Introduction
Primary pulmonary lymphoma (PPL) is a rare disease
that reportedly accounts for 0.45% of all pulmonary malignant
tumors (1). The current definition
of PPL covers low-grade B-cell PPL (the most common), high-grade
B-cell PPL, and lymphomatoid granulomatosis (2). The incidence of PPL peaks in the sixth
and seventh decades, and the male: female ratio is ~1:1 (3). Certain cases are diagnosed in small
specimens, such as those obtained by bronchoscopic biopsy, and
bronchoalveolar lavage (BAL) is occasionaly of use (4). However, PPL is usually difficult to
diagnose in small specimens unless there are visible endobronchial
lesions (5) and BAL alone does not
contribute to the morphologic analysis, several studies have
recommended performing surgery to make the diagnosis, which led to
the treatment itself (1,6). Diagnostic radiological findings,
namely tumor spread without the destruction of the existing lung
structure, have been reported (2).
However, these features are not initially apparent in certain
cases. Therefore, patients who do not undergo surgery can be
misdiagnosed and consequently treated inappropriately. The current
study presents a case of PPL mimicking a refractory lung
abscess.
Case report
In April 2012, an 80-year-old male who had been
institutionalized five years prior to admission to the Kobe City
Medical Center General Hospital (Kobe, Japan) was referred due to a
persistent low-grade fever. Prior to this referral, the patient had
been treated as for right-sided pneumonia and pleuritis; however, a
right-sided pleural effusion and low-grade fever persisted. The
patient was admitted to the hospital with a diagnosis of a lung
abscess and empyema. The medical history showed curative surgery
had been performed for esophageal cancer 10 years previously and
that the patient was currently receiving treatment for diabetes
mellitus. The patient was a carrier of the hepatitis C virus and
was suffering from pneumoconiosis. No history of tuberculosis
pleuritis or an artificial pneumothorax procedure was evident.
Upon physical examination, diminished lower right
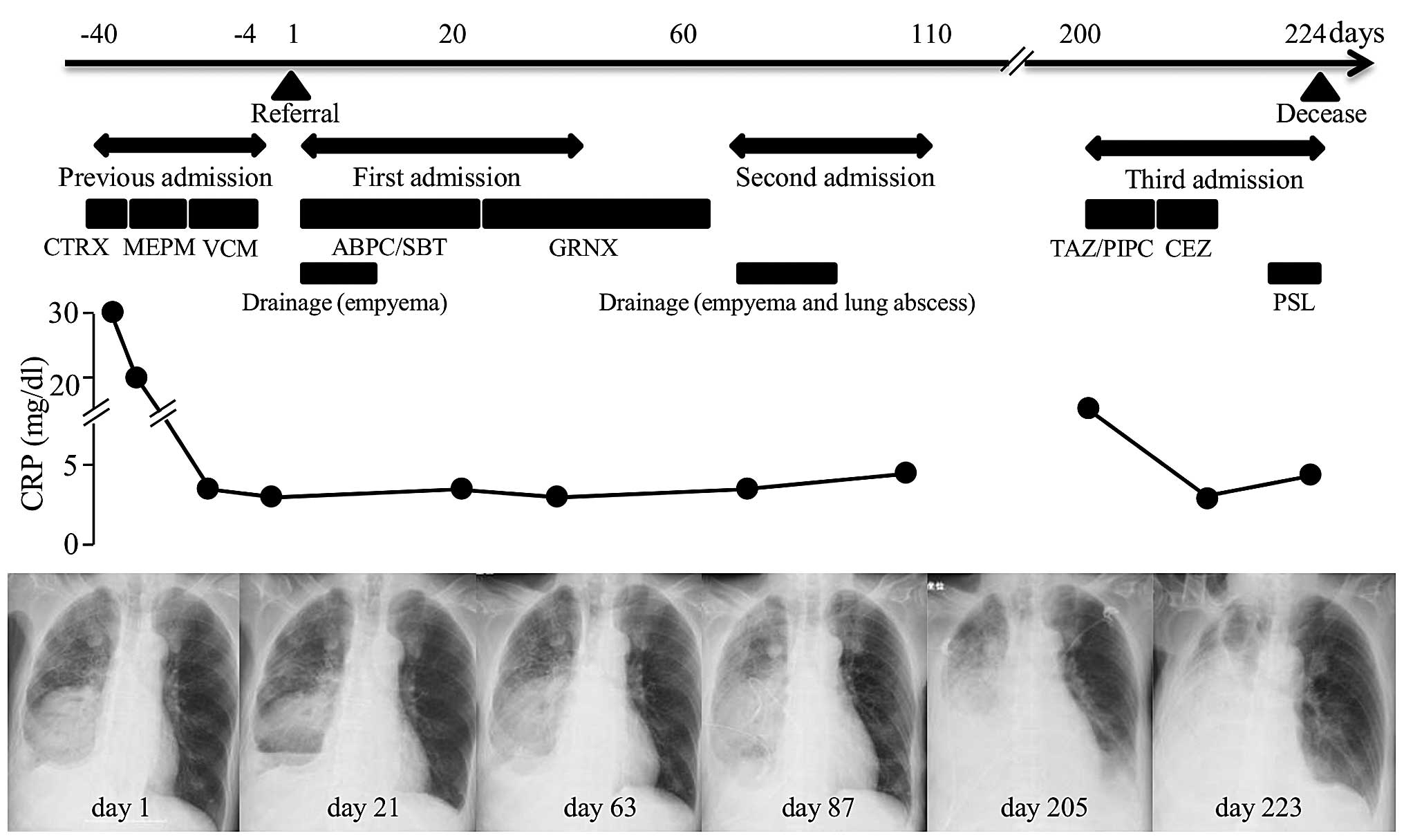
lung sounds were heard. A chest radiograph showed a large mass with
multiple air-fluid levels and pleural effusion in the right lower
