Introduction
Retinoblastoma (Rb) is a common primary intraocular
malignancy that affects children. Rb can cause serious damage to
the eyes and vision, or may lead to mortality as a result of
intracranial and systemic metastases at a later stage (1). Previous studies have identified that
multiple genes have an important role in the incidence and
development of Rb (2,3). The Rb gene demonstrates good prospects
for the early diagnosis, treatment, pathological classification and
prognosis of Rb (4). Therefore, the
search for novel genes, which are associated with Rb, has become a
topic of interest in recent years (5,6). The
sex-determining region Y box 2 (SOX2) is an embryonic stem cell
gene, which has a key role in embryonic tissue development
(7). An abnormal expression of SOX2
has been identified in lung, stomach, liver and breast cancers, and
in other tumors, which suggests that SOX2 may function in tumor
development, invasion and metastasis (8–10). At
present, the role of SOX2 expression in Rb, and its underlying
mechanisms, are unclear. The present study aimed to analyze the
gene and protein expression of SOX2 in the Rb tissues of 45
children, and in the peripheral blood of 15 children with Rb, and
also identify any association between SOX2 expression and the
clinicopathological features of the disease.
Materials and methods
Tissue samples
Tissue samples were collected from 45 children with
Rb (28 male and 17 female; aged between 1 month and 108 months,
with a mean age of 32 months) who had undergone treatment at the
The Second Xiangya Hospital (Changsha, China), between December
2010 and December 2013. Of these patients, 32 had unilateral Rb and
13 had bilateral Rb. The children were grouped according to the
International Intraocular Retinoblastoma Classification (10) as follows: i) N0 stage, no invasion
of the optic nerve by the tumors; ii) N1 stage, tumor invasion of
the optic nerve head that did not exceed the sieve; iii) N2 stage,
tumor penetrated through the sieve, but no tumor cell invasion of
the optic nerve stump; and iv) N3 stage, tumor cells at optic nerve
stump. The tissues were divided into a well-differentiated group
and a poorly differentiated group, depending upon whether the tumor
cells were arranged into a Flexner-Winterstein rosette. In the
present study, six cases were at N0 stage, five cases were at N1
stage, 16 cases were at N2 stage and 18 cases were at N3 stage. In
total, 31 samples belonged to the well-differentiated group and 14
samples belonged to poorly differentiated group. In addition, 15
pieces of normal retinal tissue, which had surrounded the Rb
tumors, were collected to represent the control group. Written
informed consents were obtained from the family members of the
patients, and the study was approved by the Ethics Review Board of
Central South University, Changsha, China.
Reagents
The serum RNA extraction reagent TRIzol LS and the
total RNA extraction reagent TRIzol were purchased from Invitrogen
(Carlsbad, CA, USA). The rabbit anti-human SOX2 polyclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The reverse transcription system was
purchased from Takara Bio, Inc. (Shiga, Japan). The
streptavidin-peroxidase immunohistochemical kits were purchased
from Beijing Zhongshan Co. (Beijing, China). The quantitative
reverse transcription polymerase chain reaction (qRT-PCR) kit for
mRNA was purchased from Kapa Biosystems, Inc. (Wilmington, MA,
USA).
Immunohistochemistry
The streptavidin-peroxidase two-step method was used
for the immunohistochemical staining. A portion of the tumor
tissues was fixed in 10% formaldehyde, embedded in paraffin, sliced
and then soaked in xylene diluent for dewaxing. Subsequent to
blocking non-specific binding with horse serum, the polyclonal
rabbit anti-human SOX2 antibody (dilution, 1:200; cat. no.
ab171380) was added, followed by a 30-min incubation with
biotin-labeled polyclonal goat anti-mouse IgG (dilution, 1:3,000;
cat. no ab6789) and polyclonal goat anti-rabit IgG (dilution,
1:2,000; cat. no. ab6721) secondary antibodies (Abcam, Cambridge,
MA, USA) at 37°C. After washing three times with phosphate-buffered
saline, the streptavidin-peroxidase complexes were added to the
samples for 30 min. Next, diaminobenzidine staining was performed.
After washing with phosphate-buffered saline three times, the
mixture was mildly stained with hematoxylin and eosin,
differentiated with hydrochloric acid, washed with tap water,
dehydrated with graded alcohol and then mounted with neutral
gum.
