Introduction
As an evolutionary process that selects for genetic
and epigenetic changes, tumorigenesis allows for the evasion of
anti-proliferative and cell death-inducing mechanisms, which
normally limit the clonal expansion of somatic cells (1). The majority of tumors develop genetic
instability, but it is unclear how early this occurs and if this
drives tumor development (2). One
of the mechanisms of tumor suppression is the DNA damage response
(DRR), which is activated in the early stages of human
tumorigenesis and leads to cell-cycle blockade or apoptosis,
thereby constraining tumor progression (3,4).
DNA double-strand breaks (DSBs) can arise from
errors that occur during DNA replication, from external agents,
including ionizing radiation, or during genomic rearrangements.
DSBs induce chromosomal aberrations that cause cells to
malfunction, resulting in cell death or tumorigenesis (5). One of the earliest steps in the
cellular response to DSBs is the phosphorylation of histone H2AX at
serine 139, the site of γ-phosphorylation, resulting in γH2AX
(6). H2AX is phosphorylated by
phosphoinositide-3 (PI3) kinases, including ataxia telangiectasia
mutated (ATM), DNA-dependent protein kinase and ataxia
telangiectasia and Rad3 related. The number of γH2AX foci is a
notable marker for DSBs. In addition to γH2AX, activated PI3
kinases also initiate other downstream events, including the
phosphorylation of BRCA1, Chk2 and p53 (7,8).
Immunohistochemical analyses of γH2AX have been performed in human
cancers of the bladder, breast, lung, colon and prostate (4,9,10).
γH2AX-positive cells have been reported to be present in colorectal
cancer (CRC) and precursor lesions, including adenoma, but not in
the normal colonic epithelium (11). Furthermore, tissue from invasive CRC
has been reported to exhibit decreased staining for γH2AX compared
with adenoma tissue (4). These
results indicate that staining of γH2AX is associated with DNA
damage checkpoint activation in premalignant lesions. Therefore,
the existence of γH2AX foci may be a useful and sensitive marker of
cancer, particularly for detecting cancers or precursor
lesions.
Worldwide, gastric cancer is the second leading
cause of cancer-associated mortality. The majority of gastric
cancers are diagnosed at a late stage, leading to a five-year
survival rate of ≤25% (12). This
late-stage diagnosis and the resulting low survival rate, in
addition to the majority of gastric cancers being preceded by
clearly recognizable precursors, has led to numerous studies
investigating the possibility of screening and surveillance
(13). The precursor lesions of
gastric cancer are well-characterized and consist of atrophic
gastritis, intestinal metaplasia or low-grade dysplasia (14). However, to the best of our
knowledge, the expression of γH2AX in gastric cancer and its
precursor lesions has not been investigated at present. The present
study investigated the effect of γH2AX expression on the
progression of the morphological spectrum between superficial and
atrophic gastritis and gastric cancer.
Materials and methods
Patients and tumor specimens
For the present immunohistochemical analysis,
formalin-fixed, paraffin-embedded archival tissues were used. The
tissues were obtained from 123 patients that were diagnosed with
superficial gastritis (n=20), atrophic gastritis (n=24) or gastric
carcinoma (n=79). The patients diagnosed with gastric carcinoma
consisted of 49 patients with moderately-differentiated gastric
adenocarcinoma, 26 patients with poorly-differentiated
adenocarcinoma, one patient with signet ring cell cancer, one
patient with salivary mucoepidermoid carcinoma and two patients
with mucoid carcinoma. All patients underwent gastroscopy and
surgery between 2011 and 2012 at Gansu Cancer Hospital (Lanzhou,
Gansu, China). There was no significant difference in the age or
gender distribution between these groups. For the gastric cancer
patients, only patients who did not undergo pre-operative radio- or
chemotherapy were enrolled in the present study. Pathological
diagnosis and classification were performed according to the
criteria of the World Health Organization (9,10). All
specimens were collected under the approval of the Ethics Comittee
of The Medical College of Northwest University for Nationalities
(Lanzhou, China) with consent from the patients.
