Introduction
Gastric cancer is the most common malignant tumor
worldwide (1,2). The most common pathological type of
gastric cancer is adenocarcinoma. Gastric adenocarcinoma is the
most common type of carcinoma in China. As a complex multifactorial
process, gastric carcinogenesis is believed to involve numerous
genes and their products (3–5). In
our previous study, comparative proteome analysis revealed that the
expression of thioredoxin domain-containing 5 (TXNDC5) was
significantly upregulated in certain precancerous lesions of
gastric cancer, such as varioliform gastritis (VG), compared with
the peripheral normal gastric mucous membrane (6). Thus, we hypothesized that upregulation
of the TXNDC5 gene may lead to increased proliferation, as well as
enhanced invasive activity, indicating a potential oncogene.
TXNDC5 was first identified in 2003 using
two-dimensional gel electrophoresis analysis of the endoplasmic
reticula of hepatic tissue (7).
TXNDC5, which is a protein disulfide isomerase-like protein, was
found to be highly expressed in endothelial cells. Furthermore,
Sullivan et al (7) reported
that TXNDC5 protects endothelial cells from stress-induced
apoptosis. An increasing number of studies have revealed that the
upregulation of TXNDC5 is found in tumors of the cervix, uterus and
lungs (7–9). According to these studies, the TXNDC5
gene is hypothesized to be a tumor-enhancing gene, however, studies
regarding the involvement of the TXNDC5 in gastric cancer remain
limited. Furthermore, the association between TXNDC5 expression and
clinicopathological factors in gastric adenocarcinoma remains
unclear. In the present study, the expression of TXNDC5 in gastric
adenocarcinoma was investigated by immunohistochemistry and the
clinicopathological significance of TXNDC5 gene expression was
investigated.
Materials and methods
Materials
Polyclonal goat anti-human TXNDC5 antibody (1:1,000)
was purchased from Cell Signaling Technology, Inc., (Danvers, MA,
USA). Rabbit anti-goat horseradish peroxidase (HRP)-conjugated
antibody (dilution 1:200) was purchased from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd., (Beijing, China).
Sample collection
Samples were obtained from 86 patients with gastric
cancer treated at the 309 Hospital of the People’s Liberation Army
(Beijing, China) between 1995 and 2008. The patients were diagnosed
by gastroscopy and biopsy, and the results were confirmed by
gastric resection, three-field lymph node dissection and
reconstruction of the digestive tract. No patients received
preoperative therapy. All samples were obtained during surgery.
Four tissue sections of the tumor and normal mucosa were obtained
from each patient and the tissue was embedded in paraffin for
future use. The tissue sections were classified as well- or
poorly-differentiated according to pathological diagnosis.
Immunohistochemical analysis of the 86 patient samples revealed
that 32 samples exhibited negative TXNDC5 staining, whereas 54
exhibited positive immunohistochemical staining (positive rate,
62.8%). Of the 54 patients, 37 were male and 17 were female, with a
mean age of 60.6 years (mean ± standard deviation, 60.6±13.1
years). Written informed consent was obtained from all patients.
This study was approved by the Ethics Committee of the 309 Hospital
of the People’s Liberation Army. The clinicopathological stages of
the tumors were assessed according to the International Union
Against Cancer tumor-node-metastasis classification (10). Follow-up of the patients was
performed after surgery until mortality. The mean duration of the
follow-up period was two years and eight months (range, 1 month and
7 days to 4 years and 8 months).
Immunohistochemical assay of TXNDC5
Tissue sections (5 μm) were cut from
paraffin-embedded tissue blocks, placed on slides precoated with
silane and incubated for 20 min at 60°C.