Microscopy
Each slice was observed under an Olympus BX53
optical microscope (Olympus Corporation, Tokyo, Japan)
(magnification, ×400). Brown or sepia granular staining of the
cytoplasm or cellular membrane indicated positive cells. In total,
five high-power fields were randomly selected for the tumor cell
count. The percentage of positive cells was represented by the
ratio of stained tumor cells to the total number of tumor cells in
the field. The average percentage of positive cells was calculated
from the five fields. The final score was calculated as follows:
Final score = degree of staining × percentage of positive cells in
each field. The final scores were presented as follows: 0–1,
negative; 2–3, weak positive; 4–6, positive; >6, strong
positive.
Western blotting
In order to extract the total proteins, Rb tissues
were lysed with radio-immunoprecipitation assay protein (Beyotime
Institute of Biotechnology, Haimen, China). Next, SDS-PAGE was
performed for 2 h. The proteins were then transferred to a
polyvinylidene difluoride membrane, and blocked with 5% skimmed
milk for 1 h at room temperature. Next, primary antibodies against
the target protein, SOX2 (dilution, 1:1,000), and the internal
reference protein, GAPDH (polyclonal rabbit anti-human antibody;
dilution, 1:3,000; cat. no. ab9485; Abcam), were added
proportionally. The horseradish peroxidase-labeled secondary
antibodies for SOX2 (goat anti-mouse; dilution, 1:3,000) and GAPDH
(goat anti-rabbit; dilution, 1:2,000) were then added, followed by
incubation at room temperature for 1 h. The membrane was then
washed with phosphate-buffered saline with Tween 20 three times for
15 min each time, and developed with electrochemiluminescence
liquid.
RT-qPCR
Overall, 6 μl total RNA was used for the reverse
transcription reaction, and the expression of SOX2 in the
peripheral blood was determined by RT-qPCR. For reverse
transcription, the reaction conditions were as follows: 42°C for 45
min. The reaction system consisted of 10 μl qRT-PCR-Mix, 0.5 μl
upstream primer, 0.5 μl downstream primer, 1 μl cDNA and 8 μl
ddH2O. The reaction conditions for PCR were as follows:
pre-denaturation at 94°C for 5 min, followed by 40 cycles of 94°C
for 30 sec, 60°C for 30 sec and 72°C for 20 sec, and a final
extension step at 72°C for 5 min. Three parallel wells were set up
for each sample. The internal reference gene was GAPDH, and the
associated primers are listed in Table
I. The 2−ΔΔCT method was used to calculate the
relative expression level of SOX2.
 | Table IPrimers for quantitative reverse
transcription polymerase chain reaction. |
Table I
Primers for quantitative reverse
transcription polymerase chain reaction.
| Primer | Sequence (5′ to
3′) |
|---|
| SOX2, forward |
GAGAGTGTTTGCAAAAGGGGG |
| SOX2, reverse |
GCTTCTCCGTCTCCGACAAA |
| GAPDH, forward |
CTCTGCTCCTCCTGTTCGAC |
| GAPDH, reverse |
GCGCCCAATACGACCAAATC |
Statistical analysis
Data was analyzed using SPSS version 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation. Group t-test was used
for the comparison of SOX2 expression between groups in the
peripheral blood of Rb children. The χ2 test was used to
analyze the association of SOX2 protein expression with the
clinicopathological features of Rb. A value of P<0.05 was used
to indicate a statistically significant difference.