Immunohistochemical analysis of
γH2AX
One representative tumor block from each patient,
including the tumor center, invasive front and tumor-associated
non-neoplastic mucosa, was examined by immunohistochemistry. In
cases of large, late-stage tumors, various sections were examined
to include representative areas of the tumor center and lateral and
deep invasive fronts.
The paraffin-embedded tissues that were used for the
original hematoxylin and eosin-stained sections were also chosen
for immunohistochemistry. Sections of paraffin-embedded tumor
tissue (5 μm thick) were prepared, and subsequent to dewaxing and
rehydrating the tissue using graded ethanol series, the slides were
immersed in high-pH target retrieval solution (Dako, Glostrup,
Denmark) in a 95°C water bath for 30 min. The slides were washed in
Tris-buffered saline, blocked for 10 min, and incubated with
polyclonal goat anti-mouse γH2AX antibody (1:500 dilution; cat. no.
115-545-003, Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) for 2 h. The slides were rinsed and incubated for 1 h with
fluorescein isothiocyanate-conjugated anti-mouse goat
F(ab′)2 fragment (Dako). To stain the nuclei, the slides
were submersed in 0.05 μg/ml DAPI for 5 min, rinsed and mounted
using 10 μl fluorescent mounting media (KPL, Gaithersburg, MD,
USA). The tumor sections were observed using an Olympus BX53
fluorescent microscope (Olympus, Tokyo, Japan). As this was
considered a feasibility study, no specific procedures were adopted
to ensure that images were obtained randomly across the section. As
sections rather than whole cells were scored, cells possessing one
or more γH2AX foci were counted as positive. Efforts were made to
score only tumor regions, and evident regions of necrosis or stroma
were not included in the analysis. The foci results were presented
as averages of the scores for several high-power images. In total,
8–12 digitized images, containing 50–100 nuclei each, were scored
by eye for the percentage of nuclei presenting γH2AX foci, and the
results were presented as averages. Finally, the sections were
scored semi-quantitatively as follows: (1+), <10% immunopositive
cells; (2+), 10–50% immunopositive cells; and (3+), >50%
immunopositive cells (15–17).
Statistical methods
