Introduction
Nature’s abundant botanical, animal and mineral
resources have provided a large supply of materials for anticancer
discovery, with >60% of anticancer agents being either natural
products or based thereon (1).
Therefore, the analysis of natural products is an important
approach in developing modern medicine, as well as an inevitable
trend of traditional medicine.
The fruit of Brucea javanica has been used
for the treatment of various types of cancer in China for
centuries. Dozens of single compounds have been isolated and
identified from B. javanica, which have demonstrated
relatively high activities and broad antitumor spectrums in
vitro (2). The anticancer
mechanisms of B. javanica or its extracts involve the
induction of apoptosis, the regulation of the cell cycle, the
reversal of multidrug resistance and the induction of cell
differentiation (3–11). However, the effect of B.
javanica or its extracts on autophagy in cancer cells has yet
to be reported. The intracellular process of autophagy involves the
transportation of cytoplasmic materials to lysosomes by
double-membrane autophagosomes for degradation.
Microtubule-associated protein light chain 3 (LC3) is the key
factor in the formation of autophagosomes and includes two forms,
cytosolic LC3-I and membrane-bound LC3-II, which are produced in
the process of autophagy. An evident correlation has been observed
between the quantity of LC3-II and the number of autophagosomes.
LC3-II therefore serves as a good indicator of autophagosome
formation, and the detection of LC3 conversion (LC3-I to LC3-II) by
immunoblot analysis is consequently widely used to monitor
autophagy (12). Additionally,
Beclin-1 is a key protein involved in the initiation of
autophagosome formation and closure, and has been indicated to be
involved in tumor development. In combination with other
biochemical factors, Beclin-1 may be used as a biomarker to monitor
the extent of autophagy (13).
Furthermore, Bim [also known as B-cell lymphoma 2 (Bcl 2)-like
protein 11] has recently been identified to inhibit autophagy by
binding to Beclin-1 independent of its proapoptotic function,
therefore, acting as a novel molecular link between autophagy and
apoptosis. Bim exists in three splicing isoforms, termed BimEL,
BimL and BimS. BimEL and BimL interact with Beclin-1, however, only
a weak interaction appears to exist between BimS and Beclin-1
(14).
Autophagy is a catabolic process involving the
degradation of the unnecessary, injured or aged proteins and
organelles in a cell, followed by the recycling of the degraded
products. Autophagy is required by the majority of organisms to
maintain survival, however, excessive autophagy results in cell
death (15). In addition to its
physiological role, autophagy is involved in cancer development
(16) and is typically considered
to be a tumor-suppressing mechanism occurring at cancer initiation.
However, in established cancer, there is much dispute regarding
whether the autophagy induced during cancer treatment is a
pro-survival or pro-death (autophagic cell death or type II
programmed cell death) mechanism (17). The aim of the current study was to
investigate whether B. javanica oil emulsion (BJOE)
modulates autophagy in HCT116 human colon cancer cells and whether
the modulation of autophagy is a mechanism by which BJOE kills
cancer cells. The variation of autophagy and apoptosis induced by
BJOE in colon cancer cells was analyzed, and an agent was
concurrently used to counteract the effect of BJOE on autophagy in
order to verify the role of autophagy in the process of
BJOE-induced apoptosis.
Materials and methods
Cell culture and reagents
Monolayer cultures of human colon cancer cells,
specifically HCT116, were maintained in Roswell Park Memorial
Institute 1640 medium (Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal calf serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China) and 1%
antibiotic, and incubated in a 5% CO2 incubator at 37°C.