Subsequent to being washed in xylene and a graded
series of ethanol to remove the paraffin, the sections were washed
with phosphate-buffered saline (PBS) for 10 min. The sections were
then treated with 2% bovine serum albumin (BSA; Beijing Huamaike
Biotechnology Co., Ltd., Beijing, China) and 0.1% Triton X-100
(Shanghai Suolaibao Biotechnology Co., Ltd., Shanghai, China) in
PBS (Shanghai Kexing Biotechnology Co., Ltd., Shanghai, China) for
1 h at room temperature. Next, the sections were treated with 3%
H2O2 for 10 min to block endogenous
peroxidase activity. Subsequent to being washed in PBS for 10 min,
polyclonal goat anti-TXNDC5 antibody was applied for 1 h. After
three washes with PBS, the biotin-labeled rabbit anti-goat HRP
secondary antibody was added for 20 min. Next, the sections were
washed in PBS for 10 min, then 3,3′-diaminobenzidine was applied as
the chromogen. Finally, the sections were counterstained with
Harris’ hematoxylin for 3 min and coverslips were applied with a
xylene-based mounting medium. For the semiquantification of TXNDC5,
the immunostaining was analyzed based on the criteria that were
presented by Kase et al (11) and Nozoe et al (12). The TXNDC5-labeling index (showing
the positive proportion of TXNDC5) was expressed as the percentage
of the number of TXNDC5-labeled cells divided by the total number
of cells examined under a microscope (20× objective). The average
value of 10 fields was calculated.
High TXNDC5 expression was represented by ≥50% of
the carcinoma cells in a specimen exhibiting positivity for TXNDC5.
Specimens with <50% of the carcinoma cells exhibiting positivity
for TXNDC5 were considered to exhibit low TXNDC5 expression.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay of TXNDC5
Total RNA (1,400 ng/μl) was extracted by
homogenization using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). cDNA was synthesized in a 20-μl reverse
transcription reaction system using 5 μg RNA. TXNDC5 was amplified
and β2-MG was used as the internal control, in a DNA thermal cycler
(PerkinElmer, Inc., Waltham, MA, USA) using equal amounts of cDNA
