Introduction
Glucagonoma is an extremely rare neuroendocrine
tumour that accounts for 1% of neuroendocrine tumours and <5% of
all primary pancreatic malignancies (1,2).
Although glucagonoma may appear as a benign neoplasia, at least 50%
of glucagonomas cause metastatic disease when diagnosed (2). If the disorder is complicated with
systemic clinical manifestations, including necrolytic migratory
erythema, hyperglucagonaemia, diabetes mellitus, anaemia, weight
loss, glossitis, cheilitis, steatorrhoea, diarrhoea, venous
thrombosis and neuropsychiatric disturbances, it is referred to as
glucagonoma syndrome (3). At
present, the standard treatment for glucagonoma syndrome is
surgical resection (2). The early
and accurate diagnosis of this syndrome may lead to positive
treatment outcomes and an improved prognosis. However, few studies
have detailed the imaging features of glucagonoma (4). In particular, the magnetic resonance
imaging (MRI) features of the lesion have not yet been reported. A
male with glucagonoma syndrome was admitted to the First Affiliated
Hospital of Shandong University (Jinan, China). Imaging modalities,
including computed tomography (CT), MRI and
18F-fludeoxyglucose (18F-FDG) positron
emission tomography (PET)-CT were performed, and imaging findings
were characterised. The present study describes and discusses these
imaging features.
Case report
A 54-year-old male was admitted to the First
Affiliated Hospital of Shandong University (Jinan, China) due to
persistent and progressive skin eruptions and weight loss of ~20 kg
over the past two years. The medical history revealed that the
patient had previously been diagnosed with Behçet’s disease and
treated with corticosteroids during multiple hospital admissions.
However, there had been no significant improvement of the symptoms.
In addition, the patient had suffered with diabetes mellitus for
five years. The patient stated that the skin erythema had recently
become progressively worse. The family history was negative for
multiple endocrine neoplasia and diabetes mellitus. Upon physical
examination, the patient exhibited cyclical itchy skin lesions on
the face, back, groin and lower limbs. The centres of the lesions
were hypopigmented or slightly scaly. There was no palpable mass
evident in the abdomen.
Laboratory analysis revealed normocytic anaemia [red
blood cell count, 2.59×1012/l (normal range,
4.0–5.0×1012/l); haemoglobin level, 81 g/l (normal
range, 120–160 g/l); mean corpuscular volume, 86.1 fl (nromal
range, 80–100 fl); mean corpuscular haemoglobin level, 29.5 pg
(normal range, 27–33 pg); and mean corpuscular haemoglobin
concentration, 343 g/l (normal range, 320–360 g/l)],
hyperglucagonaemia (181.00 pg/ml; normal range, 50–50 pg/ml) and
hyperglycaemia (fasting blood glucose level, 165.6 mg/dl; normal
range, 70–100 mg/dl). In addition, the expression of the tumour
marker, carbohydrate antigen 19–9, was markedly increased (180.10
U/ml; normal range, 0–37 U/ml), whereas the α-fetoprotein and
carcinoembryonic antigen levels were within the normal ranges. The
exocrine function was also normal.
