Introduction
Lung cancer is divided into non-small cell lung
cancer (NSCLC) and SCLC according to its morphology. Accounting for
~80% of all lung cancers, NSCLC is clinically a heterogeneous
entity with major histological subtypes, including adenocarcinoma,
squamous cell carcinoma and large cell carcinoma (1). Slower rates of growth and spread
compared with SCLC are common features of all NSCLC subtypes. This
enables early eradication of the cancer by surgery, however, only a
small fraction of cases are currently diagnosed in clinical stages
I-IIb, where surgical removal is the preferred therapeutic option
(2). Therefore, there is a
requirement for more sensitive NSCLC biomarkers, which can predict
prognosis and guide the effective targeted therapy.
The oncofetal protein, insulin-like growth factor II
mRNA-binding protein 3 (IMP3), is a member of the IMP family that
has recently become a focus of attention, as it appears to be
significant in the migration and adhesion of cells in range of
malignant neoplasms (3). IMP3 is a
580-amino acid protein, with four K homology domains and two RNA
recognition motifs, and is encoded by a gene on chromosome 7p11.5
(4) that has been known in previous
studies as K homology domain-containing protein overexpressed in
cancer or L523S. L523S is a regulatory binding protein that is
believed to be involved in the facilitation of insulin-like growth
factor (IGF)-II production by stabilizing and trafficking it
intracellularly (5). IMP3 is
expressed in numerous cells of the developing fetus, but not in the
majority of adult cells, with the exception of the gonads. IMP3
overexpression has been observed in a number of malignant tumors,
including renal carcinomas (6),
malignant pancreatic lesions (7),
endometrial carcinomas (8), and
uterine cervical (9) and testicular
(10) cancer.
Our previous studies showed that that IMP3
expression is correlated with the prognosis of a variety of human
tumors, such as hepatocellular carcinoma and colorectal cancer
(11,12). Thus, IMP3 is expected to be a novel
molecular target for cancer therapy. However, the role of IMP3 in
prognostic evaluation and its association with survival in NSCLC is
unknown. As IMP3 is known to play a critical role in numerous
cancers, the present study analyzed its function in NSCLC.
Materials and methods
Clinical samples
Fresh samples from eight cases of NSCLC were paired
with adjacent non-cancerous tissues, and 186 NSCLC cases with
routinely processed and paraffin-embedded tissue samples, which met
the strict follow-up criteria, were selected at random from
patients undergoing surgery between 2004 and 2008 at the Dandong
Centre Hospital (Dandong, Liaoning, China). Pathological
parameters, including age, gender, smoking status, tumor size,
pathological stage, differentiation, subtype, CEA level, metastasis
status, and disease-free and overall survival data, were carefully
reviewed. The ages of the patients ranged between 32 and 79 years,
with a mean age of 62.4 years. The male to female ratio was 112:74.
Tumors were staged according to the 6th edition of the American
Joint Committee on Cancer (13). Of
the 186 NSCLC samples, 97 were determined to be early stage (I-II)
and 89 were late stage (III-IV). A total of 43 samples were
well-differentiated cancer, 87 were moderately-differentiated
cancer and 56 were poorly-differentiated cancer. No patients
received chemotherapy or radiotherapy prior to surgery. By March
2013, 66 patients had succumbed and 120 patients remained alive.
The median survival time was 69 months. This study was approved by
the Ethics Committee of Eastern Liaoning University (Dandong,
China) and written informed consent was obtained from all
patients.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA (2 μl) from fresh tissues was extracted
using TRIzol reagent (Takara Biotechnology, Dalian, China).
First-strand cDNA was synthesized using PrimeScript reverse
transcriptase (Takara Biotechnology) and oligo(dT) following the
manufacturer’s instructions. All PCR reactions were performed in a
20 μl reaction mixture (10× PCR Buffer II 2 μl, dNTP mixture (2.5
mM each) 1.6 μl, primer-forward 1 μl, primer-reverse 1 μl, Takara
Ex Taq HS (5 U/μl) 0.15 μl, cDNA 1 μl and RNAse Free <20 μl).
