Introduction
The human ribophorin II (RPN2) gene has been
localized to chromosome 20ql2-13.1, a region that is frequently
deleted in patients with myeloid malignancies (1–4). The
gene, which was cloned in 1987 (5),
encodes a type I integral membrane protein that is found only in
the rough endoplasmic reticulum (ER). Analysis of the structural
and topological features of the gene has revealed RPN2 to be a
unique integral rough ER membrane glycoprotein that is involved in
translocation and the maintenance of the structural uniqueness of
the rough ER (5,6). Subsequent biochemical studies have
demonstrated that the RPN2 protein is a component of an
N-oligosaccharyl transferase complex that conjugates high mannose
oligosaccharides to asparagine residues in the N-X-S/T consensus
motif of nascent polypeptide chains (7,8).
In addition to its association with myeloid
disorders, RPN2 has been demonstrated to be a prognostic marker of
human breast (9) and pancreatic
cancers (10). RPN2 has also been
revealed to contribute to the resistance of tumor cells to
chemotherapeutic agents, including docetaxel and taxane, in animal
models of breast (11) and ovarian
(12) cancers, and in clinical
studies of breast (11) and
esophageal squamous cell carcinoma (13). In an RNA interference (RNAi)-based
screening study, Honma et al identified RPN2 as a molecular
target for therapy (11). In this
animal model of orthotopically implanted, docetaxel-resistant
breast tumors, it was revealed that RPN2 silencing effectively
facilitated the accumulation of docetaxel in tumor cells, augmented
docetaxel-induced apoptotic cell death, and suppressed tumor
growth. These studies indicated that RPN2 confers drug resistance
by N-glycosylation, which stabilizes the transporter P-glycoprotein
(P-gp) in the cellular membrane, and by regulating antiapoptotic
genes. This study further demonstrated that the RPN2 expression
status in patients with breast cancer was associated with the
response to docetaxel, proposing RPN2 as a candidate predictive
marker for resistance to docetaxel-based chemotherapy (11,13).
Future studies on genes involved in clinical
anticancer drug resistance offer the possibility of identifying
early prognostic markers and developing personalized therapeutic
targets that can improve the efficacy of therapies against human
cancers. There is little current information regarding RPN2
expression in gastric cancer or a possible correlation between its
expression and responses to clinical anticancer drugs. Utilizing
gastric cancer cell lines as a model, the present study was
undertaken to elucidate the role of RPN2 in the response of cells
to six common chemotherapeutic agents.
Materials and methods
Cell culture
The human gastric AGS, TMC-1, SNU-1, TMK-1, SCM-1,
MKN-45 and KATO III carcinoma cell lines were gifted from Dr.
Chun-Ying Wu (Division of Gastroenterology, Taichung Veterans
General Hospital, Taichung, Taiwan). Cells were cultured in
RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS), 2%, w/v sodium bicarbonate, 0.29
mg/ml L-glutamine, 100 units/ml penicillin and 100 μg/ml
streptomycin (Invitrogen) in a humidified 5% CO2
incubator at 37°C.
Antibodies
Specific monoclonal antibodies against RPN2 (H300),
P-gp (G-1) and β-actin were obtained from Santa Cruz Biotechnology
(Dallas, TX, USA). Polyclonal antibodies against poly(ADP-ribose)
polymerase (PARP; catalog no. 9542), caspase 3 (catalog no. 9661)
and Bcl-2 (catalog no. 2872), and monoclonal antibodies against p21
(catalog no. 12D1) were obtained from Cell Signaling Technology
(Beverly, MA, USA). The monoclonal anti-p53 antibody (catalog no.
BP53-12) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Treatment
Cells (1×105) were seeded in 6 cm culture
dishes and incubated overnight at 37°C in medium containing 10%
FBS. Cells then subsequently treated with oxaliplatin (20, 40 and
80 μM), irinotecan (10, 20 and 40 μM), doxorubicin (100, 200 and
400 nM), docetaxel (2.5, 5 and 10 nM), cisplatin (2, 4 and 8 μg/ml)
and 5 fluorouricil (5-FU; purchased from Sigma-Aldrich) (50, 100
and 200 μM) for 48 h and cell viability was determined by MTS
assay.
MTS assay
Cells (5 × 103) were seeded in 96-well
culture plates and incubated overnight at 37°C in medium containing
10% FBS. At the end of treatment, the cell viability was determined
using a rapid, tetrazolium-based MTS colorimetric assay (CellTiter
96 cell proliferation assay kit; Promega, Madison, WI, USA)
according to the manufacturer’s instructions. All experiments were
performed at least in triplicate on three separate occasions. A
dose-response curve was plotted, and the concentration of each drug
that resulted in a 50% decrease in color development was calculated
and classed as the IC50 value for each drug. The data
are presented as the mean ± standard deviation.