field (Fig. 1A). Chest computed
tomography (CT) showed a large mass with a cavity and fluid in the
right lower lung lobe, and pleural effusion with multiple gas
bubbles (Fig. 1B and C). Laboratory
studies revealed a normal white blood cell count of
7,200/mm3, a marginal increase in neutrophils (75%;
normal range, 37–72%), an increased C-reactive protein level of 3.4
mg/dl (<0.3 mg/dl) and a normal lactate dehydrogenase level of
150 U/l. Aspirated pleural fluid appeared gray-white and sludgy,
with an increased lactate dehydrogenase level of 10,630 U/l (normal
range, <200 U/l) and a decreased glucose concentration of 16
mg/dl (normal range, >60 mg/dl). A cell count could not be
performed, as the cells had disintegrated.
With the diagnosis of a lung abscess and empyema,
antibiotics (1.5 g ampicillin/sulbactam every 6 h) were
administered for 21 days, and drainage and washing out of the
empyema through a 20-Fr drainage tube was performed. Cultures for
bacteria, including acid-fast bacteria, and the cytology of the
pleural effusion were negative. Three weeks later, a CT scan showed
that the lung abscess had become slightly enlarged, in spite of
improvement of the pleural effusion. Bronchoscopy and gastroscopy
revealed no specific lesions. Inapparent aspiration was
demonstrated by videofluorography, and was considered to have
caused the lung diseases and to have contributed to their
exacerbation. Surgery was considered to be contraindicated by due
to the poor general condition of the patient. An ultrasound-guided
centesis of the lung abscess proved difficult and no biopsy sample
was obtained. As the patient refused an intravenous infusion or
CT-guided centesis, the antibiotic regime was changed to 400 mg
oral garenoxacin (GRNX) daily. Subsequent to the patient being
discharged, the lung abscess remained unchanged in size during a
further two months of GRNX treatment. The patient then consented
once more to CT-guided drainage of the lung abscess. Again, the
cultures for bacteria, including acid-fast bacteria, and the
cytology were negative. Antibiotics were discontinued for a month,
during which time the lung abscess remained the same size; it was
therefore believed to be scar tissue. A transfer to another
hospital was completed, as the patient’s condition was no longer
acute.