Firstly, cDNA was denatured for 4 min at 94°C. Next, PCR
amplification was performed for 23–36 cycles at 94°C for 15 sec and
53–58°C for 30 sec to anneal the primers, with a final extension
step at 72°C for 1 min. Aliquots (10 μl) of the PCR reaction
mixture were removed after 6, 9, 12 and 15 cycles and separated by
electrophoresis on 3% agarose gels. Images were captured using the
Champchemi Professional image analysis system (Sagecreation,
Beijing, China), and quantitation was performed using LANE 1D
software (Sagecreation). To examine expression, qPCR was performed
with a Bio-Rad sequence detection system (Hercules, CA, USA),
according to the manufacturer’s instructions, using a
double-stranded DNA-specific SYBR Premix Ex Taq™ II kit (Takara
Biotechnology). Double-stranded DNA-specific expression was tested
by the comparative Ct method using 2-ΔΔCt. The primers were as
follows: IMP3 forward, 5′-CCTTTGCTGCTGGCAGAGTT-3′ and reverse,
5′-AACAAAGGGAAGTGCAGAGC-3′; and GAPDH forward,
5′-GGTCTCCTCTGACTTCAACA-3′ and reverse, 5′-ATACCAGGAAATGAGCTTGA-3′.
All assays were performed in triplicate and repeated at least three
times.
Immunohistochemical analysis
For immunohistochemical study using the DAKO Labeled
Streptavidin Biotin kit (DAKO, Glostrup, Denmark), 4-μm thick
tissue sections were deparaffinized, rehydrated and incubated with
3% H2O2 in methanol for 15 min at room
temperature to eliminate endogenous peroxidase activity. The
antigen was retrieved at 95°C for 20 min by placing the slides in
0.01 M sodium citrate buffer (pH 6.0). The slides were then
incubated with the polyclonal goat anti-IMP3 antiserum (1:150;
N-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and monoclonal
mouse anti-Ki-67 antiserum (MAB-0129; Maixin Technology Co., Ltd.,
Shenzen, Guangdong, China) primary antibodies at 4°C overnight.
Following incubation at room temperature for 30 min with
biotinylated secondary antibodies; rabbit anti-goat and goat
anti-mouse (dilution, 1:200; ZSGB Biotechnology Co., Ltd., Beijing,
China), the slides were incubated with streptavidin-peroxidase
complex at room temperature for 30 min. Immunostaining was
developed by using 3,3′-diaminobenzidine as a chromogen and then
counterstained with Mayer’s hematoxylin. Goat immunoglobulin G
isotope controls were used, which showed negative staining.
Additionally, the positive tissue sections were processed without
the primary antibody to create the negative controls.
All specimens were examined by two pathologists who
were blinded to the clinical data. In cases of discrepancy, a final
score was established when an agreement was reached following
reassessment of the samples under a double-headed microscope.
Briefly, immunostaining for IMP3 was semi-quantitatively scored as
follows: −, 0 to <5% positive cells; +, 5–50% positive cells;
and ++, >50% positive cells. Only the cytoplasmic expression
pattern was considered as positive staining. For the survival
analysis, IMP3 expression levels were denoted as either positive (+
and ++) or negative (−) expression.
Statistical analyses
Statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Correlations
between IMP3 expression and clinicopathological characteristics
were evaluated using the χ2 test and Fisher’s exact
test. The disease-free and overall survival rates following tumor
removal were calculated using the Kaplan-Meier method, and
differences in survival curves were analyzed using log-rank tests.
Multivariate survival analysis was performed on all significant
characteristics measured by univariate survival analysis with Cox’s
proportional hazard regression model. P<0.05 was considered to
indicate a statistically significant difference.