Apoptosis determination
Apoptosis was measured using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD
Pharmingen, San Jose, CA, USA). The cells cultured in 6-cm dishes
were trypsinized and collected by centrifugation. The cell pellet
was washed, resuspended in 1X binding buffer and stained with
Annexin V-FITC, according to the manufacturer’s instructions. The
cells were also stained with propidium iodide (PI) to detect
necrosis or late apoptosis. The distribution of viable (FITC/PI
double-negative), early apoptotic (FITC-positive), late apoptotic
(FITC/PI double-positive) and necrotic (PI-positive/FITC-negative)
cells was analyzed using a Beckman Coulter FC500 flow cytometer
(Beckman Coulter, Brea, CA, USA). The results are reported as a
percentage of the total cells.
Transfection of small interfering RNA
(siRNA)
The RPN2 siRNA duplex was purchased from Dharmacon
Research (Lafayette, CO, USA). The gastric cancer cells cultured in
glucose-free Opti-MEM were transfected with the siRNA using
Lipofectamine RNAiMAX (Invitrogen), according to the manufacturer’s
instructions.
Western blot analysis
The cell extracts were prepared in lysis buffer,
which consisted of 20 mM Tris-HCl (pH 7.4), 100 mM NaCl, 5 mM EDTA,
2 mM phenylmethylsulfonyl fluoride, 10 ng/ml leupeptin and 10 μg/ml
aprotinin. Volumes of extract containing equal amounts of proteins
were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The proteins were then transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA,
USA), and the membranes were blocked, washed, and probed with
primary antibodies. The antibodies used were monoclonal antibodies
against RPN2, P-gp (G 1), β-actin (C4), p21 and p53, and polyclonal
antibodies against poly(ADP ribose) polymerase (PARP), caspase 3,
Bcl-2. Subsequent to the removal of the primary antibody by
washing, the membranes were incubated with horseradish peroxidase
conjugated goat anti-mouse or anti-rabbit secondary antibody (Santa
Cruz Biotechnology) for 1 h. The blots were washed again, and were
developed using enhanced chemiluminescence (ECL) reagents,
according to the manufacturer’s instructions (Millipore).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
RNA was isolated from the cultured cells using
TRIzol reagent (Invitrogen), according to the manufacturer’s
instructions. cDNA was synthesized from 2 μg of total RNA by
reverse transcription, using the ImProm-II Reverse Transcriptase
kit (Promega, Madison, WI, USA) and oligo(d) 12–18 primers. The
resulting cDNA was used for the subsequent PCR assays. RPN2 was
amplified by using the primers with the following sequences:
Forward, 5′-GCCAGGAAGTGGTGTTTGTT-3′ and reverse,
5′-ACAGAGCGAAGAGCAGAAGC-3′, in conjunction with a thermal cycling
program consisting of 95°C for 1 min, 55°C for 1 min, and 72°C for
1 min for 30 cycles. β-actin was amplified as an internal control.
The β-actin primers were: Forward, 5′-AGAGCTACGAGCTGCCTGAC-3′ and
5′-CACCTTCACCGTTCCAGTTT-3′.
Statistical analysis
The differences in the data between the groups were
analyzed to determine the significance using the Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RPN2 expression and anticancer
drug-induced cytotoxicity
It has been proposed that RPN2 expression status is
a predictive marker for drug resistance in breast cancer. However,
little is known about the correlation between RPN2 expression and
the response of gastric cancer cells to clinical anticancer drugs.
In the present study, RPN2 expression was analyzed in seven gastric
cancer cell lines by western blot analysis (Fig. 1B). Among these lines, MKN-45 and
TMK-1 cells revealed high levels of RPN2 expression at the protein
level, whereas AGS and SNU-1 cells exhibited much lower levels of
RPN2 protein expression (Fig. 1A).
Therefore, AGS, MKN-45, TMK-1 and SCM-1 cells were used for the
subsequent analysis. Notably, RPN2 expression was similar at the
transcriptional level in all seven gastric cancer line (Fig. 1B). The cytotoxicity of six common
anticancer drugs, oxaliplatin, irinotecan, doxorubicin, docetaxel,
cisplatin and 5-FU, was then determined in these four gastric
cancer lines by exposing the cells to various concentrations of
anticancer drugs for 48 h and then performing MTS assays, which
measured the reduction of the MTS dye to formazan by enzymes in
living cells.
 | Figure 1RPN2 expression in the gastric cancer
AGS, TMC-1, SNU-1, TMK-1, SCM-1, MKN-45 and KATO III cell lines.