BJOE containing 10% refined B. Javanica oil was obtained
from Zhejiang Jiuxu Pharmaceutical Co. Ltd. (Jiuxu, China; batch
no. 20111113) and an equal volume of dimethyl sulfoxide was added
to the controls of all the experiments. The polyclonal rabbit
anti-human antibody against LC3 was obtained from Sigma-Aldrich
(St. Louis, MO, USA), while polyclonal rabbit anti-human anti-Bim
and anti-Beclin-1, and monoclonal mouse anti-human GAPDH were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Biotinylated goat anti-rabbit and -mouse immunoglobulin G (IgG)
were obtained from Wuhan Sanying Biotechnology (Wuhan, China). The
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI) Apoptosis Detection kit was obtained from BestBio (Shanghai,
China), and the trehalose was purchased from Difco, BD Biosciences
(Franklin Lakes, NJ, USA).
Detection of apoptosis using flow
cytometry
The HCT116 cells were cultured in the abovementioned
medium containing 2 mg/ml BJOE in the presence and absence of 50mM
trehalose. Following 0, 8, 16 or 24 h of treatment, the cells were
harvested by trypsinization, washed twice in
Ca2+/Mg2+-free phosphate-buffered saline
[PBS(−)], and stained with a combination of Annexin V-FITC and -PI,
according to the manufacturer’s instructions. The samples were
immediately analyzed by performing flow cytometry (Cytomics FC500;
Beckman Coulter, Fullerton, CA, USA). This process allows the
distinction between cells in early (Annexin
V-FITC+/PI−) and late (Annexin
V-FITC+/PI+) apoptosis, however, in the
present study, the two subpopulations were counted together and
expressed as the total fraction of apoptotic cells.
Western blot analysis of LC3 protein
expression levels
The HCT116 cells were treated with 0, 1, 2 and 4
mg/ml BJOE or 2 mg/ml BJOE in the presence and absence of 50mM
trehalose. Subsequent to being washed twice with PBS, the cells
were lysed with 0.5 ml lysis buffer [50 mM Tris-HCl (pH 7.6), 150
mM sodium chloride, 0.1% SDS, 1 mM EDTA and 1% Triton X-100)
containing various protease inhibitors (1 mM phenylmethylsulfonyl
fluoride, 5 μg/ml pepstain, 5 μg/ml leupeptin and 5 μg/ml
aprotinin) for 0.5 h on ice. The cells were subsequently harvested
using a cell scraper and centrifuged at 13,000 × g for 15 min at
4°C. The clear supernatant was collected and used as the cell
protein extract, the total protein concentration of which was
determined by performing a bicinchoninic acid assay (Beyotime
Institute of Biotechnology, Shanghai, China). The whole-cell
lysates were electrophoresed on a 15% SDS-polyacrylamide gel and
the separated proteins were electrophoretically transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The remaining reactive sites of the membrane were blocked by
incubating the membrane in Tris-buffered saline with Tween 20
containing 5% skimmed milk. Subsequently, the membrane strips were
incubated with theanti-LC-3, -Beclin-1, -Bim and -GAPDH primary
antibodies (dilution, 1:1,000) overnight at 4°C. Following primary
antibody incubation, the blots were washed and incubated with a
1:3,000 dilution of biotinylated anti-rabbit IgG and biotinylated
anti-mouse IgG, respectively. Finally, the proteins were detected
and visualized using the Enhanced Chemiluminescence Plus Western
Blotting Detection system (Beyotime Institute of
Biotechnology).
Statistical analysis
For the statistical analysis, Student’s t-test was
used as appropriate and a one-way analysis of variance was used for
multiple comparisons. All statistical analysis was performed using
SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. All of the experiments were conducted a minimum of
three times.
Results
BJOE-inhibited autophagy in HCT116 cancer
cells
Abundant levels of LC3-II, a specific protein marker
of autophagy, was observed in the HCT116 cells under basal
conditions, as indicated in Fig. 1A and
B. Following a 24-h exposure to different concentrations of
BJOE, a significant decrease in the protein expression levels of
LC3-II (P=0.02) and a marginal decrease in the protein expression
levels of LC3-I were identified (Fig.