as a template. The PCR products were separated by 1.5% agarose gel
electrophoresis, then scanned and analyzed using an ImageMaster VDS
System (GE Healthcare Life Sciences, Uppsala, Sweden).
Statistical analysis
Student’s t-test and the χ2 test were
used to compare the data. A survival analysis was performed using
the Life Tables method. All statistical analyses were performed
using SPSS version 11.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
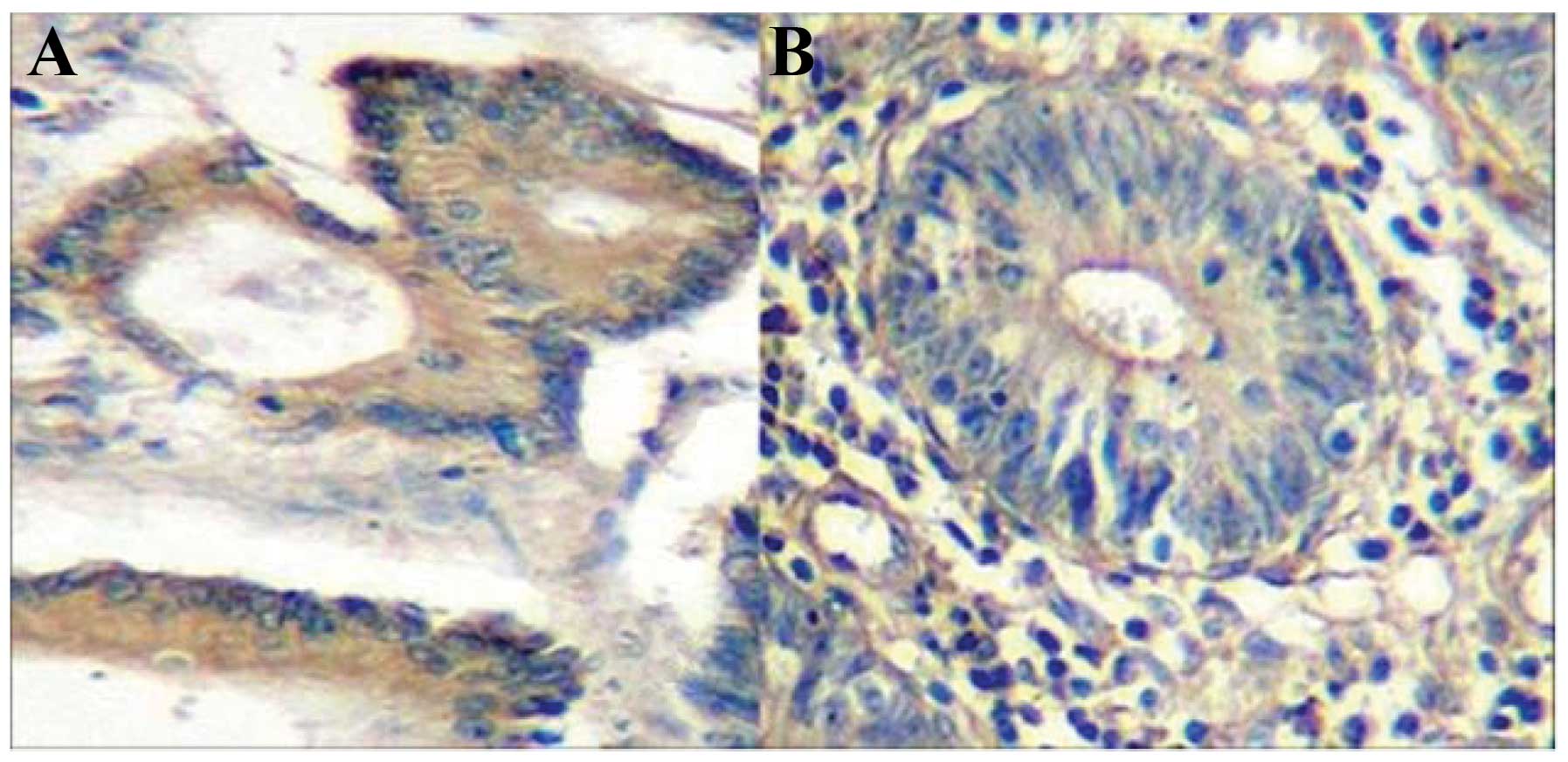
The results of the immunohistochemical assay
revealed brown staining, representing positive signals,
predominantly distributed in the cytoplasm of the cancer cells,
with a small proportion of in the nuclei and cell membrane
(Fig. 1).
The results of the statistical analysis of the
association between TXNDC5 expression and the clinicopathological
characteristics are shown in Table
I. A total of 30 patients (55.6%) exhibited high TXNDC5
expression, whereas 24 patients (44.4%) exhibited low levels of
expression. No significant differences were identified between the
patients with high TXNDC5 expression and those with low expression
with regard to gender or age. The proportion of primary tumors
located in the cardia was significantly higher in the specimens
with high TXNDC5 expression (P<0.05). Furthermore, the
proportion of poorly-differentiated adenocarcinomas was
significantly higher in the specimens with high TXNDC5 expression
compared with the specimens exhibiting low TXNDC5 expression
(P<0.05). The proportion of lymph node metastases and the depth
of the tumors in the specimens with high TXNDC5 expression was
significantly higher than that in the low TXNDC5 expression group
(P<0.05). No significant differences were identified between the
high and low expression groups with regard to vascular invasion.
Furthermore, a significant difference was identified between the
two groups with regard to tumor stage.
 | Table IAssociation between TXNDC5 expression
and clinicopathological characteristics. |
Table I
Association between TXNDC5 expression
and clinicopathological characteristics.