(A) The cell extracts were prepared from exponentially growing
cells, and extracts containing equal amounts of protein were
resolved by SDS-PAGE, followed by western blot analyses using an
antibody specific for RPN2. Among these lines, MKN 45 and TMK 1
cells revealed high levels of RPN2 expression at the protein level,
whereas AGS and SNU 1 cells exhibited much lower levels of RPN2
protein expression. (B) The RPN2 mRNA levels in each cell line were
determined by reverse transcription-polymerase chain reaction using
total RNA isolated from cultured cells. RPN2 expression was similar
at the transcriptional level in all seven gastric cancer lines..
RPN2, ribophorin II. |
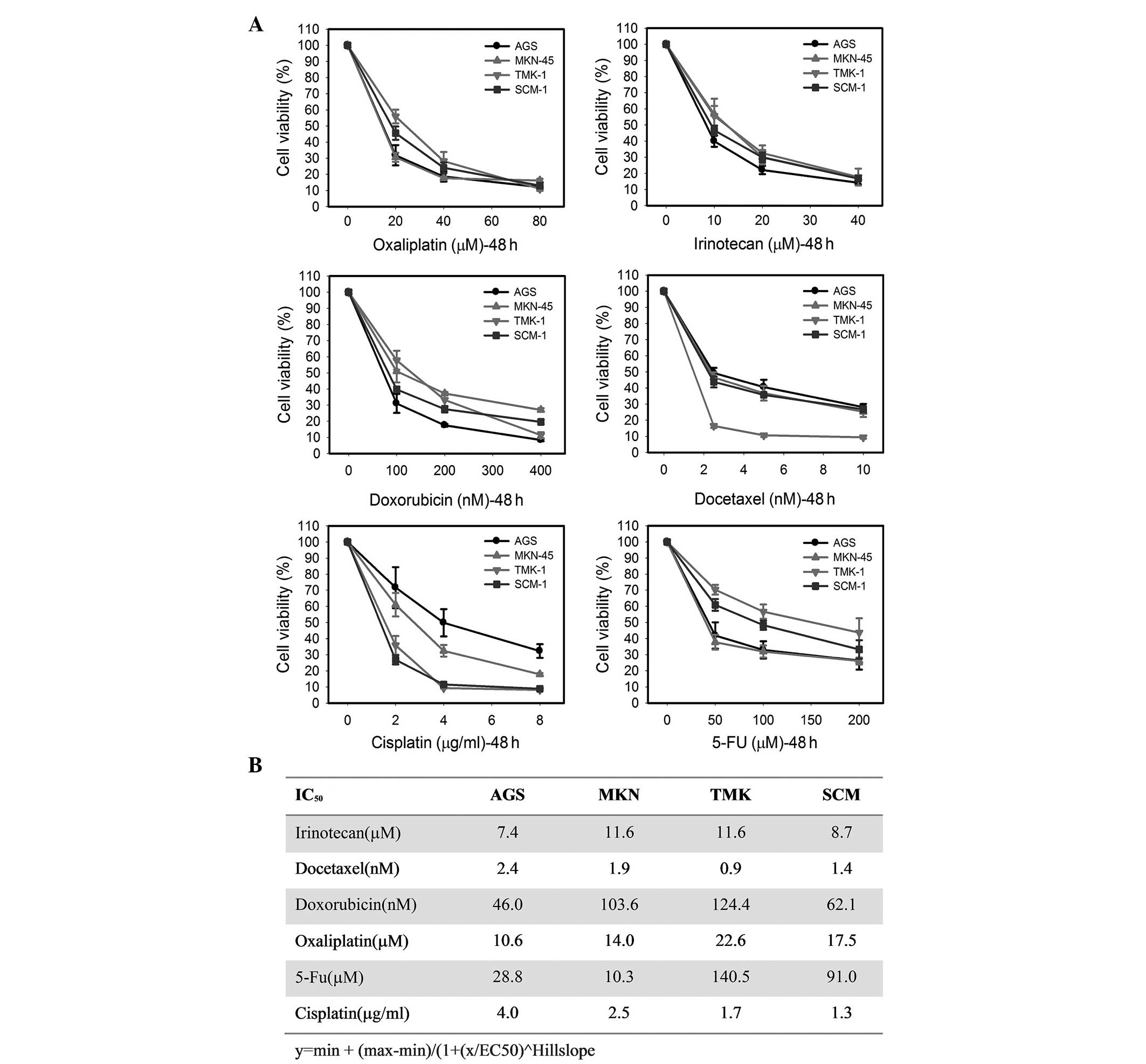
In these MTS assays, all six anticancer drugs
induced a concentration-dependent inhibition of cell survival in
all tested cell lines (Fig. 2A). To
evaluate the role of RPN2 in the drug responsiveness of gastric
cancer cell lines, the half-maximal inhibitory concentration
(IC50) was measured for each anticancer drug. The
IC50 values calculated from the MTS assays, presented in
Fig. 2B, indicate a substantial
difference in the sensitivity to anticancer drugs of these four
cell lines. For example, the AGS cells were moderately resistant to
cisplatin exposure compared with the other three cell lines,
whereas the SCM-1 cells showed the lowest IC50 for
cisplatin. By contrast, the AGS cells showed the lowest
IC50 for irinotecan, doxorubicin and oxaliplatin,
whereas the TMK-1 cells exhibited the highest IC50 value
for these agents out of the four cell lines (Fig. 2B). Compared with the other cell
lines, the TMK-1 cells showed the lowest IC50 value and
were the most sensitive to docetaxel. Additionally, the MKN-45
cells were the most sensitive to 5-FU among the four cell lines
with an IC50 of 10.3 μM. In contrast, MKN-45 exhibited
the most resistanance to irinotecan with an IC50 of 11.6
μM. Taken together, the results indicated that RPN2 expression
levels were not related to the response to anticancer drugs used in
this study.