Three month later, the patient was admitted to the
Kobe City Medical Center General Hospital due to respiratory
failure. A CT scan showed that the lung mass had increased in size
and was infiltrating the blood vessels. A new solid lesion was also
identified in the spleen (Fig. 2A and
B). Accordingly, lymphoma was considered in the differential
diagnosis of the mass that had previously been diagnosed as a
refractory lung abscess. Due to its increased size, the mass could
now be biopsied with ultrasound guidance. The biopsy revealed
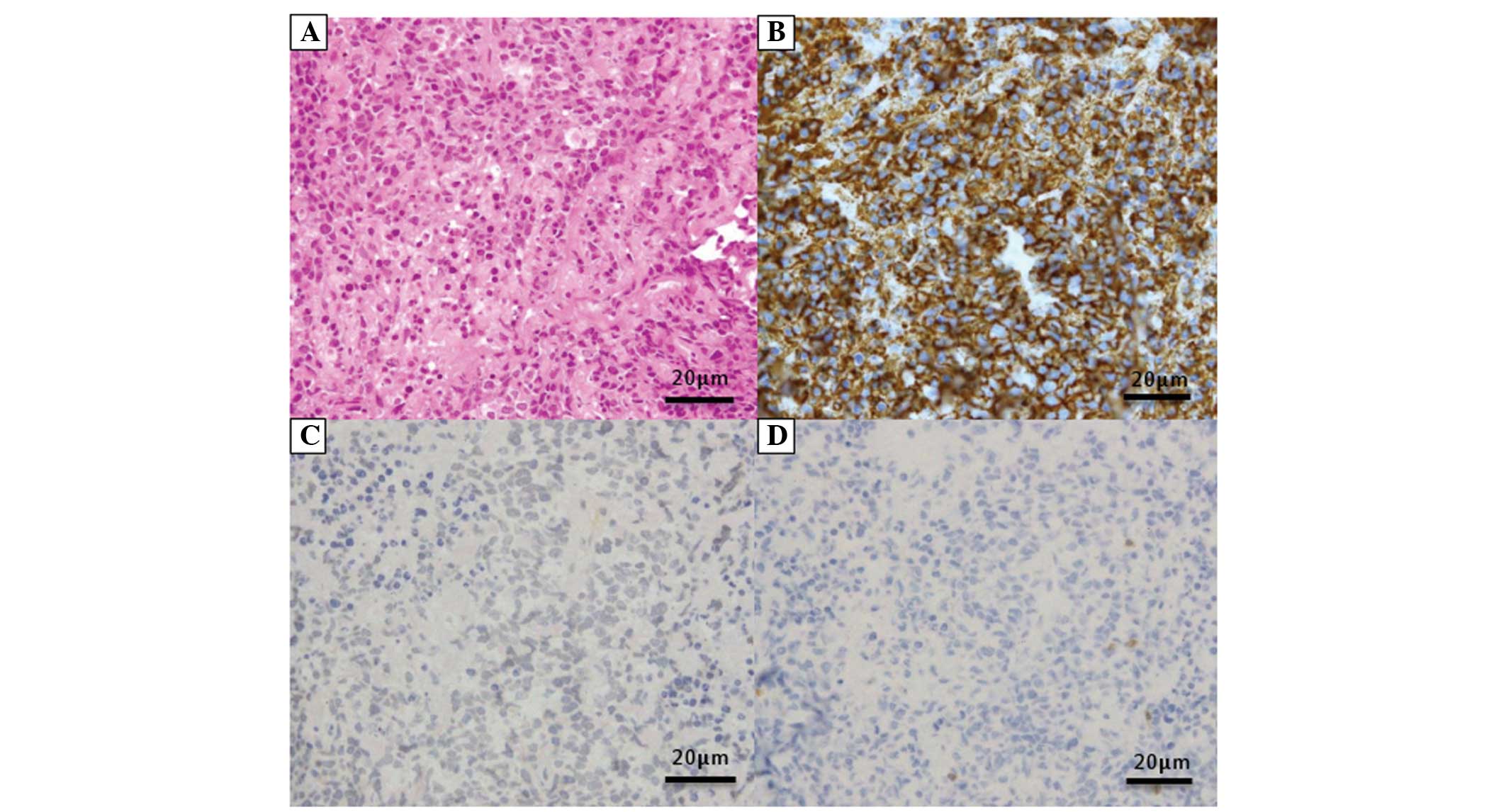
atypical lymphocytes that were positive for cluster of
differentiation (CD)20 and IgH rearrangement, and negative for CD3
and Epstein-Barr virus-encoded early RNA in situ
hybridization, all of which resulted in a diagnosis of diffuse
large B-cell lymphoma (DLBCL), stage IIIB (Fig. 3A–D). However, the general condition
of the patient was too poor to tolerate aggressive treatment, and
so antibiotics for bacterial pneumonia (4.5 g
tazobactam/piperacillin, every 8 h for five days and 1 g cefazolin,
every 8 h for nine days) and palliative steroids (20 mg
prednisolone, daily for five days) were administered. The patient
subsequently succumbed to sudden respiratory failure (Fig. 4).
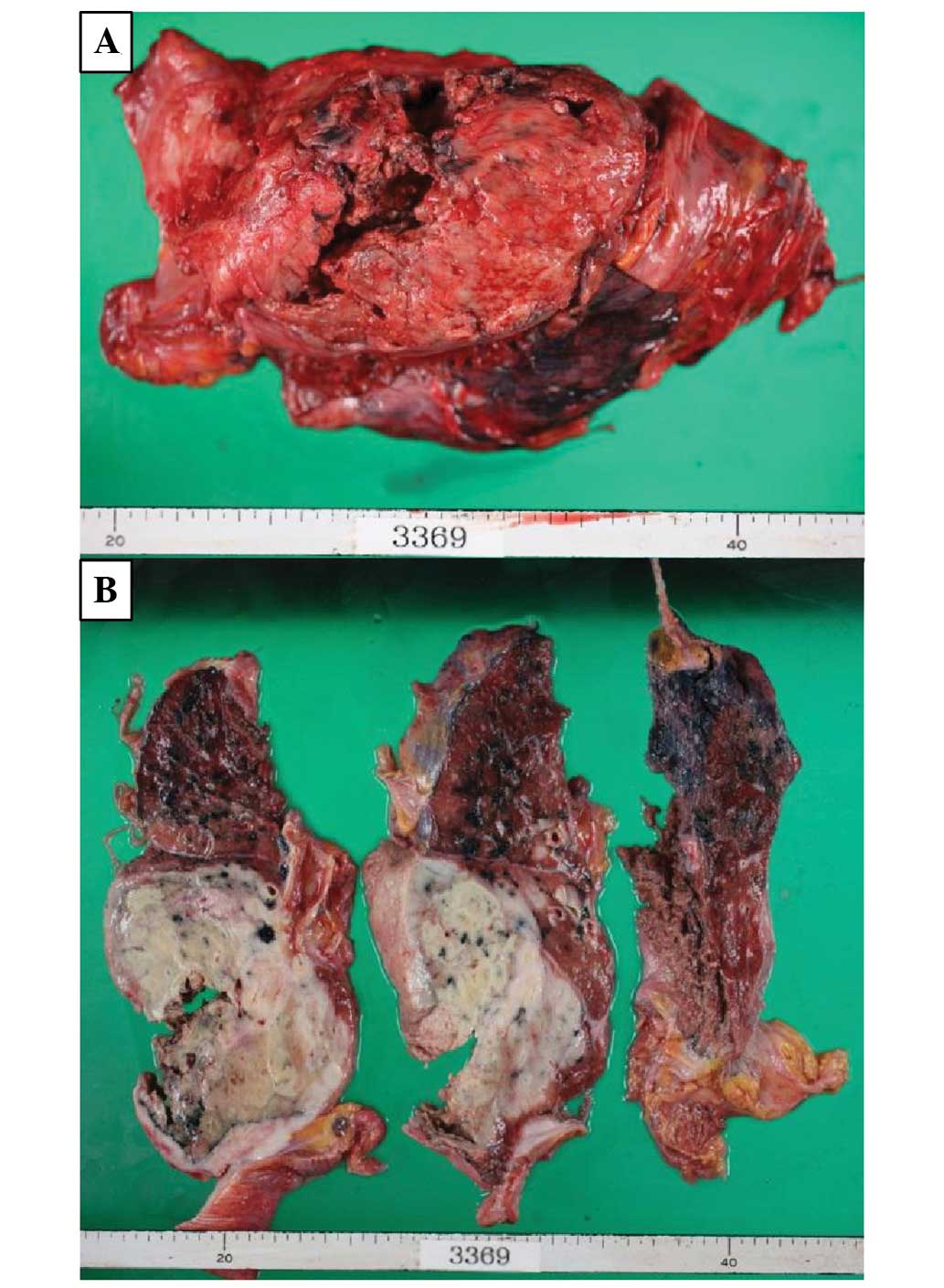
An autopsy revealed multiple lymphoma lesions
located in the right lung, esophagus, duodenum, ileum, mesenteru,