1A), indicating that autophagy was inhibited. This inhibition
of autophagy was most evident at a BJOE concentration of 2 mg/ml
and therefore, this concentration was used in all subsequent
experiments. Additionally, the protein expression levels of
Beclin-1 were decreased by BJOE administration in a dose-dependent
manner. Numerous studies have recorded the apoptosis-inducing
activity of BJOE (or other forms of B. javanica extract) on
cancer cells (2–6); hence, the present study determined
whether BJOE effected the protein expression levels of Bim, which
possesses anti-autophagy and pro-apoptosis properties, in HCT116
cancer cells. As indicated in Fig.
1A, a dose-dependent increase in BimL expression was observed,
however, no marked change in BimEL expression was observed.
Considering that autophagy is a dynamic process, the
present study evaluated the protein expression levels of LC3 in
HCT116 cells treated with 2 mg/ml BJOE for 0, 8, 16 and 24 h. As
indicated in Fig. 1B, BJOE
treatment for 8 h resulted in a marked decrease in LC3-II
expression and a marginal decrease in LC3-I expression, which
remained at a low level up to 24 h, indicating that short-term
exposure to BJOE may inhibit autophagy in HCT116 cells for at least
one day.
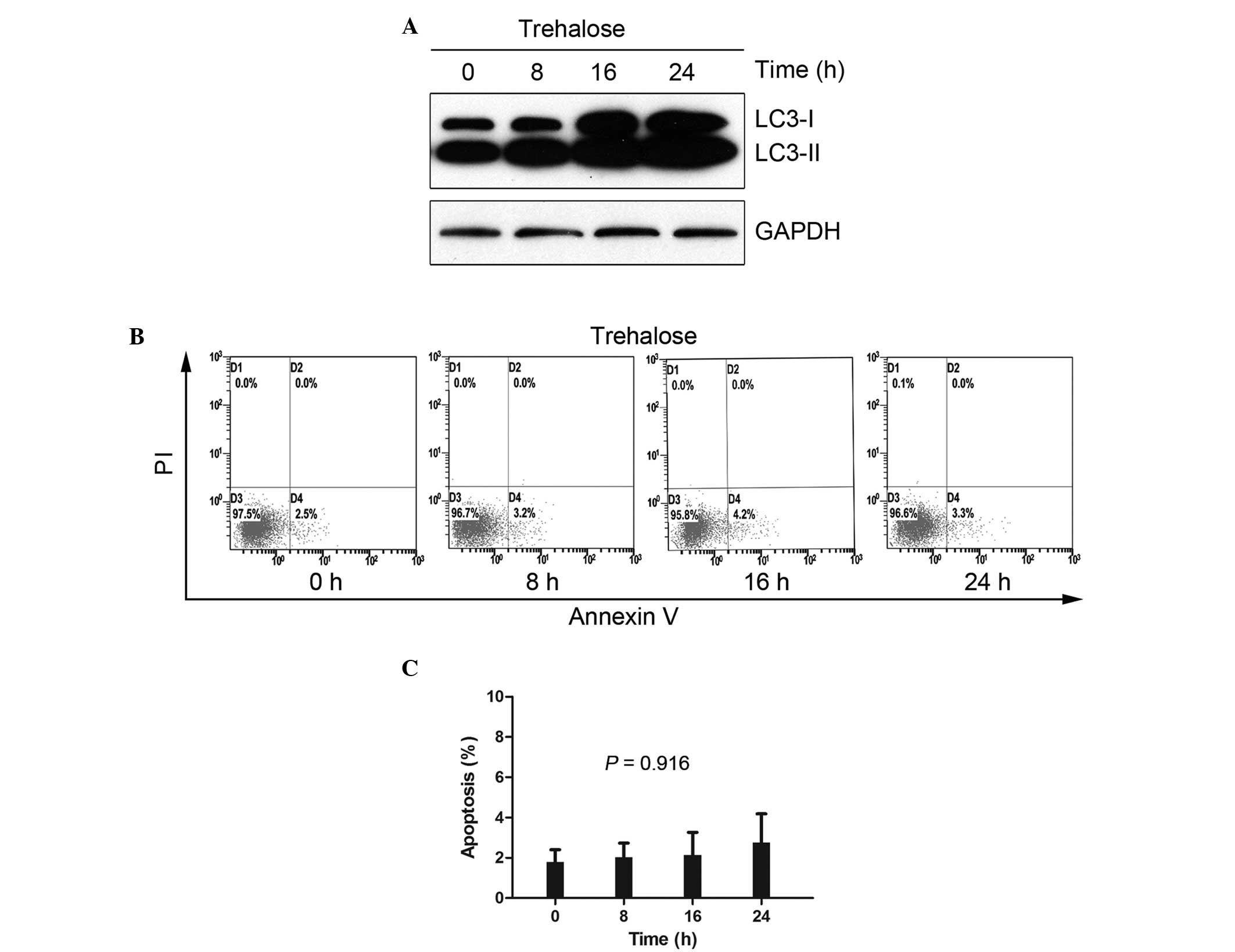
Trehalose-induced autophagy in HCT116
cells without cytotoxicity
Rapamycin is a classical autophagy inducer that
blocks mammalian target of rapamycin (mTOR), a critical signaling
pathway for cell survival and proliferation. The simultaneous used
of rapamycin and other agents results in unclear data, therefore,
the present study used trehalose, an mTOR-independent autophagy
inducer with little cell toxicity (18). In the current study, a
time-dependent increase in LC3-II was consistently observed in the
50 mM trehalose-treated HCT116 cells, indicating that trehalose is
an effective inducer of autophagy (Fig.
2A). To clarify whether trehalose induces apoptosis at a
concentration of 50 mM, an apoptosis detection assay was performed
on the trehalose-treated HCT116 cells for 8, 16 and 24 h using
Annexin V-FITC/PI staining (Fig.