Anticancer drug-induced cytotoxicity
through apoptosis in gastric cancer cell lines
To further analyze whether the anticancer
drug-induced growth inhibition was attributable to apoptosis, the
cells were examined for apoptosis-associated protein expression by
western blot analysis. At 48 h post-exposure to 2 μg/ml cisplatin,
the expression of the cleaved, active form of caspase 3 was
significantly enhanced in the AGS, TMK-1 and SCM-1 cells (Fig. 3A). Consistent with this result, 2
μg/ml cisplatin also enhanced PARP cleavage (Fig. 3A). In addition, the expression of
p53 protein was induced by 2 μg/ml cisplatin in the AGS and MKN-45
cells, leading to increased p21 expression, indicating a possible
p53-mediated growth-inhibition pathway (Fig. 3B). The present study also evaluated
the cytotoxic effect of docetaxel and found that docetaxel
significantly induced the activation of caspase 3, leading to
enhanced PARP cleavage in all cell lines (Fig. 3C). The downregulation of Bcl-2
observed in the TMK-1 and SCM-1 cells is also consistent with the
induction of apoptosis caused by 2 nM docetaxel (Fig. 3C). Similarly, 2 nM docetaxel
increased the expression of the p53 and p21 proteins in the AGS and
MKN-45 cells (Fig. 3D). Notably,
although MKN-45 cells exhibited significant induction of p53 and
p21 in response to cisplatin and docetaxel, the levels of activated
caspase 3 and cleaved PARP were lower compared with other cell
lines. In addition, p53 expression was lower in SCM-1 cells
compared with the other cell lines, even subsequent to treatment
with cisplatin and docetaxel.
Effect of siRNA-mediated RPN2 silencing
on anticancer-drug sensitivity in various gastric cancer cell
lines
To directly study the importance of the RPN2 protein
level in drug responsiveness of gastric cancer cells, a loss of
function approach was employed, using siRNA to knock down RPN2
expression in four gastric cancer cell lines. The RPN2 protein
level was markedly downregulated by RPN2 siRNA after 72 h in the
tested gastric cancer cell lines (Fig.
4A). The subsequent experiments revealed that siRNA-mediated
RPN2 knockdown in the AGS cells increased the percentage of
apoptotic cells from 5.9% in the siRNA control cells to 8.6% in the
RPN2-knockdown cells. Additional induction of apoptosis was
observed after treatment with 4 μg/ml cisplatin, which increased
the percentage of apoptotic cells between 5.9% in the control siRNA
group and 14.4% in the RPN2-knockdown cells (Fig. 4B). Therefore, knockdown of RPN2
significantly enhanced cisplatin-induced apoptosis in the AGS cells
compared with the siRNA control group (Fig. 4C). Furthermore, western blot
analyses demonstrated that the depletion of RPN2 increased the
level of activated caspase 3 and downregulated Bcl-2 expression,
which supports the hypothesis of enhanced induction of apoptosis by
RPN2 knockdown alone (Fig. 4D).