Results
IMP3 protein expression in NSCLC
samples
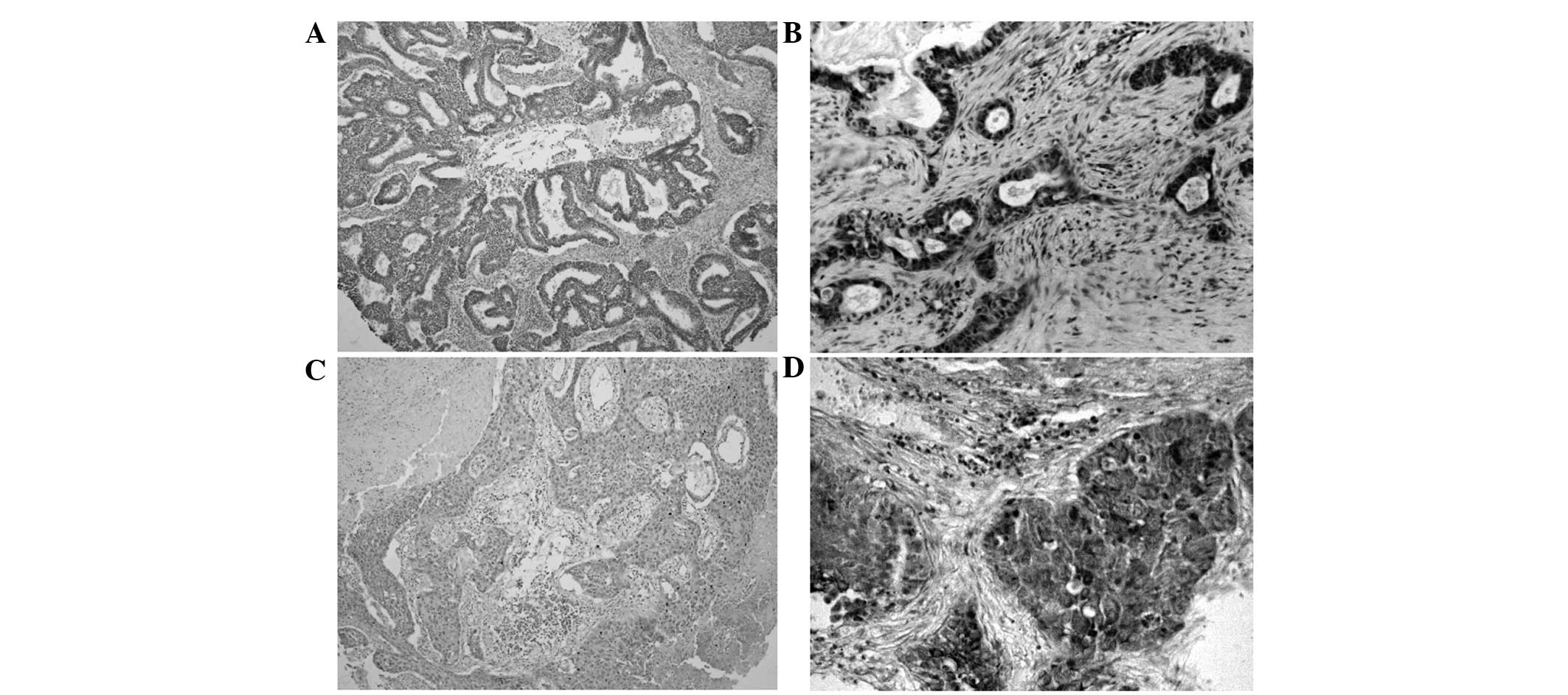
IMP3 protein expression exhibited a strict nuclear
staining pattern in the NSCLC tissues upon immunohistochemistry
analysis, with the exception of three cases of adenocarcinoma,
which showed a mainly cytoplasmic staining pattern. IMP3 protein
expression was negative in the normal lung tissues, but was usually
upregulated in the NSCLC tissues. The positive rate of IMP3 protein
was 74.7% (139/186) in the NSCLC tissues, and was significantly
higher than the 19.9% (37/186) found in the normal lung tissues
(P<0.01) (Fig. 1; Table I).
 | Table IInsulin-like growth factor II
mRNA-binding protein 3 protein expression in NSCLC. |
Table I
Insulin-like growth factor II
mRNA-binding protein 3 protein expression in NSCLC.
| | IMP3 protein
expression, n | | |
|---|
| |
| | |
|---|
| Diagnosis | No. of cases | − | + | ++ | Positive rate, % | P-value |
|---|
| NSCLC | 186 | 47 | 60 | 79 | 74.7 | 0.000a |
| Adjacent
non-tumor | 186 | 149 | 22 | 15 | 19.9 | |
The RT-qPCR data confirmed increased levels of IMP3
mRNA expression in the NSCLC samples compared with the adjacent
non-tumor tissues (Fig. 2).