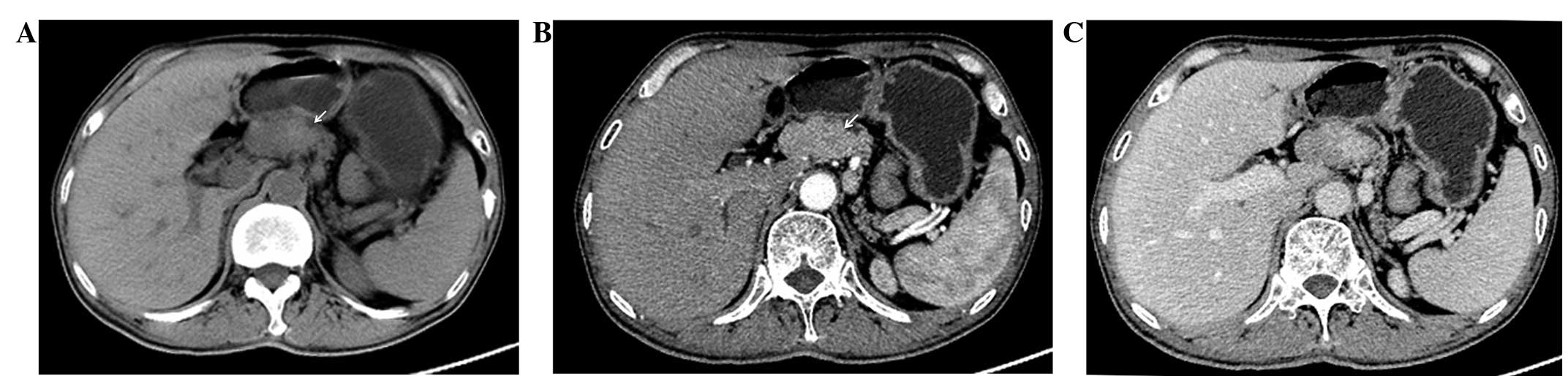
The plain abdominal CT scan identified an obscure
mass in the neck of the pancreas with a vague margin. Upon enhanced
CT, the lesion was slightly enhanced during the arterial phase and
washed out during the portal venous phase. The body and tail of the
pancreas were atrophied. There was no evidence of enlarged lymph
nodes or liver metastases (Fig. 1).
Upon MRI, the lesion exhibited a low signal intensity on
T1-weighted imaging (WI), and a slightly high signal intensity on
T2WI and half-Fourier acquisition single-shot turbo spin echo
sequence imaging, which measured ~4.5×3.0×3.0 cm in size. Upon
diffusion-WI (DWI), the lesion demonstrated heterogeneous
hyperintensity, which was mildly reinforced during the arterial
phase and washed out during the portal venous phase of
gadopentetate dimeglumine-enhanced imaging (Fig. 2). The 18F-FDG PET-CT
revealed mild 18F-FDG uptake by the lesion (standardised
uptake value, 3.8) in the neck of the pancreas, which corresponded
to the location of the tumour identified by the CT and MRI scans
(Fig. 3).
Based upon the clinical presentation and imaging
findings, the patient was diagnosed with glucagonoma syndrome. A
distal pancreatectomy and splenectomy were subsequently performed.
The regional lymph nodes were also dissected. The histopathological
examination revealed that the tumour was composed of uniform round
and polygonal cells, with pale cytoplasm and round nuclei. The
tumour cells exhibited nest- and belt-like arrangements. The
immunohistochemical staining identified positive reactions for
glucagon, synaptophysin and chromogranin A, a weakly positive
reaction for insulin (Fig. 4), and
negative reactions for gastrin and somatostatin. The 12 dissected
regional lymph nodes were not affected.
During the eight-month post-surgery follow-up
period, the skin lesions disappeared and the plasma glucagon levels
returned to normal.
The present study was approved by the Institute
Ethics Committee of the First Affiliated Hospital of Shandong
University, and written informed consent was obtained from the
patient for the publication of the study and any accompanying
images.
Discussion
Glucagonoma may appear as a benign or slow-growing
metastasising malignant tumour (5,6). The
first case of glucagonoma was described by Becker et al
(7) in 1942, in which the patient
suffered from pancreatic neoplasia complicated with skin erythema,
diabetes mellitus and anaemia.
In the present study, the CT scans identified an
obscure mass in the head and neck of the pancreas, but no
distinctive features. However, the MRI scans using T1WI or T2WI
identified morphological characteristics, including the contour and
internal structures of the lesion. DWI is extremely sensitive to
the motion of water protons at the microscopic level in response to
thermal energy (8). In contrast to
the low signal intensity of the normal pancreas, pancreatic tumours
may exhibit high signal intensities (8). Upon DWI, the present case demonstrated
certain features typical of a tumour.
Teixeira et al (5) reported that glucagonomas demonstrate
significant hypervascularity, and that selective celiac and
superior mesenteric arteriographies were the most reliable ways to
detect the primary neoplasm. Although selective arteriographies
were not performed in the present study, the lesion exhibited
slight enhancement upon enhanced CT and MRI, which was not a result
of hypervascularity. These findings are not consistent with the
previous literature, and therefore suggest that glucagonoma may
exhibit additional haemodynamic patterns.