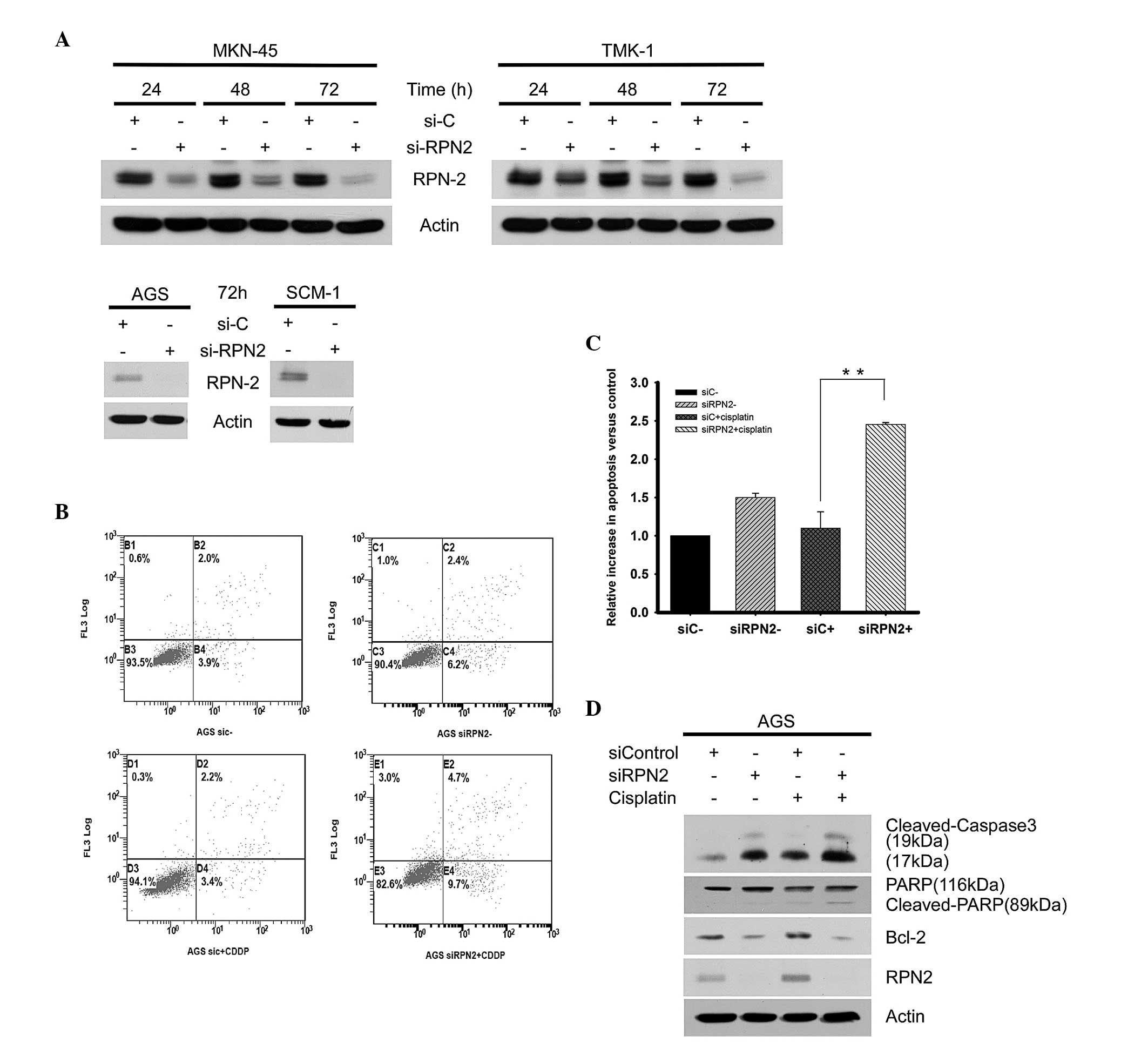 | Figure 4Knockdown of RPN2 enhances apoptotic
cell death in the AGS cell line. (A) The MKN-45, TMK-1, AGS and
SCM-1 cells were transfected with RPN2 siRNA duplex to specifically
silence RPN2 expression. (B) The AGS cells were exposed to 4 μg
cispatin for 48 h and the induction of apoptosis was analyzed. The
percentage of apoptotic cells was determined by flow cytometry, and
the results were expressed as the percentage of total cells in
apoptotic populations. (C) Increases in apoptosis were calculated
as fold-induction compared to the control, and the values,
presented as the mean ± standard deviation, were obtained from at
least three independent experiments. (D) The protein levels of
activated caspase 3, cleaved PARP, Bcl-2, RPN2 and β-actin in
control and RPN2-knockdown cells subsequent to 2μg/ml cisplatin
treatment were determined by western blot analysis.
**Indicates P<0.01 compared with cisplatin-treated
siC. PARP, poly(ADP-ribose) polymerase; Bcl-2, B-cell lymphoma 2;
RPN2, ribophorin II; siRNA, small interfering RNA; si-C, control
siRNA; si-RPN2, RPN2 siRNA; CDDP, cisplatin. |
The functional significance of RPN2 in cell survival
in response to six anticancer drugs was then investigated using MTS
assays. The AGS cells were transfected with siRPN2 for 24 h and
then treated with anticancer drugs for 48 h. In the presence of
anticancer drugs, treatment with RPN2 siRNA slightly reduced the
viability of AGS cells relative to the siRNA control. This effect
of RPN2-knockdown was significant for all anticancer drugs with the
exception of 5-FU, indicating that RPN2 may exert a protective role
in cell survival (Fig. 5). RPN2
knockdown in MKN-45 cells also enhanced caspase 3 activation and
Bcl-2 downregulation (Fig. 6A).