Results
SOX2 expression in Rb tissues is
dependent upon the degree of Rb differentiation and optic nerve
invasion
In order to analyze the expression of SOX2 in Rb
tissues, immunohistochemical staining was performed. The results
revealed that SOX2 was expressed either in the cytoplasm or the
cell membrane of Rb cells, or in both (Fig. 1). SOX2 expression in Rb tissues was
positive, and included 10 weak positive cases (22.2%), 14 positive
cases (31.1%) and 21 strong positive cases (46.7%). By contrast,
SOX2 expression in the control group was negative. The comparison
of SOX2 expression between groups of varying Rb differentiation
revealed that the expression was strong positive in the 14 cases of
poorly differentiated Rb. However, in the well-differentiated
group, there were seven cases of strong positive expression, 14
cases of positive expression, and 10 cases of weak positive
expression. The comparison of SOX2 expression between groups of
different Rb stages revealed that there were nine cases of weak
positive SOX2 expression, and two cases of positive SOX2 expression
in the N0–N1 stage tissues; four cases of strong positive SOX2
expression, 11 cases of positive SOX2 expression and one case of
weak positive SOX2 expression in the N2 stage tissues; and 17 cases
of strong positive SOX2 expression and one case of positive SOX2
expression in the N3 stage tissues. The comparison between genders
revealed that there were no statistically significant differences
between the SOX2 positive expression rates (P<0.05) (Table II). These data suggest that SOX2
expression in Rb tissues is dependent upon the degree of Rb
differentiation and optic nerve invasion.
 | Table IICorrelation of sex-determining region
Y box 2 (SOX2) expression and clinicopathological features of
retinoblastoma. |
Table II
Correlation of sex-determining region
Y box 2 (SOX2) expression and clinicopathological features of
retinoblastoma.
| Variables | n | Percentage of
positive SOX2 expression | χ2 | P-value |
|---|
| Gender |
| Male | 28 | 100 | | |
| Female | 17 | 100 | | |