| Clinicopathological
parameter | Low TXNDC5
expression | High TXNDC5
expression | P-value |
|---|
| Age, years | 59.3±10.4 | 61.6±15.2 | P>0.05 |
| Gender, n (%) |
| Male | 17 (70.8) | 20 (66.7) | P>0.05 |
| Female | 7 (29.2) | 10 (33.3) | P>0.05 |
| Body weight, kg | 66±12.3 | 69±19.4 | P>0.05 |
| Height, m | 1.69±0.21 | 1.71±0.16 | P>0.05 |
| Primary tumor
diameter, cm | 4.3±2.6a | 6.2±1.8a | P<0.05 |
| Depth of invasion of
primary tumor, n (%) |
| T0 | 0 (0.0) | 0 (0.0) | P>0.05 |
| T1 | 3 (12.5) | 3 (10.0) | P>0.05 |
| T2 | 9 (37.5)a | 5 (16.7) | P<0.05 |
| T3 | 7 (29.6) | 12 (40.0)a | P<0.05 |
| T4 | 5 (20.8) | 10 (33.3)a | P<0.05 |
| Location of the
primary tumor, n (%) |
| Cardia | 4 (16.7) | 9 (30.0)a | P<0.05 |
| Gastric body | 6 (25.0) | 8 (26.7) | P>0.05 |
| Gastric antrum | 9 (37.5) | 10 (33.3) | P>0.05 |
| Pylorus | 5 (20.8)a | 3 (10.0) | P<0.05 |
| Lymph node
metastasis, n (%) | 7 (29.2) | 16 (53.3)a | P<0.05 |
| Vascular invasion, n
(%) | 10 (41.7) | 14 (46.7) | P>0.05 |
| Pathological type, n
(%) |
| Well-differentiated
adenocarcinoma | 9 (37.5) | 10 (33.3) | P>0.05 |
|
Poorly-differentiated adenocarcinoma | 7 (29.2) | 13 (43.3)a | P<0.05 |
| Signet ring cell
carcinoma | 3 (12.5) | 3 (10.0) | P>0.05 |
| Mucinous
adenocarcinoma | 4 (16.7) | 3 (10.0) | P>0.05 |
| Undifferentiated
carcinoma | 1 (4.2) | 1 (3.3) | P>0.05 |
Using semiquantitative RT-PCR, 476-bp fragments of
TXNDC5 and 876-bp control fragments of β2-MG were amplified
(Fig. 2A and B). The mean ratios of
the absorbency of the TXNDC5 band normalized to the control band
were 1.29±0.16 and 0.71±0.20 in the high and low expression groups,
respectively. This difference was significant when analyzed using
Student’s t-test (P<0.05; Fig.
2C). The results also identified significant differences in
TXNDC5 expression at the mRNA level between the high and low
expression groups.
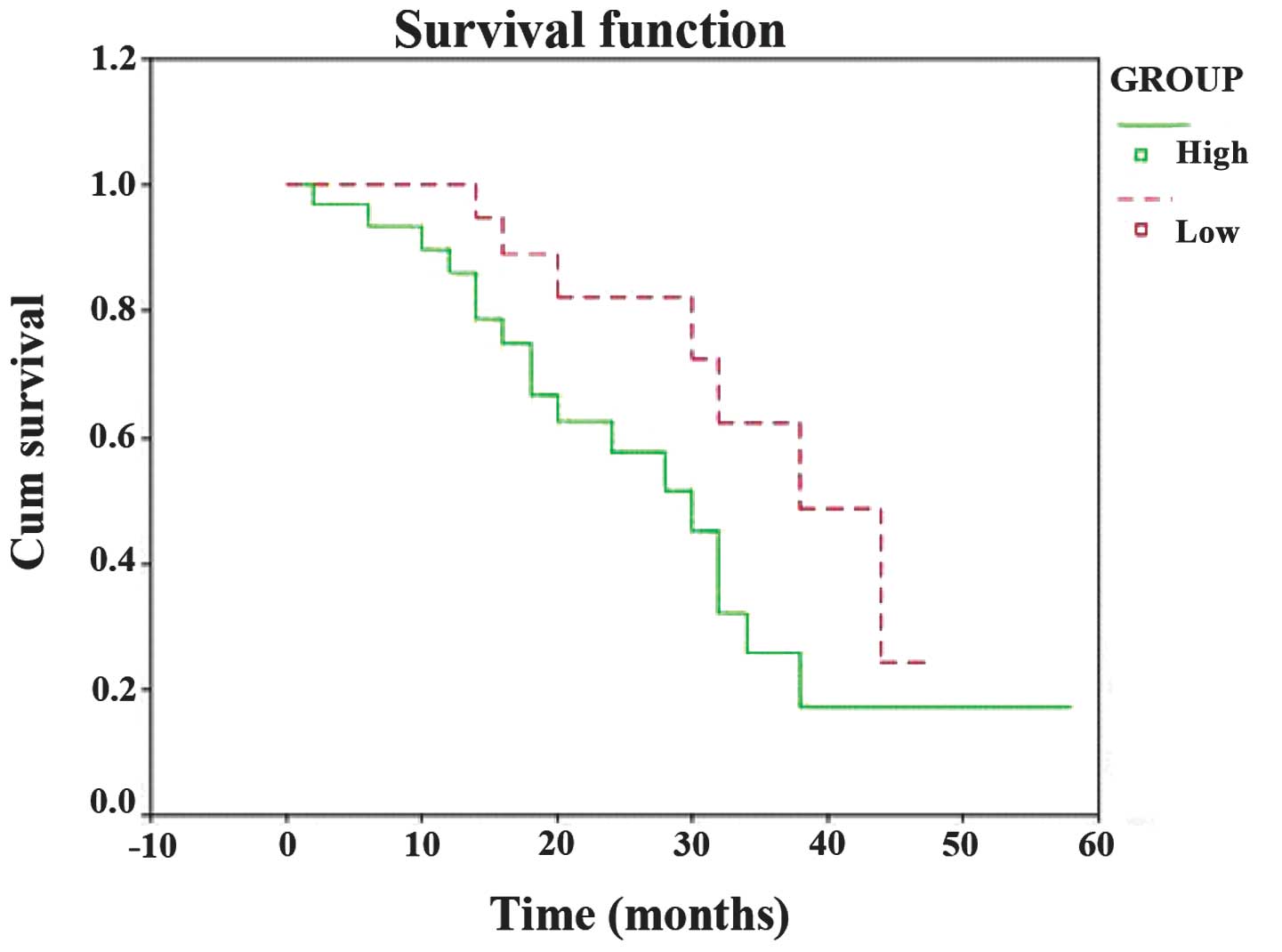
The results of the survival analysis are shown in
Fig. 3. The 10-, 20-, 30- and
40-month survival rates of the patients with high TXNDC5 expression
were 83.3, 46.7, 23.3 and 6.7%, respectively, and those of the
patients with low TXNDC5 expression were 87.5, 50.0, 29.2 and 8.3%,
respectively. The median survival time for the group with high
TXNDC5 expression was 28.47 months and that of the group with a
weak TXNDC5 expression was 37.77 months. The prognosis of the
patients with high TXNDC5 expression was significantly worse than
those with low TXNDC5 expression (P=0.035).
Discussion
At present, gastric cancer remains a common disease