2B). No significant changes were noted following 24 h of
exposure, with apoptosis rates of 1.8±1.0, 2.0±1.2, 2.1±2.0 and
2.8±2.4% identified at 0, 8, 16 and 24 h, respectively (P=0.916;
Fig. 2C).
Trehalose administration counteracts the
anti-autophagy effect of BJOE and attenuates the apoptosis-inducing
activity of BJOE
To investigate whether the autophagy-inhibiting
effect of BJOE can be counteracted by trehalose, the protein
expression levels of LC3 were determined by western blot analysis
in HCT116 cells treated with 2 mg/ml BJOE with or without 50 mM
trehalose for 0, 8, 16 and 24 h. As indicated in Fig. 3A and B, the time-dependent decrease
in LC3-II protein expression observed in the HCT116 cells treated
with BJOE alone disappeared in the presence of trehalose,
indicating that the autophagy-inhibiting ability of BJOE was
impeded in the presence of trehalose.
To determine whether autophagy inhibition was an
antitumor mechanism, an apoptosis detection assay using Annexin
V-FITC/PI staining was performed on the HCT116 cells following
exposure to BJOE with or without trehalose. As indicated in
Fig. 3C, 2 mg/ml BJOE alone
resulted in time-dependent apoptosis in the HCT116 cells. Following
a 24-h treatment with BJOE, ~50% apoptotic cells were observed,
indicating that BJOE is a strong inducer of apoptosis. However, the
apoptotic cell rates induced by BJOE and trehalose were
significantly reduced at 8h (18.6±3.2 vs. 11.0±3.0%; P=0.041), 16h
(34.5±3.3 vs. 19.7±3.0%; P=0.005) and 24 h (51.0±6.3 vs. 35.6±5.1%;
P=0.031) compared with BJOE treatment alone (Fig. 3D).
Discussion
As an evolutionarily conserved, intracellular
self-defense mechanism, authophagy occurs in organisms at basal
levels in all cells and is responsible for homeostatic functions,
such as protein and organelle turnover. Autophagy is upregulated
when intracellular nutrients and energy are required and in the
presence of specific stress or pathological conditions. The
majority of anticancer treatment strategies, including
chemotherapy, radiotherapy, endocrine therapy and targeted therapy,
result in the upregulation of autophagy as a method of inducing
cancer cell apoptosis (17).