| Optic nerve
invasion | | | 29.152 | 0.000 |
| N0–1 | 11 | 0a | | |
| N2 | 16 | 25a | | |
| N3 | 18 | 94a | | |
| Differentiation | | | 23.226 | 0.000 |
| Well | 31 | 23a | | |
| Poor | 14 | 100a | | |
SOX2 protein expression is correlated
with the development and invasion of Rb
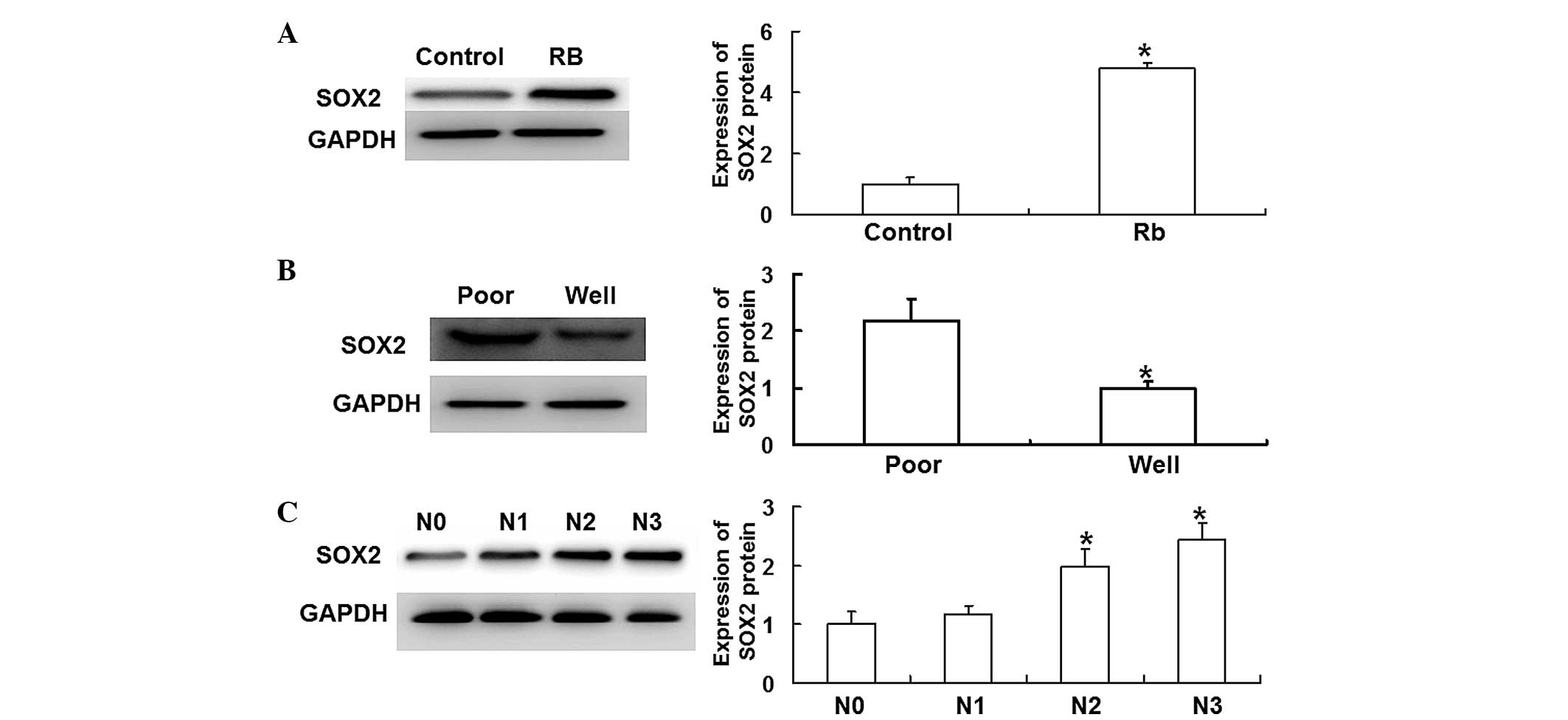
Western blot analysis was performed in order to
measure the expression of SOX2 protein within the Rb tissues. The
results revealed that SOX2 protein expression was significantly
higher in the Rb tissues than in the control group tissues
(P<0.05) (Fig. 2A). In addition,
SOX2 protein expression was significantly higher in the poorly
differentiated group than in the well-differentiated group
(P<0.05) (Fig. 2B). Furthermore,
SOX2 protein expression increased as the optic nerve invasion
progressed from stage N0 to N3, with the expression significantly
higher at the N2 and N3 stages than the N0 stage (P<0.05)
(Fig. 2C). These results suggest
that SOX2 protein expression is correlated with the development and
invasion of Rb.
SOX2 gene is highly expressed in the
peripheral blood of Rb children, with expression in the poorly
differentiated group higher than that in well-differentiated
group
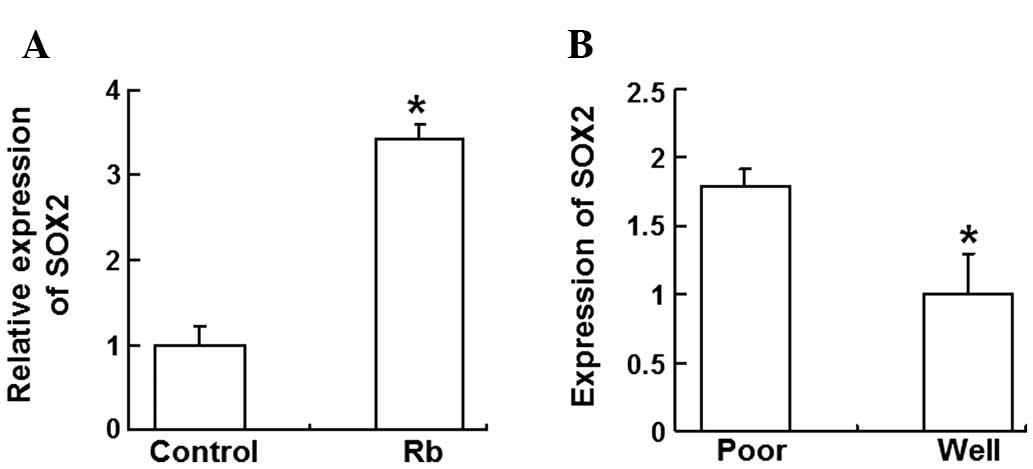
In order to determine the SOX2 gene expression in
the peripheral blood of children with Rb, RT-qPCR was performed. In
total, SOX2 gene expression was detected in 15 Rb children,
including five poorly differentiated cases and eight
well-differentiated cases. The quantitative results demonstrated
that SOX2 gene expression was significantly higher in the
peripheral blood of Rb children than in individuals from the
control group (P<0.05) (Fig.
3A). In addition, SOX2 gene expression in the poorly
differentiated group was higher than that observed in the
well-differentiated group (P<0.05) (Fig. 3B). These data indicate that the SOX2
gene was highly expressed in the peripheral blood of children with
Rb, with expression higher in the poorly differentiated group
compared with the well-differentiated group.