Associations between the clinicopathological
variables and immunostaining for γH2AX were analyzed using a
χ2-test. SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for the statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
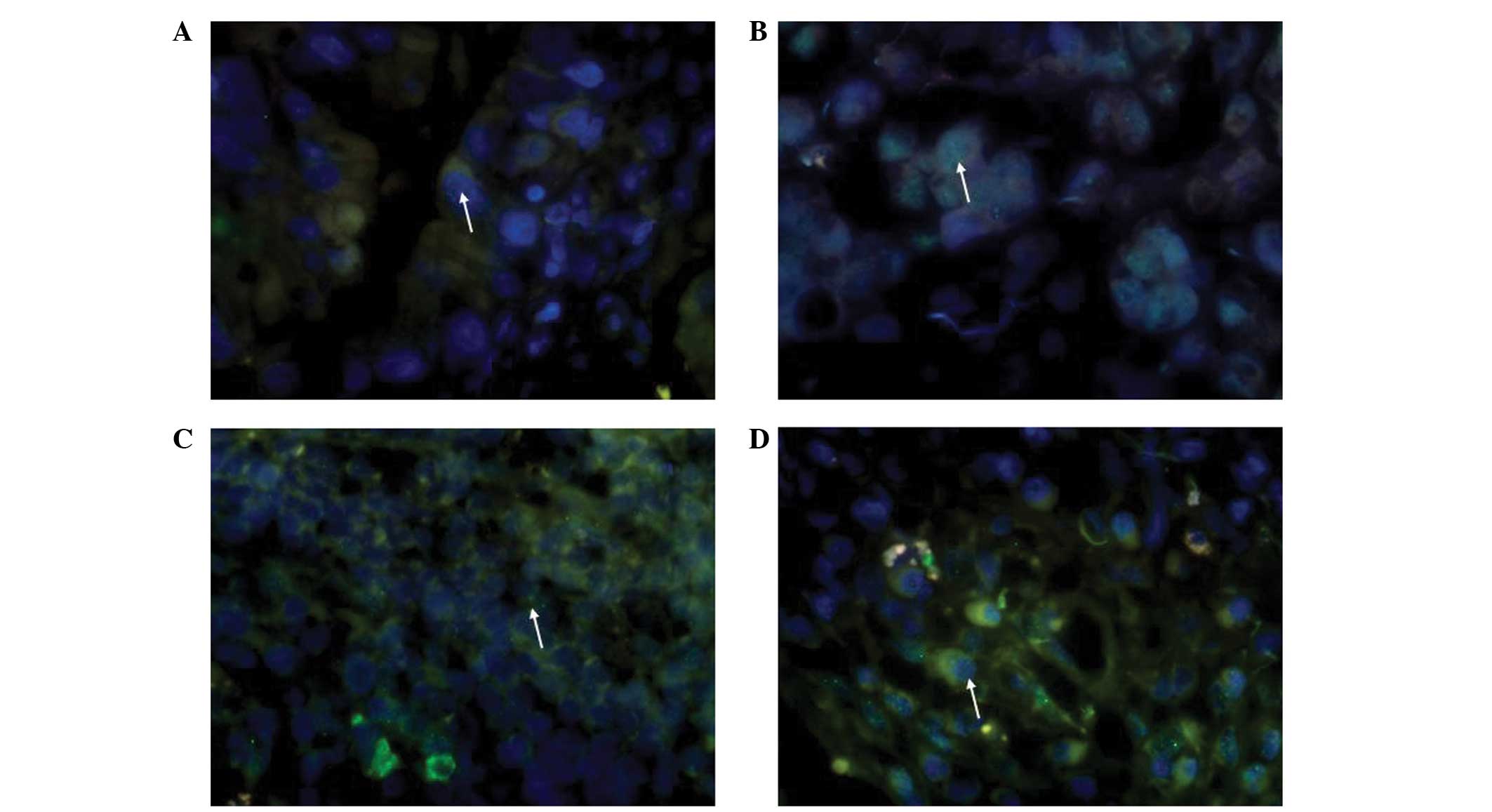
High-power images of these biopsies were analyzed
visually to determine the fraction of nuclei that were
γH2AX-positive, which was defined as cells possessing one or more
foci per nucleus. γH2AX produced granular nuclear staining. It was
confirmed that γH2AX demonstrated ubiquitous staining in gastric
adenocarcinoma, particularly in moderately-differentiated gastric
adenocarcinoma, and that superficial and atrophic gastritis
demonstrate only partial staining (Fig.
1A–D). It has previously been reported that γH2AX is expressed
during early apoptosis triggered through the caspase
3/caspase-activated DNase pathway (18) and that γH2AX-positive tumor cells in
superficial portions or necrotic debris were also positive for the
activated form of caspase-3, which is a marker of apoptosis.
Therefore, cases with superficial staining and staining of necrotic
debris were not classified as positive for γH2AX expression in the
present study.
Gastric carcinoma tissue expresses a higher level of
γH2AX compared with superficial and atrophic gastritis tissue
(Table I; χ2=68.712;
P<0.001). On average, 34.43% nuclei in gastric carcinoma tissues
possess one or more γH2AX foci, ranging between 5.34 and 72.71%.
However, in superficial and atrophic gastritis, only 6.35 and 9.75%
of nuclei, respectively, contained foci. Poorly-differentiated
gastric adenocarcinoma expressed lower levels of γH2AX compared
with moderately-differentiated adenocarcinoma (Table II; χ2=14.241;
P<0.01). In poorly-differentiated gastric adenocarcinoma, 26.55%
of nuclei contained one or more γH2AX foci, ranging between 5.64
and 66.71%, and in moderately-differentiated adenocarcinoma, 39.31%
of nuclei contained one or more γH2AX foci, ranging between 5.75
and 72.73%.
 | Table IImmunohistochemical analysis of γH2AX
expression in superficial gastritis, atrophic gastritis and gastric
carcinoma. |
Table I
Immunohistochemical analysis of γH2AX
expression in superficial gastritis, atrophic gastritis and gastric
carcinoma.