Correlation between IMP3 expression and
the clinicopathological features of NSCLC
To evaluate the role of IMP3 protein in NSCLC
progression, the correlations between IMP3 protein expression and
the major clinicopathological features of NSCLC were analyzed. The
results showed that IMP3 expression was significantly associated
with tumor size, differentiation, lymph node metastasis and the
clinical stage of NSCLC (P=0.013, P<0.001, P=0.004 and
P<0.001, respectively). However, IMP3 expression levels were not
associated with age, gender, CEA level, smoking status or
pathological subtype of NSCLC (P>0.05) (Table II).
 | Table IICorrelation between IMP3 expression
and the clinicopathological features of non-small cell lung
cancer. |
Table II
Correlation between IMP3 expression
and the clinicopathological features of non-small cell lung
cancer.
| IMP3 protein
expression, n (%) | | |
|---|
|
| | |
|---|
| Variables | +/++ | − | χ2 | P-value |
|---|
| Age, years | | | 0.458 | 0.500 |
| <62 | 66 (72.5) | 25 (27.5) | | |
| ≥62 | 73 (76.8) | 22 (23.2) | | |
| Gender | | | 2.624 | 0.106 |
| Male | 79 (70.5) | 33 (29.5) | | |
| Female | 60 (81.1) | 14 (18.9) | | |
| Tumor size, cm | | | 6.214 | 0.013a |
| ≤3 | 51 (65.4) | 27 (34.6) | | |
| >3 | 88 (81.5) | 20 (18.5) | | |
| Differentiation | | | 19.170 | 0.000b |
| Well | 22 (51.2) | 21 (48.8) | | |
| Moderately | 67 (77.0) | 20 (23.0) | | |
| Poorly | 50 (89.3) | 6 (10.7) | | |
| Pathological
subtype | | | 1.018 | 0.314 |
| SCC | 68 (78.2) | 19 (21.8) | | |
| AC | 71 (71.7) | 28 (28.3) | | |
| Clinical stage | | | 20.761 | 0.000b |
| I–II | 59 (60.8) | 38 (39.2) | | |
| III–IV | 80 (89.9) | 9 (10.1) | | |
| LN metastasis | | | 8.331 | 0.004b |
| Positive | 84 (83.2) | 17 (16.8) | | |
| Negative | 55 (64.7) | 30 (35.3) | | |
| CEA level | | | 0.037 | 0.849 |
| Normal | 54 (74.0) | 19 (26.0) | | |
| Increased | 85 (75.2) | 28 (24.8) | | |
| Smoking status | | | 0.364 | 0.547 |
| Yes | 94 (73.4) | 34 (26.6) | | |
| No | 45 (77.6) | 13 (22.4) | | |
Correlation between survival rates and
IMP3 expression using the Kaplan-Meier method
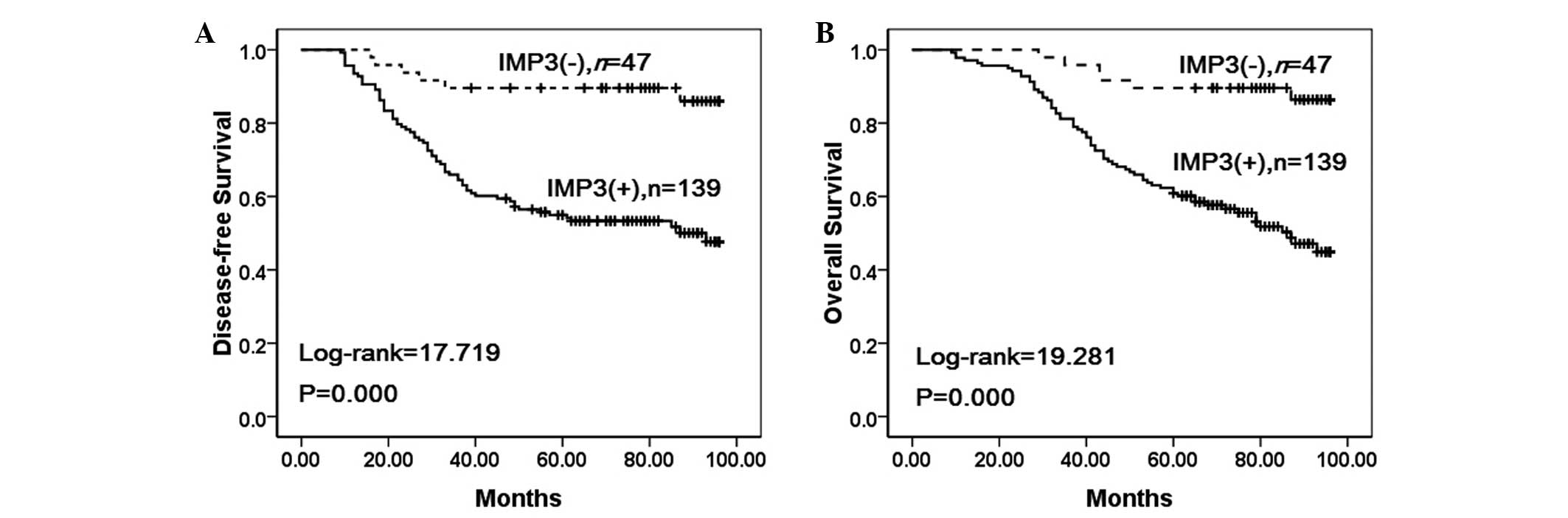
To further confirm the role of IMP3 expression in
NSCLC progression, the disease-free and overall survival rates of
186 patients with NSCLC were analyzed using the Kaplan-Meier
method. It was found that the NSCLC patients with IMP3 expression
exhibited lower disease-free (log-rank=17.719, P<0.001) and
overall (log-rank=19.281, P<0.001) survival rates compared with
those patients without IMP3 expression (Fig. 3).