Discussion
In recent years, genes have been discovered that are
closely associated with the development of Rb (12,13).
Previous studies have demonstrated that Rb gene mutations and
deletions occur in the majority children with Rb (14,15).
Furthermore, in vitro experiments have indicated that the Rb
gene has important roles in the incidence, metastasis, apoptosis
and other aspects of Rb. Vascular endothelial growth factor has
been demonstrated to promote Rb tumor angiogenesis (16). The melanoma differentiation
associated gene-7 has been shown to selectively induce the
apoptosis and inhibit the growth of Rb tumor cells (17). The identification of these novel
biomarkers has led studies to investigate the molecular mechanism
of Rb, and has provided an alternative approach for clinical
diagnosis and treatment.
The SOX2 gene, a member of the SOX gene family, is
an important transcription factor that regulates embryotic
development and cell differentiation, and which functions in the
embryotic development of the brain, nerves, lens and other tissue
structures. The abnormal expression of SOX2 can often lead to
cellular and tissue differentiation in developmental disorders
(18,19). Therefore, the function of the SOX2
gene in abnormally differentiated tumor tissues has become of
particular interest. Previous studies have demonstrated that the
expression of SOX2 is increased in lung, liver and stomach cancers,
and in other tumor tissues, and that it is positively correlated
with the clinicopathological stages and degrees of differentiation
of Rb (20,21). In vitro experiments have
confirmed that SOX2 functions in the malignant biological behaviors
of a variety of tumor cells (22,23).
In the present study, immunohistochemistry, western blotting and
RT-qPCR were performed in order to detect the expression of SOX2 in
Rb tissues and peripheral blood. In addition, the correlations
between SOX2 expression and the degree of differentiation and
clinical stage of Rb were preliminarily analyzed using the
clinicopathological data. It has been reported that SOX-2
expression is elevated in Rb tissues (14,24–27).
For example, Wadhwa et al (24) observed that SOX2 is expressed in the
inner retina and the ganglion cells of human RB tumors. Zhang et al
(25) identified Sox2 as one of the
upregulated genes in Rb tissues using a chromatin
immunoprecipitation-on-chip analysis. Our results were consistent
with these previous reports.
The results of the present study indicate that the
SOX2 protein, as a key transcription factor in the embryotic
development and tissue cell differentiation, has an important role
in the incidence and development of Rb. Due to the diversity of the
downstream target genes that can be regulated by SOX2, the specific
molecular mechanism of SOX2 requires further study. The peripheral
blood results suggested that SOX2 gene expression may be clinically
useful for the early diagnosis and treatment of children with
Rb.
To summarize, the SOX2 gene was highly expressed in
Rb tissues and in the peripheral blood. Furthermore, its expression
increased with the progression of clinical stage and with the
lowering of the degree of differentiation. The present study
indicates that SOX2 has an important role in the incidence and
development of Rb. However, the downstream molecular mechanism
requires further study in order to provide a theoretical basis for
the clinical diagnosis and treatment of Rb.
Acknowledgements
The present study was supported by a grant from the
National Nature Science Foundation of China (no. 81371013).
References
|
1
|
Villegas VM, Hess DJ, Wildner A, et al:
Retinoblastoma. Curr Opin Ophthalmol. 24:581–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jehanne M, Brisse H, Gauthier-Villars M,
et al: Retinoblastoma: recent advances. Bull Cancer. 101:380–387.
2014.(In French). PubMed/NCBI
|
|
3
|
Aerts I, Lumbroso-Le Rouic L,
Gauthier-Villars M, et al: Retinoblastoma. Orphanet J Rare Dis.
1:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Temming P, Eggert A, Bornfeld N, et al:
Diagnosis and treatment of retinoblastoma: current strategies for
effective tumour control and preservation of vision. Klin Monbl
Augenheilkd. 230:232–242. 2013.(In German). PubMed/NCBI
|
|
5
|
Hilgendorf KI, Leshchiner ES, Nedelcu S,
et al: The retinoblastoma protein induces apoptosis directly at the
mitochondria. Genes Dev. 27:1003–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai D, Visser-Grieve S and Yang X: Tumour
suppressor genes in chemotherapeutic drug response. Biosci Rep.
