by S-1 administration, the incidence of
diarrhea (3.6 vs. 42.9%; P<0.001) was significantly higher in
arm B than in arm A, as anticipated.
The rate of discontinued cases was higher in arm A
than in arm B, however, notably, four patients refused treatment
subsequent to finishing 6 cycles of therapy in arm A for grade 1
asthenia. The cumulative rate of the relative total administration
dose of S-1 in the 6th treatment course at 100% was 71.4% (95% CI,
56.5–90.3%) in arm A, which was significantly higher than the 21.4%
(95% CI, 10.5–43.6%) in arm B (P=0.001). Additionally, when
calculated in the 8th treatment course, the rate was 32.1% (95% CI,
18.8–55.1%) in arm A, which was higher than the 14.3% (95% CI:
5.77–35.4%) in arm B (NS, P=0.276). The relative total
administration dose of S-1 in the 6th treatment course and the 8th
treatment course were higher in arm A than arm B. However, no
significant difference in the administration dose of oxaliplatin
was found between the two arms.
These results suggested that a regimen using S-1 at
a dose of 70 mg/m2 twice daily for 14 days followed by a
7-day rest period is more acceptable compared with the regimen of
S-1 at 80 mg/m2 twice daily for 14 days, when combined
with oxaliplatin at 130 mg/m2 on day 1 every 3 weeks.
Owing to the lack of a survival analysis and the small sample size
in the current study, the potential to reduce relapse following
treatment in GC patients post-surgery should be carefully examined
in the future. Patients in the present study will continue to be
observed for recurrence and survival.
Acknowledgements
S-1 was kindly provided by Taiho Parmaceutical
Company (Princeton, NJ, USA).
References
|
1
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunderson LL: Gastric cancer - patterns of
relapse after surgical resection. Semin Radiat Oncol. 12:150–161.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallo A and Cha C: Updates on esophageal
and gastric cancers. World J Gastroenterol. 12:3237–3242.
2006.PubMed/NCBI
|
|
5
|
Mari E, Floriani I, Tinazzi A, et al:
Efficacy of adjuvant chemotherapy after curative resection for
gastric cancer: a meta-analysis of published randomised trials. A
study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi
dell’Apparato Digerente). Ann Oncol. 11:837–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Earle CC and Maroun JA: Adjuvant
chemotherapy after curative resection for gastric cancer in
non-Asian patients: revisiting a meta-analysis of randomised
trials. Eur J Cancer. 35:1059–1064. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasako M, Sakuramoto S, Katai H, et al:
Five-year outcomes of a randomized phase III trial comparing
adjuvant chemotherapy with S-1 versus surgery alone in stage II or
III gastric cancer. J Clin Oncol. 29:4387–4393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunningham D, Starling N, Rao S, et al:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higuchi K, Koizumi W, Yamada Y, et al:
Randomized phase III study of S-1 plus oxaliplatin versus S-1 plus
cisplatin for first-line treatment of advanced gastric cancer. J
Clin Oncol. 31(Suppl 4): astr 60. 2013.
|
|
11
|
Yang L, Song Y, Zhou AP, et al: A phase II
trial of oxaliplatin plus S-1 as a first-line chemotherapy for
patients with advanced gastric cancer. Chin Med J (Engl).
126:3470–3474. 2013.
|
|
12
|
Koizumi W, Takiuchi H, Yamada Y, et al:
Phase II study of oxaliplatin plus S-1 as first-line treatment for
advanced gastric cancer (G-SOX study). Ann Oncol. 21:1001–1005.
2010. View Article : Google Scholar
|
|
13
|
Narahara H, Iishi H, Imamura H, et al:
Randomized phase III study comparing the efficacy and safety of
irinotecan plus S-1 with S-1 alone as first-line treatment for
advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer.
14:72–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Yang Y, Qin Q, et al: Dose-finding
study on adjuvant chemotherapy with S-1 plus oxaliplatin for
gastric cancer. Mol Clin Oncol. 2:93–98. 2014.PubMed/NCBI
|
|
15
|
Edge S, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th edition. Springer; New York, NY:
2009
|
|
16
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events [v.4.03]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed June 11, 2011
|
|
18
|
Schulz KF, Altman DG and Moher D: CONSORT
Group: CONSORT 2010 statement: updated guidelines for reporting
parallel group randomised trials. PLoS Med. 7:e10002512014.
View Article : Google Scholar
|
|
19
|
Nakajima T, Nashimoto A, Kitamura M, et
al: Adjuvant mitomycin and fluorouracil followed by oral uracil
plus tegafur in serosa-negative gastric cancer: a randomised trial.
Gastric Cancer Surgical Study Group. Lancet. 354:273–277. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakajima T, Takahashi T, Takagi K, Kuno K
and Kajitani T: Comparison of 5-fluorouracil with ftorafur in
adjuvant chemotherapies with combined inductive and maintenance
therapies for gastric cancer. J Clin Oncol. 2:1366–1371.
1984.PubMed/NCBI
|
|
21
|
Nashimoto A, Nakajima T, Furukawa H, et
al: Gastric Cancer Surgical Study Group, Japan Clinical Oncology
Group: Randomized trial of adjuvant chemotherapy with mitomycin,
Fluorouracil, and Cytosine arabinoside followed by oral
Fluorouracil in serosa-negative gastric cancer: Japan Clinical
Oncology Group 9206-1. J Clin Oncol. 21:2282–2287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kodera Y, Ishiyama A, Yoshikawa T, et al:
Chubu Clinical Cancer Group: A feasibility study of postoperative
chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma
(CCOG0703). Gastric Cancer. 13:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang BW, Kim JG, Chae YS, et al: Pilot
study of adjuvant chemotherapy with 3-week combination of S-1 and
cisplatin for patients with stage II–IV (M0) gastric cancer. Invest
New Drugs. 30:1671–1675. 2012. View Article : Google Scholar
|