Notably, in the present study, the anticancer agent BJOE exhibited
anti-autophagy and pro-apoptosis effects on HCT116 cancer cells
with elevated autophagy under basal conditions. The pro-apoptotic
activity of BJOE in the HCT116 colon cancer cells was attenuated
when autophagy was enhanced by trehalose, indicating that autophagy
is an anti-apoptosis mechanism and thus, may exhibit a
cytoprotective role. This observation supports the hypothesis that
the inhibition of autophagy may be a potential antitumor mechanism
of BJOE.
Although numerous arguments exist regarding whether
the autophagy induced in cancer treatment is a pro-survival or
pro-death mechanism, increasing evidence indicates that autophagy
serves a largely cytoprotective role in physiologically relevant
conditions. Therefore, previous studies have argued that autophagic
cell death is a misnomer, preferring the concept of cell death with
autophagy as opposed to cell death by autophagy (19). The activation of autophagy in cancer
cells causes resistance to treatment to develop, and predicts the
invasiveness and prognosis of tumors (20). Numerous preclinical investigations
of various types of cancer have demonstrated that autophagy
inhibition augments the efficacy of conventional treatment
strategies (17), however, rare
agents have been identified that exhibit anti-autophagy and
pro-apoptosis effects. For example, the present study identified
that BJOE has these two roles, which may aid in explaining why BJOE
administration is able to increase the efficacy of conventional
anticancer treatments in clinical practice (21). Considering the clinical evidence
that has emerged regarding the use of autophagy inhibitors to treat
refractory malignancies, studies have been conducted with the aim
of developing more powerful and specific autophagy inhibitors
(22–24). In China, BJOE is widely used in
combination with conventional therapy to treat cancer,
demonstrating efficacy-enhancing and toxicity-reducing effects,
hematopoietic protection, and immunoregulation activity (21). Furthermore, the present study
indicated that BJOE-inhibition of the autophagic process in HCT116
cancer cells contributes to an increasing rate of cell death. Thus,
we propose that B. javanica may contain a useful autophagy
inhibitor for the treatment of colon cancer. Although autophagy is
commonly activated in various types of cancer, previous studies
have identified that Ras-driven and mTOR inhibitor-treated tumors
exhibit prominently high levels of autophagy contributing to drug
resistance, which may be overcome by the administration of
autophagy inhibitors, indicating that autophagy inhibition is a
potential strategy for overcoming drug resistance in these types of
cancer (25–31). Considering that the inhibition of
autophagy and apoptosis were induced by BJOE administration in the
present study, future studies should be conducted to determine if
tumors (such as pancreatic cancer) that are Ras-driven and tumors
that are mTOR inhibitor-treated (such as everolimus) are
appropriate candidates for BJOE treatment.
The present study indicated that the apoptosis
induced by BJOE may be partially attributed to autophagy inhibition
in the present study, however, the key factor for their interaction
is unknown. The association between autophagy and apoptosis is
complex, with the two processes appearing to antagonize each other;
autophagy targets caspases for degradation, while caspases cleave
autophagy-related proteins (32).
Bim, the first Bcl-2 family member identified to possess
anti-autophagy and pro-apoptosis functions, was investigated in the
present study. BimEL and BimL appear to inhibit autophagy by
sequestering Beclin-1 (33); in the
present study, a simultaneous, dose-dependent increase in BimL and
decrease in Beclin-1 was observed in the BJOE-treated HCT116 cancer
cells, indicating that BimL may be important in the association
between BJOE-mediated apoptosis induction and autophagy inhibition.
The complex signaling network between apoptosis and autophagy has
yet to be fully elucidated, for example, it is not known which
targets in the signaling network are modulated by BJOE, or whether
specific compounds in BJOE inhibit autophagy while others promote
apoptosis to establish BJOE as a ‘double killer’.