spleen and left anterior superior iliac spine (Fig. 5A and B). As the right lung lesion
was so large and the other lesions had not previously been
detected, the final diagnosis was of PPL.
Discussion
The present case of PPL, which is rare and difficult
to diagnose, and was therefore misdiagnosed as a lung abscess, is
reported in order to highlight the requirement for considering this
diagnosis in patients with refractory lung abscesses.
PPL is defined as clonal lymphoid proliferation that
affects one or both lungs (parenchyma and/or bronchi) in patients
with no detectable extrapulmonary involvement at the time of
diagnosis or during the subsequent three months (6). PPL is a rare tumor, comprising 0.45%
of pulmonary malignant tumors, <1% of all lymphomas and 3.6% of
extranodal lymphomas (1,6,7).
Mucosa-associated lymphoid tissue lymphoma is the most frequent
type of PPL, accounting for 58–87% of cases, whereas DLBCL
reportedly accounts for 5–20% of cases (2,7,9,10).
As PPL is difficult to diagnose, the majority of
diagnoses are made incidentally; 90% by surgical intervention and
only 10% by non-surgical procedures, such as transbronchial lung
biopsy or CT-guided percutaneous needle biopsy (5). Two cases of PPL that were difficult to
diagnose and were consequently treated as other diseases have
previously been reported (11,12).
In one case, a transbronchial lung biopsy and CT-guided
percutaneous needle biopsy failed to yield a diagnosis, and