worldwide, with a poor prognosis and low survival rate. VG is a
unique type of gastritis and a major precursor lesion of gastric
cancer. Previous studies have demonstrated that VG is a significant
step in gastric carcinogenesis (13–15).
In a recent study, we detected a difference in protein expression
between VG and the morphologically normal mucosa tissues near the
lesions using the proteomic analysis (6,16).
Results of these studies revealed that TXNDC5 could be a novel
protein in those differentially-expressed proteins and that the
cell cycle, cell death or proliferation-modifying processes may be
involved in the precancerous change. However, the molecular
mechanism behind the action of TXNDC5 is poorly understood.
Located on chromosome 6p24, the TXNDC5 gene encodes
a protein-disulfide isomerase. To date, studies regarding the
TXNDC5 gene are limited. It has been reported that the expression
of the TXNDC5 gene is upregulated in a number of carcinoma tissues
compared with normal tissues (17–20).
However, few studies have investigated the expression of this gene
in gastric cancer tissues. In the current study, the expression of
the TXNDC5 gene in gastric adenocarcinoma tissues was detected
using immunohistochemistry, and the association between TXNDC5
expression and clinicopathological features was analyzed. The
results indicated that the TXNDC5 gene was expressed in gastric
adenocarcinoma and that the positive signals were predominantly
located in the cytoplasm of the tumor cells. The upregulated
expression of TXNDC5 may correlate with poorly-differentiated
adenocarcinoma, lymph node metastasis and deeper tumor invasion.
Nissom et al (18)
demonstrated that poorly-differentiated hepatocellular carcinoma
(HCC) exhibits upregulated TXNDC5 expression, but that the level is
unchanged in well-differentiated HCC, and thus, it was hypothesized
that the gene is involved in tumor progression. In the present
study, the proportion of the primary tumors located in the cardia
was significantly higher in specimens exhibiting high TXNDC5
expression levels compared with those exhibiting low expression
levels. The may be due to the fact that tumors located in cardia
are often considered to be poorly-differentiated. A previous study
indicated that the TXNDC5 gene may affect certain biological
characteristics of cancer cells, promoting the growth and
proliferation of tumor cells, or preventing their apoptosis
(7). However, the exact molecular
mechanism of this remains unclear and thus, further study to
investigate the functional role of the gene in vitro and
in vivo is required.