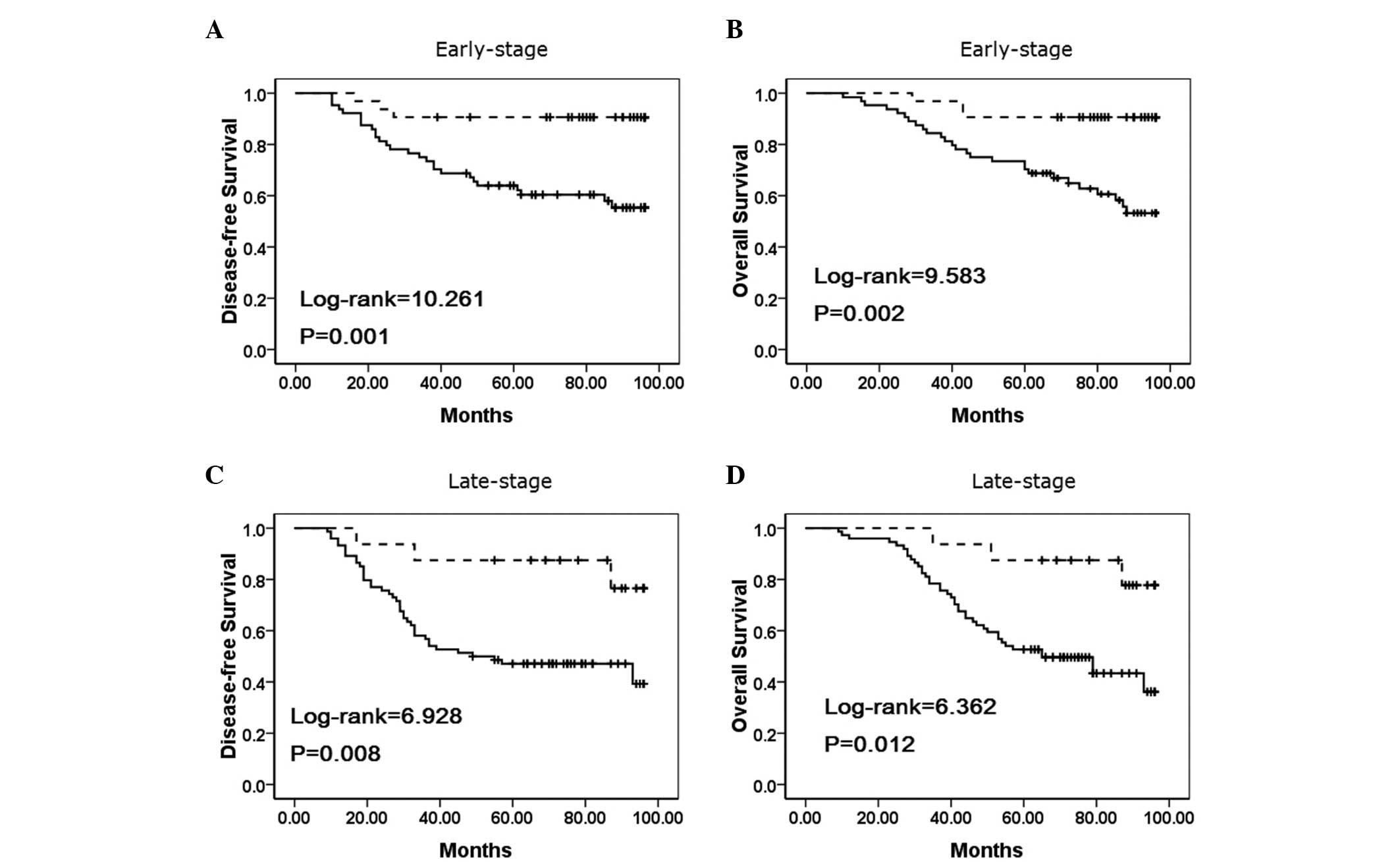
To substantiate the significance of IMP3 expression
in NSCLC progression, the correlations between IMP3 expression and
the clinical stage of NSCLC were analyzed. In early-stage NSCLC,
the patients with IMP3 expression exhibited lower disease-free and
overall survival rates compared with the patients without IMP3
expression (P=0.001 and P=0.002, respectively) (Fig. 4A–B). Additionally, the disease-free
and overall survival rates were also correlated with IMP3
expression status (P=0.008, and P=0.012, respectively) in
late-stage NSCLC (Fig. 4C–D).
IMP3 is an independent prognostic factor
in NSCLC, as determined by Cox’s proportional hazard regression
model
Univariate analysis showed that the patients with
NSCLC tumors that expressed IMP3 exhibited significantly lower
overall survival rates (P<0.001) compared with the patients with
NSCLC tumors that did not express IMP3. Additionally, patient age
(P=0.024), pathological stage (P<0.001), differentiation
(P=0.029), and lymph node metastasis (P=0.002) were all associated
with the overall survival rate. Therefore, multivariate survival
analysis was performed using Cox’s proportional hazards model for
all the significant variables found with the univariate survival
analysis. The results suggested that clinical stage [hazard ratio
(HR), 1.734; 95% confidence interval (CI), 1.279–2.351;
P<0.001), and lymph node metastasis (HR, 1.431; 95% CI,
1.054–1.944; P=0.022) were independent prognostic factors for
overall survival rates in NSCLC. Significantly, IMP3 expression
also emerged as a significant independent prognostic factor in the
prognosis of NSCLC (HR, 1.608; 95% CI, 1.134–2.281; P=0.008)
(Table III).
 | Table IIIUnivariate and multivariate survival
analysis of clinicopathological factors for the overall survival
rate of 186 patients with non-small cell lung cancer. |
Table III