treatment of the apparent lung abscess was ineffective. Surgery was
therefore performed, resulting in a diagnosis of PPL (11). In the other case, a transbronchial
biopsy suggested a diagnosis of granulomatosis with polyangiitis,
and sulfamethoxazole-trimethoprim therapy was temporarily
effective. However, subsequent to the appearance of new nodular
lesions, a final diagnosis of PPL was established by open biopsy
(12). In the present case, the
poor general condition of the patient precluded surgery, making the
diagnosis more difficult.
PPL has diverse radiological findings, and 70–79% of
patients reportedly present with multiple lesions (13). PPL spreads without destroying the
existing lung structure, air bronchograms often being found within
these tumors (2). There is
generally no evidence of tissue destruction and the formation of
cavities is rare. However, as in this case, formation of cavities
reportedly occurs most often in DLBCL and is associated with a poor
prognosis (14). In the present
patient, a large cavitated mass was found, with no air bronchogram
and with destruction of the existing lung structure. Thus, the
typical radiological findings of PPL were absent. By the third
admission, the lung mass had infiltrated the blood vessels,
resulting in the so-called CT-angiogram sign, which is diagnostic
of PPL (15). As illustrated by
this case, the differential diagnosis is extremely important,
particularly in patients with apparent lung abscesses (11,14).