Numerous preclinical studies have determined that
autophagy inhibition is able to augment apoptosis induced by
anticancer agents (34–37). In the current study, when autophagy
was activated by trehalose in the HCT116 cancer cells, the
apoptosis induced by BJOE was attenuated, indicating that autophagy
is an anti-apoptosis and pro-apoptosis mechanism. The present study
indicated that BJOE inhibits autophagy in HCT116 human colon cancer
cells, which demonstrated elevated levels of autophagy under basal
conditions. Excluding the induction of apoptosis, the inhibition of
autophagy may be a potential anticancer mechanism of BJOE.
In conclusion, to maintain consistency with clinical
practice, BJOE was selected for use in the present study. However,
the use of BJOE, a complex mixture of B. javanica extract,
was a limitation of the present study as it hindered the accurate
interpretation of the results and feasibility of subsequent studies
of the mechanisms involved due to the fact that it is made from a
mixture of numerous chemical ingredients, which may exert different
pharmacological effects, thus making it difficult to identify the
compound causing autophagy inhibition. Additional investigations
are warranted to identify the single chemical compound with the
ability to exert this anti-autophagy effect and elucidate the
molecular mechanisms underlying autophagy inhibition.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81302141), the
Administration of Traditional Chinese Medicine of Guangdong
Province (no. 20111169), the Science and Technology Planning
Project of Guangdong Province (no. 2010B031600317) and the
Fundamental Research Funds for the Sun Yat-sen University Young
Teacher Training Project (no. 12ykpy56).
References
|
1
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JH, Jin HZ, Zhang WD, Yan SK and Shen
YH: Chemical constituents of plants from the genus Brucea. Chem
Biodivers. 6:57–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Yang JY, Zhou F, et al: Seed oil
of Brucea javanica induces apoptotic death of acute myeloid
leukemia cells via both the death receptors and the
mitochondrial-related pathways. Evid Based Complement Alternat Med.
2011:1–14. 2011.
|
|
4
|
Lau ST, Lin ZX, Zhao M and Leung PS:
Brucea javanica fruit induces cytotoxicity and apoptosis in
pancreatic adenocarcinoma cell lines. Phytother Res. 22:477–486.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou GG, Yao HP and Xie LP: Brucea javanica
oil induces apoptosis in T24 bladder cancer cells via upregulation
of caspase-3, caspase-9, and inhibition of NF-κB and COX-2
expressions. Am J Chin Med. 38:613–624. 2010. View Article : Google Scholar
|
|
6
|
Lau FY, Chui CH, Gambari R, et al:
Antiproliferative and apoptosis-inducing activity of Brucea
javanica extract on human carcinoma cells. Int J Mol Med.
16:1157–1162. 2005.PubMed/NCBI
|
|
7
|
Xuan YB, Yasuda S, Shimada K, Nagai S and
Ishihama H: Growth inhibition of the emulsion from to Brucea
javanica cultured human carcinoma cells. Gan To Kagaku Ryoho.
21:2421–2425. 1994.(In Japanese). PubMed/NCBI
|
|
8
|
Mata-Greenwood E, Cuendet M, Gustin D,
Stock W and Pezzuto JM: Brusatol-mediated induction of leukemic
cell differentiation and G1 arrest is associated with
down-regulation of c-myc. Leukemia. 16:2275–2284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren D, Villeneuve NF, Jiang T, et al:
Brusatol enhances the efficacy of chemotherapy by inhibiting the
Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA.
108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murakami C, Fukamiya N, Tamura S, et al:
Multidrug-resistant cancer cell susceptibility to cytotoxic
quassinoids, and cancer chemopreventive effects of quassinoids and
canthin alkaloids. Bioorg Med Chem. 12:4963–4968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuendet M, Gills JJ and Pezzuto JM:
Brusatol-induced HL-60 cell differentiation involves NF-κB
activation. Cancer Lett. 206:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabeya Y, Mizushima N, Ueno T, et al: LC3,
a mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang XH, Jackson S, Seaman M, et al:
Induction of autophagy and inhibition of tumorigenesis by beclin 1.