32:361–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu K, Lin B, Zhao M, et al: The multiple
roles for Sox2 in stem cell maintenance and tumorigenesis. Cell
Signal. 25:1264–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teicher BA: Targets in small cell lung
cancer. Biochem Pharmacol. 87:211–219. 2014. View Article : Google Scholar
|
|
10
|
Lipka AF, Verschuuren JJ and Titulaer MJ:
SOX1 antibodies in Lambert-Eaton myasthenic syndrome and screening
for small cell lung carcinoma. Ann N Y Acad Sci. 1275:70–77. 2012.
View Article : Google Scholar
|
|
11
|
Linn Murphree A: Intraocular
retinoblastoma: the case for a new group classification. Ophthalmol
Clin North Am. 18:41–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cecchini MJ, Thwaites M, Talluri S, et al:
A retinoblastoma allele that is mutated at its common E2F
interaction site inhibits cell proliferation in gene targeted mice.
Mol Cell Biol. 34:2029–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu R, Zhang XH, Zhang K, et al:
5-Aza-2′-deoxycytidine inhibits retinoblastoma cell by reactivating
epigenetically silenced RASSF1A gene. Int J Ophthalmol. 7:51–56.
2014.
|
|
14
|
Thériault BL, Dimaras H, Gallie BL and
Corson TW: The genomic landscape of retinoblastoma: a review. Clin
Experiment Ophthalmol. 42:33–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Madhavan J, Ganesh A and Kumaramanickavel
G: Retinoblastoma: from disease to discovery. Ophthalmic Res.
40:221–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xin GH, Zhao XH, Liu D, et al: Effect of
VEGF-targeted antisense gene therapy on retinoblastoma cell line
SO-RB50 in vitro and in vivo. Int J Ophthalmol. 5:440–447.
2012.PubMed/NCBI
|
|
17
|
Mhashilkar AM, Schrock RD, Hindi M, et al:
Melanoma differentiation associated gene-7 (mda-7): a novel
anti-tumor gene for cancer gene therapy. Mol Med. 7:271–282.
2001.PubMed/NCBI
|
|
18
|
Popovic J, Stanisavljevic D, Schwirtlich
M, et al: Expression Analysis of SOX14 during retinoic acid induced
neural differentiation of embryonal carcinoma cells and assessment
of the effect of its ectopic expression on SOXB members in HeLa
Cells. PLoS One. 9:e918522014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin IY, Chiu FL, Yeang CH, et al:
Suppression of the SOX2 neural effector gene by PRDM1 promotes
human germ cell fate in embryonic stem cells. Stem Cell Reports.
2:189–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weina K and Utikal J: SOX2 and cancer:
current research and its implications in the clinic. Clin Transl
Med. 3:192014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia X, Li X, Xu Y, et al: SOX2 promotes
tumorigenesis and increases the anti-apoptotic property of human
prostate cancer cell. J Mol Cell Biol. 3:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujii T, Shimada K, Tatsumi Y, et al:
Syndecan-1 responsive microRNA-126 and 149 regulate cell
proliferation in prostate cancer. Biochem Biophys Res Commun. Nov
22–2014.(Epub ahead of print).
|
|
23
|
Chou MY, Hu FW, Yu CH and Yu CC: Sox2
expression involvement in the oncogenicity and radiochemoresistance
of oral cancer stem cells. Oral Oncol. 51:31–39. 2015. View Article : Google Scholar
|
|
24
|
Wadhwa L, Bond WS, Perlaky L, et al:
Embryonic retinal tumors in SV40 T-Ag transgenic mice contain
CD133+ tumor-initiating cells. Invest Ophthalmol Vis Sci.
53:3454–3462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Benavente CA, McEvoy J, et al: A
novel retinoblastoma therapy from genomic and epigenetic analyses.
Nature. 481:329–334. 2012.PubMed/NCBI
|
|
26
|
Seigel GM, Campbell LM, Narayan M and
Gonzalez-Fernandez F: Cancer stem cell characteristics in
retinoblastoma. Mol Vis. 11:729–737. 2005.PubMed/NCBI
|
|
27
|
Seigel GM, Hackam AS, Ganguly A, et al:
Human embryonic and neuronal stem cell markers in retinoblastoma.
Mol Vis. 13:823–832. 2007.PubMed/NCBI
|