However, subsequent treatment with cisplatin exhibited no evident
effect on caspase 3 activation and demonstrated little synergetic
effect on Bcl-2 downregulation. These observations were further
supported by MTS assays, which revealed no significant decrease in
survival in the cisplatin-exposed RPN2-knockdown MKN-45 cells
compared with the cisplatin-exposed control siRNA cells (Fig. 6B).
 | Figure 6RPN2 silencing does not further
increase cisplatin-induced cell death in the MKN-45 cell line. (A)
The MKN-45 cells were transfected with siRPN2 to silence RPN2
expression. The protein levels of activated caspase-3, cleaved
PARP, Bcl-2, RPN2, and β-actin in control and RPN2-knockdown cells
following treatment with 2 μg/ml cisplatin were determined by
western blot analysis. (B) The RPN2-knockdown MKN-45 cells were
treated with 2.5 μg/ml cisplatin (IC50 for MKN-45) for
48 h, and the cell viability was determined using a MTS assay. The
values were obtained from at least three independent experiments.
PARP, poly(ADP-ribose) polymerase; Bcl-2, B-cell lymphoma 2; RPN2,
ribophorin II; si, small interfering RNA; siC, control siRNA;
siRPN2, RPN2 siRNA. |
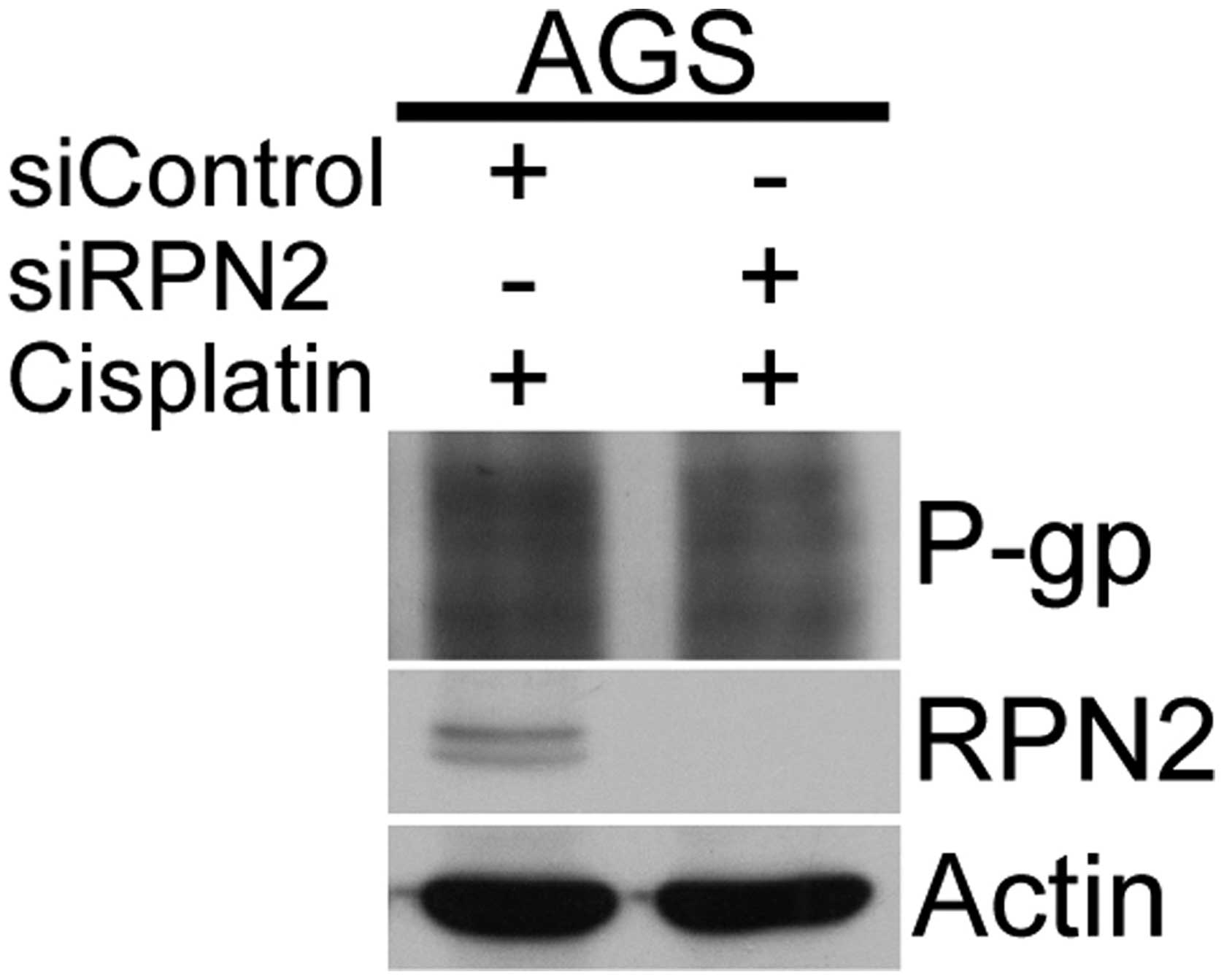
RPN2-knockdown decreased the level of
N-glycosylation on P-gp in response to cisplatin
The potential effect of RPN2 on P-gp 1 function was
examined via N-glycosylation in the mechanism of anticancer drug
resistance. Expression of the multidrug transporter P-gp, encoded
by the multidrug resistance 1 (MDR1) gene, is a major mechanism
leading to multidrug resistance in cancer cells. To test the role
of P-gp glycosylation in anticancer drug resistance in gastric
cancer cells, the AGS cells were transfected with RPN2 siRNA and
the glycosylation status of the P-gp protein was determined. A
western blot analysis of P-gp revealed that the smear pattern of
P-gp, which has previously been demonstrated to reflect the
presence of various sizes of intermediately glycosylated forms
(11), was slightly decreased upon
cisplatin treatment in the RPN2-knockdown cells (Fig. 7).