Nature. 402:672–676. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo S and Rubinsztein DC: BCL2L11/BIM: a
novel molecular link between autophagy and apoptosis. Autophagy.
9:104–105. 2013. View Article : Google Scholar :
|
|
15
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: a double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar S, Davies JE, Huang Z, Tunnacliffe
A and Rubinsztein DC: Trehalose, a novel mTOR-independent autophagy
enhancer, accelerates the clearance of mutant huntingtin and
α-synuclein. J Biol Chem. 282:5641–5652. 2007. View Article : Google Scholar
|
|
19
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma XH, Piao S, Wang D, et al: Measurements
of tumor cell autophagy predict invasiveness, resistance to
chemotherapy, and survival in melanoma. Clin Cancer Res.
17:3478–3489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Wang M, He X, et al: Meta-analysis
on treatment of non-small cell lung cancer with Brucea javanica oil
emulsion in combination with platinum-contained first-line
chemotherapy. Zhongguo Zhong Yao Za Zhi. 37:2022–2029. 2012.(In
Chinese). PubMed/NCBI
|
|
22
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: a randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rangwala R, Chang YC, Hu J, et al:
Combined MTOR and autophagy inhibition: phase I trial of
hydroxychloroquine and temsirolimus in patients with advanced solid
tumors and melanoma. Autophagy. 10:1391–1402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rangwala R, Leone R, Chang YC, et al:
Phase I trial of hydroxychloroquine with dose-intense temozolomide
in patients with advanced solid tumors and melanoma. Autophagy.
10:1369–1379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo JY, Chen HY, Mathew R, et al:
Activated Ras requires autophagy to maintain oxidative metabolism
and tumorigenesis. Genes Dev. 25:460–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim MJ, Woo SJ, Yoon CH, et al:
Involvement of autophagy in oncogenic K-Ras-induced malignant cell
transformation. J Biol Chem. 286:12924–12932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang S, Wang X, Contino G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moad AI, Tengku Muhammad TS, Oon CE and
Tan ML: Rapamycin induces apoptosis when autophagy is inhibited in
T-47D mammary cells and both processes are regulated by Phlda1.
Cell Biochem Biophys. 66:567–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan QW, Cheng C, Hackett C, et al: Akt and
autophagy cooperate to promote survival of drug-resistant glioma.
Sci Signal. 3:ra812010.PubMed/NCBI
|
|
30
|
Huang S, Yang ZJ, Yu C and Sinicrope FA:
Inhibition of mTOR kinase by AZD8055 can antagonize
chemotherapy-induced cell death through autophagy induction and
down-regulation of p62/sequestosome 1. J Biol Chem.
286:40002–40012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosich L, Xargay-Torrent S, López-Guerra
M, Campo E, Colomer D and Roué G: Counteracting autophagy overcomes
resistance to everolimus in mantle cell lymphoma. Clin Cancer Res.
18:5278–5289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gordy C and He YW: The crosstalk between
autophagy and apoptosis: where does this lead? Protein Cell.
3:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo S, Garcia-Arencibia M, Zhao R, et al:
Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol
Cell. 47:359–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, An Y, Wang Y, et al: miR-101
inhibits autophagy and enhances cisplatin-induced apoptosis in
hepatocellular carcinoma cells. Oncol Rep. 29:2019–2024.
2013.PubMed/NCBI
|
|
35
|
Li Y, Zhu H, Zeng X, et al: Suppression of
autophagy enhanced growth inhibition and apoptosis of interferon-β
in human glioma cells. Mol Neurobiol. 47:1000–1010. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang Q, Li G, Wei X, et al:
Resveratrol-induced apoptosis is enhanced by inhibition of
autophagy in esophageal squamous cell carcinoma. Cancer Lett.
336:325–337. 2013. View Article : Google Scholar : PubMed/NCBI
|