Univariate and multivariate survival
analysis of clinicopathological factors for the overall survival
rate of 186 patients with non-small cell lung cancer.
| | | | | 95% CI | |
|---|
| | | | |
| |
|---|
|
Characteristics | β | SE | Wald | HR | Lower | Upper | P-value |
|---|
| Univariate |
| Gender | 0.100 | 0.148 | 0.459 | 1.105 | 0.827 | 1.477 | 0.498 |
| Age, years | 0.349 | 0.155 | 5.071 | 1.418 | 1.046 | 1.921 | 0.024a |
| Smoking
status | 0.103 | 0.177 | 0.340 | 1.109 | 0.784 | 1.569 | 0.560 |
| Tumor size,
cm | 0.242 | 0.149 | 2.634 | 1.274 | 0.951 | 1.708 | 0.105 |
| Clinical
stage | 0.588 | 0.151 | 15.198 | 1.801 | 1.340 | 2.420 | 0.000b |
|
Differentiation | 0.229 | 0.105 | 4.746 | 1.257 | 1.023 | 1.544 | 0.029a |
| CEA | 0.023 | 0.147 | 0.024 | 1.023 | 0.767 | 1.365 | 0.876 |
| Pathological
subtype | 0.050 | 0.147 | 0.116 | 1.051 | 0.788 | 1.404 | 0.734 |
| LN metastasis | 0.478 | 0.151 | 10.029 | 1.614 | 1.200 | 2.170 | 0.002b |
| IMP3 | 0.618 | 0.169 | 13.382 | 1.856 | 1.333 | 2.585 | 0.000b |
| Multivariate |
| Age, years | 0.283 | 0.157 | 3.251 | 1.328 | 0.976 | 1.806 | 0.071 |
| Clinical
stage | 0.551 | 0.155 | 12.583 | 1.734 | 1.279 | 2.351 | 0.000b |
|
Differentiation | 0.137 | 0.112 | 1.511 | 1.147 | 0.922 | 1.428 | 0.219 |
| LN metastasis | 0.359 | 0.156 | 5.277 | 1.431 | 1.054 | 1.944 | 0.022a |
| IMP3 | 0.475 | 0.178 | 7.096 | 1.608 | 1.134 | 2.281 | 0.008b |
Discussion
IMP3 is considered as the overexpressed K homology
protein in carcinoma and also the activator of IGF-II mRNA
translation. IGF-II, an embryonic growth factor, is structurally
homologous to proinsulin and has also been found to be one of the
endogenous genes. IGF-II plays a significant role in embryonic
development and cell growth, and can promote cell proliferation and
inhibit apoptosis (14). The
expression of IGF-II is affected by various factors and its
transcript contains six types of mRNA (15,16).