Discussion
A previous study revealed that downregulation of
RPN2 efficiently induced apoptosis in docetaxel-resistant human
breast cancer cells in the presence of docetaxel (11). This study reported that silencing of
RPN2 reduced the glycosylation and membrane localization of P-gp,
thereby sensitizing cancer cells to docetaxel (11). Considering that numerous anticancer
drugs are commonly used in the clinic to treat various human
cancers, there is an urgent requirement for an efficient assessment
of the curative effects of these agents in individuals. Cell lines
are highly useful for preclinical physiological and toxicological
studies and are commonly used in a wide range of biomedical
studies. Accordingly, the current study used the AGS, SCM-1, TMK-1
and MKN-45 gastric cancer cell lines to investigate whether RPN2
expression is a candidate target for chemotherapy in gastric
cancers, one of the most frequent human cancers worldwide. In
particular, the role of RPN2 in the efficacy of the clinically used
anticancer drugs 5-FU, docetaxel, doxorubicin, irinotecan,
cisplatin and oxaliplatin was examined.
Normally, cells possess several mechanisms that
protect the cell against a noxious environment. These mechanisms
ultimately underlie resistance to cancer chemotherapy. The
mechanisms that have been reported to contribute to anticancer drug
resistance include decreased drug uptake, increased drug efflux,
drug detoxification, induction of anti-apoptotic factors,
suppression of pro-apoptotic factors, enhanced DNA repair and
increased tolerance to DNA damage (14). Of these, decreases in the
intracellular accumulation of hydrophobic chemotherapeutics due to
members of the adenosine triphosphate-binding cassette (ABC)
transporter superfamily constitute a major mechanism of drug
resistance (15). P-gp is one of
the key molecules that cause multidrug resistance in cancer cells
(16). Overall, the strategy of
inhibiting drug efflux transporters, including P-gp, depends on the
hypothesis that cancer cells are more dependent on drug efflux or
overexpression of the transporter compared with normal cells. In
this context, numerous clinical trials of various inhibitors of
P-gp have been conducted in an attempt to reverse drug resistance.
However, a large majority of these inhibitors have yielded
non-significant results, and only a few have demonstrated evidence
of a clinical benefit (17,18). Studies of the malignant
transformation process have implicated P-gp expression in several
oncogene signaling pathways and epigenetic mechanisms (17). It has also been demonstrated that
over-activating the P-gp transporter through post-transcriptional
modification contributes to increased drug efflux. On the basis of
previous studies and the data obtained in the present study, it is
indicated that knockdown of RPN2 alone may only result in a limited
effect on anticancer drug-induced cell death. This limited efficacy
reflects that modulation of P-gp function through N-glycosylation
is only one of the numerous mechanisms resulting in drug
resistance. Other members of the ABC transporter family and non-ABC
mediated drug resistance may also contribute to drug resistance in
gastric cancers (18).
Tumor progression is driven by a sequence of
randomly occurring mutations and epigenetic alterations of DNA that
affect the genes controlling cell proliferation and survival, as
well as other traits associated with the malignant cell phenotype.
Therefore, tumor cells exhibit heterogeneity that is reflected
histologically and genetically. In addition, human cancers express
multiple redundant drug-resistance mechanisms. Drug resistance
acquired by cancer cells is the leading cause of chemotherapy
failure. The identification of promising biomarkers for determining
the diagnosis or predicting the responsiveness of tumors to
anticancer agents is a compelling and urgent objective, as these
biomarkers may improve the assessment of individual treatment
requirements and aid in the development of molecular-targeted
therapies. Provided that the tumor formation process exhibits
features that are specific for distinct organs, future studies may
aim to identify specific molecular targets responsible for gastric
cancers.