In conclusion, the immunohistochemical expression of
TXNDC5 may correlate with poor differentiation of the tumors and
with a poor prognosis in gastric cancer patients.
Acknowledgements
The authors would like to thank Dr. Yanmei Wang, Dr.
Kai Wu, Nurse Na Li, Nurse Xianlei Kong and their associated staff
for handling the patient contacts, and the Fourth Military Medical
University of the People’s Liberation Army (Xi’an, China) for
providing the equipment for the current investigation.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.PubMed/NCBI
|
|
3
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
4
|
González CA, Sala N and Capellá G: Genetic
susceptibility and gastric cancer risk. Int J Cancer. 100:249–260.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue FB, Xu YY, Wan Y, et al: Association
of H. pylori infection with gastric carcinoma: a Meta analysis.
World J Gastroenterol. 7:801–804. 2001.
|
|
6
|
Zhang L, Hou YH, Wu K, et al: Proteomic
analysis reveals molecular biological details in varioliform
gastritis without Helicobacter pylori infection. World J
Gastroenterol. 16:3664–3673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sullivan DC, Huminiecki L, Moore JW, et
al: EndoPDI, a novel protein-disulfide isomerase-like protein that
is preferentially expressed in endothelial cells acts as a stress
survival factor. J Biol Chem. 278:47079–47088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang X, Xu B, Wang L, et al:
Investigating a pathogenic role for TXNDC5 in tumors. Int J Oncol.
43:1871–1884. 2013.PubMed/NCBI
|
|
9
|
Vincent EE, Elder DJ, Phillips L, et al:
Overexpression of the TXNDC5 protein in non-small cell lung
carcinoma. Anticancer Res. 31:1577–1582. 2011.PubMed/NCBI
|
|
10
|
Sobin LH and Wittekind C: TNM
classification of malignant tumours. International Union Against
Cancer. 5th edition. Wiley-Liss; New York, NY: pp. 54–58. 1997
|
|
11
|
Kase S, Osaki M, Honjo S, et al:
Expression of cyclo-oxygenase-2 is correlated with high
intratumoral microvessel density and low apoptotic index in human
esophageal squamous cell carcinomas. Virchows Arch. 442:129–135.
2003.PubMed/NCBI
|
|
12
|
Nozoe T, Ezaki T, Kabashima A, Baba H and
Maehara Y: Significance of immunohistochemical expression of
cyclooxygenase-2 in squamous cell carcinoma of the esophagus. Am J
Surg. 189:110–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallina F and Benedetti-Valentini F:
Varioliform gastritis associated with gastric ulcer simulating a
neoplasm. Riv Gastroenterol. 15:85–94. 1963.(In Italian).
PubMed/NCBI
|
|
14
|
Vandenborre KM, Ghillebert GL, Rutgeerts
LJ, et al: Hypertrophic lymphocytic gastritis with a gastric
carcinoma. Eur J Gastroenterol Hepatol. 10:797–801. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosnier JF, Flejou JF, Amouyal G, et al:
Hypertrophic gastropathy with gastric adenocarcinoma: Menetrier’s
disease and lymphocytic gastritis? Gut. 32:1565–1567. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Hou Y, Li N, et al: The influence
of TXNDC5 gene on gastric cancer cell. J Cancer Res Clin Oncol.
136:1497–1505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Ma Y, Lü B, et al: Differential
expression of mimecan and thioredoxin domain-containing protein 5
in colorectal adenoma and cancer: a proteomic study. Exp Biol Med
(Maywood). 232:1152–1159. 2007. View Article : Google Scholar
|
|
18
|
Nissom PM, Lo SL, Lo JC, et al: Hcc-2, a
novel mammalian ER thioredoxin that is differentially expressed in
hepatocellular carcinoma. FEBS Lett. 580:2216–2226. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Claudio JO, Masih-Khan E, Tang H, et al: A
molecular compendium of genes expressed in multiple myeloma. Blood.
100:2175–2186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Q, Li M, Fu X, et al: Global analysis
of differentially expressed genes in androgen-independent prostate
cancer. Prostate Cancer Prostatic Dis. 10:167–174. 2007. View Article : Google Scholar : PubMed/NCBI
|