| | γ-H2AX expression,
n | | |
|---|
| |
| | |
|---|
| Diagnosis | n | + | ++ | +++ | χ2 | P-value |
|---|
| Superficial
gastritis | 20 | 19 | 1 | 0 | | |
| Atrophic
gastritis | 26 | 21 | 5 | 0 | 68.712 | 0.000 |
| Gastric
carcinoma | 79 | 10 | 49 | 20 | | |
 | Table IIImmunohistochemical analysis of the
difference in γH2AX expression between moderately- and
poorly-differentiated gastric adenocarcinoma. |
Table II
Immunohistochemical analysis of the
difference in γH2AX expression between moderately- and
poorly-differentiated gastric adenocarcinoma.
| | γH2AX expression,
n | | |
|---|
| |
| | |
|---|
| Gastric
adenocarcinoma | n | + | ++ | +++ | χ2 | P-value |
|---|
|
Moderately-differentiated | 49 | 1 | 34 | 14 | 14.241 | 0.007 |
|
Poorly-differentiated | 26 | 8 | 13 | 5 | | |
The association between γH2AX staining and
clinicopathological parameters in gastric adenocarcinoma was also
analyzed. γH2AX staining demonstrated no significant association
with age, depth of invasion, lymph node metastasis or the
tumor-node-metastasis (TNM) stage in gastric carcinoma (Table III).
 | Table IIICorrelation between γH2AX expression
and the clinicopathological features of gastric carcinoma. |
Table III
Correlation between γH2AX expression
and the clinicopathological features of gastric carcinoma.
| | γH2AX expression,
n | | |
|---|
| |
| | |
|---|
| Clinicopathological
features | n | + | ++ | +++ | χ2 | P-value |
|---|
| Age, years |
| <55 | 28 | 6 | 16 | 6 | 3.019 | 0.221 |
| ≥55 | 51 | 4 | 34 | 13 | | |
| Depth of
invasion |
| Tis-1 | 11 | 3 | 7 | 1 | 3.346 | 0.188 |
| T2–4 | 68 | 7 | 43 | 18 | | |
| Lymph node
metastasis |
| Present | 69 | 10 | 43 | 16 | 1.699 | 0.221 |
| Absent | 10 | 0 | 7 | 3 | | |
| Distant
metastasis |
| Present | 30 | 3 | 19 | 8 | 0.117 | 0.943 |
| Absent | 49 | 6 | 31 | 12 | | |
| TNM stage |
| 0–I | 11 | 1 | 9 | 1 | 1.838 | 0.399 |
| II–IV | 68 | 8 | 42 | 18 | | |
Discussion
In previous years, evidence has emerged to support
the hypothesis that there is aberrant activation of the DDR
checkpoint in human epithelial pre-cancerous lesions. Bartek et
al (11) reported extensive
abnormalities of the ATM-Chk2 axis in non-invasive precursor
lesions of bladder, colon and breast cancers. By contrast,
Gorgoulis et al (9) reported
similar results obtained from lung and epidermal tissues. In all
these instances, DDR checkpoint activation was accompanied by
evidence of DNA double-strand breaks, as assessed by γH2AX
expression. In addition, DDR has been observed in pre-cancerous
lesions prior to the onset of the genomic instability that
characterizes invasive cancer, indicating that widespread allelic
imbalances were not the underlying basis for checkpoint activation
within the epithelium (19).
While DDR is a major component in tumor suppression,
the mechanism behind this response actively participating in the
suppression of gastric tumorigenesis remains elusive (20). In the present study, the expression
of γH2AX in gastric tissues was investigated and a significant
immunoexpression was noted and confirmed by statistical
evaluation.
The current study provided evidence for the presence
of a DDR in gastric cancer tissue, which is characterized by
staining for γH2AX. The γH2AX levels, which were assessed by
nuclear staining scores, were significantly higher in gastric
cancer tissues compared with superficial and atrophic gastritis
tissues. Certain cells exhibited a notable increase in nuclear
staining for γH2AX and others demonstrated distinct nuclear foci,
which were confirmed by fluorescence microscopy. The majority of
tumors develop genetic instability, but the rapidity with which
this occurs and the ability to drive tumor development remains
unclear (21). From the initial
changes, cancer development is associated with DNA replication
stress, leading to DNA double-strand breaks, genomic instability
and selective pressure for p53 mutations. Several mechanisms to
constrain oncogenesis have been proposed, including hypoxia,
telomere attrition and reactive oxygen species (22).