To conclude, the commonly used anticancer drugs
examined in the present study effectively decreased cell survival
rates in all the tested cell lines in a concentration-dependent
manner, although the levels of RPN2 in the various cell lines were
not generally correlated with responses to clinical anticancer
drugs, calculated as the IC50. siRNA-mediated RPN2
downregulation increased the sensitivity of the AGS cells to
anticancer drug-induced apoptotic cell death. However, the overall
decrease in survival produced by RPN2 silencing was modest and
varied between the cell lines. In the AGS cells, siRNA-mediated
RPN2 knockdown significantly decreased the survival rate compared
with the control siRNA for all the tested drugs, whereas RPN2
silencing did not alter the response to cisplatin in MKN-45 cells.
Taken together, these data indicate that RPN2 expression may not be
a viable, stand-alone target for gastric cancer therapy.
Acknowledgements
This study was supported the Research Project of the
Department of Health (grant number, 10006), Taiwan.
Abbreviations:
|
RPN2
|
ribophorin II
|
|
MDR1
|
multidrug resistance 1
|
|
P-gp
|
P-glycoprotein 1
|
References
|
1
|
Davis MP, Dewald GW, Pierre RV and
Hoagland HC: Hematologic manifestations associated with deletions
of the long arm of chromosome 20. Cancer Genet Cytogenet. 12:63–71.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Löffler C, Rao VV and Hansmann I: Mapping
of the ribophorin II (RPN II) gene to human chromosome 20q12-q13.1
by in-situ hybridization. Hum Genet. 87:221–222. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roulston D, Espinosa R III, Stoffel M,
Bell GI and Le Beau MM: Molecular genetics of myeloid leukemia:
identification of the commonly deleted segment of chromosome 20.
Blood. 82:3424–3429. 1993.PubMed/NCBI
|
|
4
|
Testa JR, Kinnealey A, Rowley JD, Golde DW
and Potter D: Deletion of the long arm of chromosome 20
[del(20)(q11)] in myeloid disorders. Blood. 52:868–877.
1978.PubMed/NCBI
|
|
5
|
Crimaudo C, Hortsch M, Gausepohl H and
Meyer DI: Human ribophorins I and II: the primary structure and
membrane topology of two highly conserved rough endoplasmic
reticulum-specific glycoproteins. EMBO J. 6:75–82. 1987.PubMed/NCBI
|
|
6
|
Hortsch M, Avossa D and Meyer DI:
Characterization of secretory protein translocation:
ribosome-membrane interaction in endoplasmic reticulum. J Cell
Biol. 103:241–253. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelleher DJ, Kreibich G and Gilmore R:
Oligosaccharyltransferase activity is associated with a protein
complex composed of ribophorins I and II and a 48 kd protein. Cell.
69:55–65. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelleher DJ and Gilmore R: An evolving
view of the eukaryotic oligosaccharyltransferase. Glycobiology.
16:47R–62R. 2006. View Article : Google Scholar
|
|
9
|
Kaushal M, Mishra AK, Sharma J, Zomawia E,
Kataki A, Kapur S and Saxena S: Genomic alterations in breast
cancer patients in betel quid and non betel quid chewers. PLoS One.
7:e437892012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, He J, Liu Y, Simeone DM and Lubman
DM: Identification of glycoprotein markers for pancreatic cancer
CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue
microarray. J Proteome Res. 11:2272–2281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honma K, Iwao-Koizumi K, Takeshita F,
Yamamoto Y, Yoshida T, Nishio K, Nagahara S, Kato K and Ochiya T:
RPN2 gene confers docetaxel resistance in breast cancer. Nat Med.
14:939–948. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Souza R, Zahedi P, Badame RM, Allen C
and Piquette-Miller M: Chemotherapy dosing schedule influences drug
resistance development in ovarian cancer. Mol Cancer Ther.
10:1289–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Nagai Y, Ishimoto T, Baba Y, Mimori K and
Baba H: RPN2 expression predicts response to docetaxel in
oesophageal squamous cell carcinoma. Br J Cancer. 107:1233–1238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanley LA, Horsburgh BC, Ross J, Scheer N
and Wolf CR: Drug transporters: gatekeepers controlling access of
xenobiotics to the cellular interior. Drug Metab Rev. 41:27–65.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: the early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen KG and Sikic BI: Molecular pathways:
regulation and therapeutic implications of multidrug resistance.
Clin Cancer Res. 18:1863–1869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaffer BC, Gillet JP, Patel C, Baer MR,
Bates SE and Gottesman MM: Drug resistance: still a daunting
challenge to the successful treatment of AML. Drug Resist Updat.
15:62–69. 2012. View Article : Google Scholar : PubMed/NCBI
|