IMP3 is mainly combined with IGF-II leader 3 mRNA, which can
increase the expression of IFG-II by promoting the IFG-II leader 3
mRNA and exert carcinogenic effects through patterns decided by
IGF-II. IMP3 promotes the proliferation of tumor cells by
increasing the translation of IGF-II mRNA (14,17).
IMP3 has been shown to be essential to cell adhesion and spread
(14). The decreased expression of
IMP1 and IMP3 is associated with the downregulation of mRNA, which
encodes the extracellular matrix (ECM) and adhesive protein. The
study by Vikesaa et al agreed that IMP3 can regulate the ECM
and expression of particular adhesion proteins (such as ALCAM).
IMP3 can also stabilize cluster of differentiation 44 mRNA and
promote pseudopod structure formation in cancer cells, i.e., IMP3
acts like an oncogene (18).
The effect of IMP3 on tumors has become a focus of
attention. Recent studies have shown that IMP3 is associated with
the occurrence and development of several carcinomas. Yamamoto
et al suggested that IMP3 may be an supplementary tool for
the identification of aggressive abdominal mesenchymal tumors other
than gastrointestinal mesenchymal tumors (19). Lee et al (20) suggested an independent association
between IMP3 expression and disease recurrence, cancer-specific
mortality and all-cause mortality in upper urinary tract urothelial
carcinoma. This may aid in improving the risk stratification and
prognostication of upper urinary tract urothelial carcinoma
patients treated with radical nephroureterectomy (20). Beljan Perak et al (21) analyzed 105 patients with advanced
lung adenocarcinoma by indirect enzyme immunohistochemistry, and
found that IMP3 expression is associated with a solid subtype and
with distant metastases, regardless of the histological subtype of
the lung adenocarcinoma.
In our previous study, it was shown that IMP3
expression predicts a poor prognosis in patients with lung squamous
cell carcinoma (22). The present
study examined IMP3 expression and the clinicopathological features
of NSCLC, and found that IMP3 expression was significantly
correlated with a large tumor size, poor differentiation, positive
node status and advanced clinical stage, but not with age, gender,
pathological subtype, CEA level or smoking status of patients with
NSCLC. With regard to survival, it was found that NSCLC patients
with IMP3 expression exhibited lower disease-free and overall
survival rates compared with patients without IMP3 expression. In
either early- or late-stage NSCLC, patients with IMP3 expression
exhibited lower disease-free and overall survival rates compared
with those without IMP3 expression. Moreover, multivariate survival
analysis demonstrated that IMP3 expression emerged as a
significantly independent hazard factor for overall survival in
NSCLC, along with clinical stage and metastasis. In conclusion,
IMP3 plays an significant role in NSCLC progression and may be an
independent biomarker for evaluating prognosis in patients with
NSCLC.
Acknowledgements
This study was supported by the Natural Science
Foundation (20140082) and the Doctoral Research Foundation
(2014BZ0801) of Eastern Liaoning University.
References
|
1
|
Brambilla E, Travis WD, Colby TV, et al:
The new World Health Organization classification of lung tumours.
Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar
|
|
2
|
Lokk K, Vooder T, Kolde R, et al:
Methylation markers of early-stage non-small cell lung cancer. PLoS
One. 7:e398132012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikenberg K, Fritzsche FR, Zuerrer-Haerdi
U, et al: Insulin-like growth factor II mRNA binding protein 3
(IMP3) is overexpressed in prostate cancer and correlates with
higher Gleason scores. BMC Cancer. 10:3412010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mentrikoski MJ, Ma L, Pryor JG, et al:
Diagnostic utility of IMP3 in segregating metastatic melanoma from
benign nevi in lymph nodes. Mod Pathol. 22:1582–1587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffmann NE, Sheinin Y, Lohse CM, et al:
External validation of IMP3 expression as an independent prognostic
marker for metastatic progression and death for patients with clear
cell renal cell carcinoma. Cancer. 112:1471–1479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Z, Chu PG, Woda BA, et al:
Combination of quantitative IMP3 and tumor stage: a new system to
predict metastasis for patients with localized renal cell
carcinomas. Clin Cancer Res. 14:5579–5584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaeffer DF, Owen DR, Lim HJ, et al:
Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3)
overexpression in pancreatic ductal adenocarcinoma correlates with
poor survival. BMC Cancer. 10:592010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mhawech-Fauceglia P, Herrmann FR, Rai H,
et al: IMP3 distinguishes uterine serous carcinoma from endometrial
endometrioid adenocarcinoma. Am J Clin Pathol. 133:899–908. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu D, Yang X, Jiang NY, et al: IMP3, a new
biomarker to predict progression of cervical intraepithelial
neoplasia into invasive cancer. Am J Surg Pathol. 35:1638–1645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hammer NA, Hansen Tv, Byskov AG, et al:
Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and
testicular cancer. Reproduction. 130:203–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LT, Lin LJ and Zheng LL: The
correlation between insulin-like growth factor II mRNA binding
protein 3 expression in hepatocellular carcinoma and prognosis.
Hepatogastroenterology. 60:553–556. 2013.
|
|
12
|
Lin L, Zhang J, Wang Y, et al:
Insulin-like growth factor-II mRNA-binding protein 3 predicts a
poor prognosis for colorectal adenocarcinoma. Oncol Lett.
6:740–744. 2013.PubMed/NCBI
|
|
13
|
Singletary SE, Greene FL and Sobin LH:
Classification of isolated tumor cells: clarification of the 6th
edition of the American Joint Committee on Cancer Staging Manual.
Cancer. 98:2740–2741. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao B, Hu Y and Brewer G: RNA-binding
protein insulin-like growth factor mRNA-binding protein 3 (IMP-3)
promotes cell survival via insulin-like growth factor II signaling
after ionizing radiation. J Biol Chem. 286:31145–31152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gredes T, Spassov A, Mai R, et al: Changes
in insulin like growth factors, myostatin and vascular endothelial
growth factor in rat musculus latissimus dorsi by
poly-3-hydroxybutyrate implants. J Physiol Pharmacol. 60(Suppl 3):
77–81. 2009.
|
|
16
|
Ozdemir NO, Türk NS and Düzcan E: IMP3
expression in urothelial carcinomas of the urinary bladder. Turk
Patoloji Derg. 27:31–37. 2011.PubMed/NCBI
|
|
17
|
Liao B, Hu Y, Herrick DJ and Brewer G: The
RNA-binding protein IMP-3 is a translational activator of
insulin-like growth factor II leader-3 mRNA during proliferation of
human K562 leukemia cells. J Biol Chem. 280:18517–18524. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vikesaa J, Hansen TV, Jønson L, et al:
RNA-binding IMPs promote cell adhesion and invadopodia formation.
EMBO J. 25:1456–1468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto H, Arakaki K, Morimatsu K, et al:
Insulin-like growth factor II messenger RNA-binding protein 3
expression in gastrointestinal mesenchymal tumors. Hum Pathol.
45:481–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DJ, Xylinas E, Rieken M, et al:
Insulin-like growth factor messenger RNA-binding protein 3
expression helps prognostication in patients with upper tract
urothelial carcinoma. Eur Urol. 66:379–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beljan Perak R, Durdov MG, Capkun V, et
al: IMP3 can predict aggressive behaviour of lung adenocarcinoma.
Diagn Pathol. 7:1652012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin L, Zhang J, Wang Y, et al: Expression
of insulin-like growth factor 2 mRNA-binding protein 3 expression
and analysis of prognosis in the patients with lung squamous cell
carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 29:694–697.
2013.PubMed/NCBI
|