The diagnosis of PPL of DLBCL type was finally made
on the third admission. As this type of PPL has a poor prognosis,
combination chemotherapy regimens are often administered following
surgical resection with curative intent (2). With regard to the present study, the
general condition of the patient was so poor upon first
presentation to the Kobe City Medical Center General Hospital that
aggressive therapy would not have been administered even if the
correct diagnosis had been made at that time. The patient was
initially diagnosed with a lung abscess and later with a refractory
lung abscess. Pyothorax-associated lymphoma also may not have been
ruled out upon first presentation. However, the patient did not
display a typical medical history, such as evidence of tuberculosis
pleuritis or an artificial pneumothorax (16). Also, a tissue sample could not be
obtained by biopsy and repeated examination of the cytology
revealed no evidence of malignancy. As there was videofluorography
evidence of repeated aspiration, it was understandable that
worsening aspiration pneumonia with formation of a lung abscess was
clinically diagnosed. However, this case is instructive, as an
earlier diagnosis may have been made if PPL had been considered in
the differential diagnosis.
In conclusion, it should be recognized that PPL can
mimic a lung abscess and that this diagnosis should consequently be
one of the differential diagnoses of a refractory lung abscess.
References
|
1
|
Papaioannou AN and Watson WL: Primary
lymphoma of the lung: an appraisal of its natural history and a
comparison with other localized lymphomas. J Thorac Cardiovasc
Surg. 49:373–387. 1965.PubMed/NCBI
|
|
2
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. Eur Resp J. 20:750–762. 2002.
View Article : Google Scholar
|
|
3
|
Li G, Hansmann ML, Zwingers T and Lennert
K: Primary lymphomas of the lung: morphological,
immunohistochemical and clinical features. Histopathology.
16:519–531. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drent M, Wagenaar SS, Mulder PH, et al:
Bronchoalveolar lavage fluid profiles in sarcoidosis, tuberculosis,
and non-Hodgkin’s and Hodgkin’s disease. An evaluation of
differences. Chest. 105:514–519. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cordier JF, Chailleux E, Laugue D, et al:
Primary pulmonary lymphomas. A clinical study of 70 cases in
nonimmunocompromised patients. Chest. 103:201–208. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saltzstein SL: Pulmonary malignant
lymphomas and pseudolymphomas: Classification, therapy and
prognosis. Cancer. 16:928–955. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferraro P, Trastek VF, Adlakha H, et al:
Primary non-Hodgkin’s lymphoma of the lung. Ann Thorac Surg.
69:993–997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiche M, Caprons F, Berger F, et al:
Primary pulmonary non-Hodgkin’s lymphomas. Histopathol. 26:529–537.
1995. View Article : Google Scholar
|
|
10
|
L’Hoste RJ Jr, Filippa DA, Lieberman PH
and Bretsky S: Primary pulmonary lymphomas. A clinicopathologic
analysis of 36 cases. Cancer. 54:1397–1406. 1984. View Article : Google Scholar
|
|
11
|
Tao H, Nakata M, Saeki H, et al:
Unsuspected primary pulmonary malignant lymphoma. Jpn J Thorac
Cardiovasc Surg. 50:533–536. 2002. View Article : Google Scholar
|
|
12
|
Miyahara N, Eda R, Umemori Y, et al:
Pulmonary lymphoma of large B-cell type mimicking Wegener’s
granulomatosis. Intern Med. 40:786–790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radin AI: Primary pulmonary Hodgkin’s
disease. Cancer. 65:550–563. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Graham BB, Mathisen DJ, Mark EJ and
Takvorian RW: Primary pulmonary lymphoma. Ann Thorac Surg.
80:1248–1253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ooi GC, Chim CS, Lie AK and Tsang KW:
Computed tomography features of primary pulmonary non-Hodgkin’s
lymphoma. Clin Radiol. 54:438–443. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakatsuka S, Yao M, Hoshida Y, et al:
Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol.
20:4255–4260. 2002. View Article : Google Scholar : PubMed/NCBI
|