Secondly, the present study revealed that the
expression of γH2AX differs between poorly- and
moderately-differentiated gastric adenocarcinoma.
Moderately-differentiated gastric adenocarcinoma contained a higher
number of γH2AX foci compared with poorly-differentiated gastric
adenocarcinoma, and the expression levels of γH2AX were independent
of the depth of invasion and TNM stage in the two gastric
adenocarcinoma types. It was hypothesized that in the
poorly-differentiation gastric adenocarcinoma tissues a serious DDC
had occurred, resulting in the lower levels of γH2AX. When the DNA
damage in cells is too serious to be repaired, γH2AX-positive cells
undergo apoptosis. An additional explanation is that the molecular
mechanisms underlying the phosphorylation of γH2AX may differ
between moderately- and poorly-differentiated gastric
adenocarcinoma tissues. It is also possible that, due to a single
DSB being able to result in chromosomal translocations, deletions
or loss of genetic information, several genes associated with tumor
progression may be deleted in H2AX-positive gastric carcinoma
tissues (17).
In the present study, the mechanism behind the
decreased expression of γH2AX in atrophic gastritis remains to be
elucidated. γH2AX has been reported to be commonly expressed in
early precursor lesions located in the bladder, breast, lung, colon
and prostate (9,10). Atrophic gastritis is considered as a
pre-malignant gastric lesion in patients at risk of progression to
gastric cancer (23). It was
therefore hypothesized that atrophic gastritis should demonstrate a
higher DDR. Certain studies support the present result (24–26).
In non-neoplastic gastric mucosa or intestinal metaplasia adjacent
to the tumor, only a few superficial cells demonstrated
immunostaining of γH2AX. Furthermore, in intestinal metaplasia
adjacent to the tumor, which is considered to be a gastric
precancerous lesion, staining of γH2AX was not observed in
epithelial or stromal cells (14).
In addition, a previous large cohort study revealed that the risk
of progression to cancer within 10 years was only 0.8% for
individuals with atrophic gastritis (12). These results suggest that DSBs are
less likely to be involved in the genesis of gastric carcinoma.
Although the number of cases examined in the present
study was relatively small, it was revealed that γH2AX is
overexpressed in gastric carcinoma, and that the expression of
γH2AX is higher in moderately-differentiated gastric adenocarcinoma
compared with poorly-differentiated gastric adenocarcinoma. These
results demonstrated that enhanced γH2AX expression may be closely
associated with gastric carcinoma, but is less likely to be
involved in the genesis of gastric carcinoma. However, additional
details require further investigation.
Acknowledgements
This study was supported in part by grants from The
National Natural Science Foundation of China (grant nos. 31060127
and 81260442), Gansu Provincial Natural Science Foundation (grant
nos. 1010RJZA078 and 1208RJZA246) and The Project Sponsored by the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars and State Education Ministry (grant no. [2011]1568).
References
|
1
|
Merlo LM, Pepper JW, Reid BJ and Maley CC:
Cancer as an evolutionary and ecological process. Nat Rev Cancer.
6:924–935. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bodmer W, Bielas JH and Beckman RA:
Genetic instability is not a requirement for tumor development.
Cancer Res. 68:3558–3560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woo RA, McLure KG, Lees-Miller SP,
Rancourt DE and Lee PW: DNA-dependent protein kinase acts upstream
of p53 in response to DNA damage. Nature. 394:700–704. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartkova J, Horejsí Z, Koed K, et al: DNA
damage response as a candidate anti-cancer barrier in early human
tumorigenesis. Nature. 434:864–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Gent DC, Hoeijmakers JH and Kanaar R:
Chromosomal stability and the DNA double-stranded break connection.