PET-CT remains as a promising imaging technique for
detecting the presence of tumours. 18F-FDG is considered
to be a tracer of glucose metabolism, as its molecular structure is
similar to that of glucose. Subsequent to injection,
18F-FDG can be transported into the cell through the
glucose transporter proteins on the cell membrane. Consequently,
PET-CT identifies high 18F-FDG uptake in rapidly growing
tumours, in which the glycolysis rate is increased (9). In the present study, the tumour
exhibited mildly increased 18F-FDG uptake, and no
metastases were detected in any other organs.
Stacpoole (10)
stated that the following criteria should be fulfilled in order to
diagnose glucagonoma syndrome: i) Detection of a tumour by direct
visualisation or imaging examination; ii) evidence that the tumour
demonstrates a preponderance of glucagon-containing cells; iii) an
increase in the level of basal circulating immunoreactive glucagon;
and iv) the presence of a skin rash, glucose intolerance and
hypoaminoacidaemia, alone or in combination. In the present study,
the results of the microscopic examination were consistent with the
features of a neuroendocrine neoplasm, and the immunohistochemical
staining was positive for glucagon, synaptophysin and chromogranin
A. These findings confirmed the diagnosis of a glucagonoma.
Surgical resection is the optimal strategy for the
treatment of glucagonoma (11).
Depending on the location, size and pathological type of the
tumour, the surgical approach can be divided into local resection,
pancreatoduodenectomy, or pancreatic body and tail resection
(12). In the present study, the
skin lesions disappeared and the plasma glucagon levels returned to
normal shortly after the surgery.
In conclusion, glucagonoma syndrome exhibits certain
typical clinical manifestations. Imaging examinations are useful
for determining the location and size of a glucagonoma, and in
particular, MRI can identify distinctive morphological features.
Immunohistochemical analysis provides diagnostic evidence based
upon the neuroendocrine features. The present study summarized the
multimodality imaging features of glucagonoma, which are of great
importance for the differential diagnosis of pancreatic
tumours.
References
|
1
|
Bhosale PR, Menias CO, Balachandran A, et
al: Vascular pancreatic lesions: spectrum of imaging findings of
malignant masses and mimics with pathologic correlation. Abdom
Imaging. 38:802–817. 2013. View Article : Google Scholar
|
|
2
|
Hellman P, Andersson M, Rastad J, Juhlin
C, et al: Surgical strategy for large or malignant endocrine
pancreatic tumors. World J Surg. 24:1353–1360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wermers RA, Fatourechi V, Wynne AG, et al:
The glucagonoma syndrome. Clinical and pathologic features in 21
patients. Medicine (Baltimore). 75:53–63. 1996. View Article : Google Scholar
|
|
4
|
Castro PG, de León AM, Trancón JG, et al:
Glucagonoma syndrome: a case report. J Med Case Rep. 5:4022011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teixeira RC, Nico MM and Ghideti AC:
Necrolrolytic migratortory erythema associated with glucagonoma: a
report of 2 cases. Clinics (Sao Paulo). 63:267–270. 2008.
View Article : Google Scholar
|
|
6
|
McGavran MH, Unger RH, Recant L, et al: A
glucagon-secreting alpha-cell carcinoma of the pancreas. N Engl J
Med. 274:1408–1413. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker SW, Kahn D and Rothman S: Cutaneous
manifestations of internal malignant tumors. Arch Dermatol
Syphilol. 45:1069–1080. 1942. View Article : Google Scholar
|
|
8
|
Nissan N, Golan T, Furman-Haran E, et al:
Diffusion tensor magnetic resonance imaging of the pancreas. PLoS
One. 9:e1157832014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naswa N, Sharma P, Kumar A, et al:
Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic
neuroendocrine tumors: a prospective single-centre study. AJR Am J
Roentgenol. 197:1221–1228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stacpoole PW: The glucagonoma syndrome:
clinical features, diagnosis, and treatment. Endocr Rev. 2:347–361.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papavramidis T and Papavramidis S: Solid
pseudopapillary tumors of the pancreas: review of 718 patients
reported in English literature. J Am Coll Surg. 200:965–972. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norton JA, Harris EJ, Chen Y, et al:
Pancreatic endocrine tumors with major vascular abutment,
involvement, or encasement and indication for resection. Arch Surg.
146:724–732. 2011. View Article : Google Scholar : PubMed/NCBI
|