Nat Rev Genet. 2:196–206. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paull TT, Rogakou EP, Yamazaki V, et al: A
critical role for histone H2AX in recruitment of repair factors to
nuclear foci after DNA damage. Curr Biol. 10:886–895. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao J, Guo Z, Zhang H, et al: The
potential value of the neutral comet assay and γH2AX foci assay in
assessing the radiosensitivity of carbon beam in human tumor cell
lines. Radiol Oncol. 30:247–257. 2013.
|
|
9
|
Gorgoulis VG, Vassiliou LV, Karakaidos P,
et al: Activation of the DNA damage checkpoint and genomic
instability in human precancerous lesions. Nature. 434:907–913.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan C, Quan R and Feng X: ATM activation
is accompanied with earlier stages of prostate tumorigenesis.
Biochim Biophys Acta. 1763:1090–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spruck CH, Won KA and Reed SI: Deregulated
cyclin E induces chromosome instability. Nature. 401:297–300. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
den Hoed CM, Holster IL, Capelle LG, et
al: Follow-up of premalignant lesions in patients at risk for
progression to gastric cancer. Endoscopy. 45:249–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Cutsem E, Dicato M, Geva R, et al: The
diagnosis and management of gastric cancer: expert discussion and
recommendations from the 12th ESMO/World Congress on
Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 5:v1–9. 2011.
View Article : Google Scholar
|
|
14
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sentani K, Oue N, Sakamoto N, et al:
Positive immunohistochemical staining of gammaH2AX is associated
with tumor progression in gastric cancers from radiation-exposed
patients. Oncol Rep. 20:1131–1136. 2008.PubMed/NCBI
|
|
16
|
Olive PL, Banuelos CA, Durand RE, Kim JY
and Aquino-Parsons C: Endogenous and radiation-induced expression
of gammaH2AX in biopsies from patients treated for carcinoma of the
uterine cervix. Radiother Oncol. 94:82–89. 2010. View Article : Google Scholar
|
|
17
|
Brustmann H, Hinterholzer S and Brunner A:
Expression of phosphorylated histone H2AX (γ-H2AX) in normal and
neoplastic squamous epithelia of the uterine cervix: an
immunohistochemical study with epidermal growth factor receptor. J
Gynecol Pathol. 30:76–83. 2011. View Article : Google Scholar
|
|
18
|
Lu C, Zhu F, Cho YY, et al: Cell
apoptosis: requirement of H2AX in DNA ladder formation, but not for
the activation of caspase-3. Mol Cell. 23:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simão ÉM, Sinigaglia M, Bugs CA, Castro
MA, Librelotto GR, Alves R and Mombach JC: Induced genome
maintenance pathways in pre-cancer tissues describe an anti-cancer
barrier in tumor development. Mol Biosyst. 8:3003–3009. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohnishi S, Ma N, Thanan R, Pinlaor S,
Hammam O, Murata M and Kawanishi S: DNA damage in
inflammation-related carcinogenesis and cancer stem cells. Oxid Med
Cell Longev. 2013:3870142013. View Article : Google Scholar
|
|
21
|
Bartek J, Bartkova J and Lukas J: The
retinoblastoma protein pathway in cell cycle control and cancer.
Exp Cell Res. 237:1–6. 1997. View Article : Google Scholar
|
|
22
|
Olive PL: Retention of γH2AX foci as an
indication of lethal DNA damage. Radiother Oncol. 101:18–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng XS, Wang YF, Hao SG, et al:
Expression of Das-1, Ki67 and sulfuric proteins in gastric cardia
adenocarcinoma and intestinal metaplasia lesions. Exp Ther Med.
5:1555–1558. 2013.PubMed/NCBI
|
|
24
|
Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB
and Chung MH: 8-Hydroxydeoxyguanosine: not mere biomarker for
oxidative stress, but remedy for oxidative stress-implicated
gastrointestinal diseases. World J Gastroenterol. 18:302–308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hardbower DM, Peek RM Jr and Wilson KT: At
the Bench: Helicobacter pylori, dysregulated host responses, DNA
damage, and gastric cancer. J Leukoc Biol. 96:201–212. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawanishi S and Hiraku Y: Oxidative and
nitrative DNA damage as biomarker for carcinogenesis with special
reference to inflammation. Antioxid Redox Signal. 8:1047–1058.
2006. View Article : Google Scholar : PubMed/NCBI
|