Introduction
Sorafenib (Bayer Pharmaceuticals, West Haven, CT,
USA) is a multikinase inhibitor, which functions by blocking
tumor-cell proliferation and angiogenesis (1). In cases of advanced hepatocellular
carcinoma (HCC) where patients received sorafenib treatment, almost
a 3-month median survival benefit was reported, as compared with
patients receiving a placebo (2).
Common adverse side effects of sorafenib treatment
include diarrhea, weight loss, skin rash (including hand-foot skin
reactions), fatigue, and hypertension. Additionally, a number of
cases of sorafenib-induced interstitial pneumonia have also been
reported (3–5). Safety information for sorafenib
therapy in patients with HCC was presented in Japan in October
2012, and six cases of acute respiratory failure were reported
among 1,045 patients with HCC who had been treated with sorafenib
(6). The current study describes an
autopsy case of interstitial pneumonia that developed after the
long-term treatment of a patient with advanced HCC with sorafenib.
Written informed consent was obtained from the family of the
patient.
Case report
A 59-year-old male with hepatitis C virus-related,
multinodular HCC, exhibited progressive disease following eight
sessions of transarterial chemoembolization (TACE) and four
sessions of ablation therapy over the previous 15 years Kansai
Medical University Takii Hospital (Osaka, Japan) and was admitted
to the Department of Gastroenterology and Hepatology, Kansai
Medical University (Osaka, Japan). Radiological studies showed
growth of the tumor in the right lobe of the liver with several
intrahepatic metastases, and further metastases to the lung.
Although the patient had smoked until 25 years of age, no
respiratory symptoms prior to the administration of sorafenib were
observed. Additional medication at the time of commencing sorafenib
treatment included ursodeoxycholic acid, branched-chain amino
acid-containing pharmaceutical granular preparation, and tamsulosin
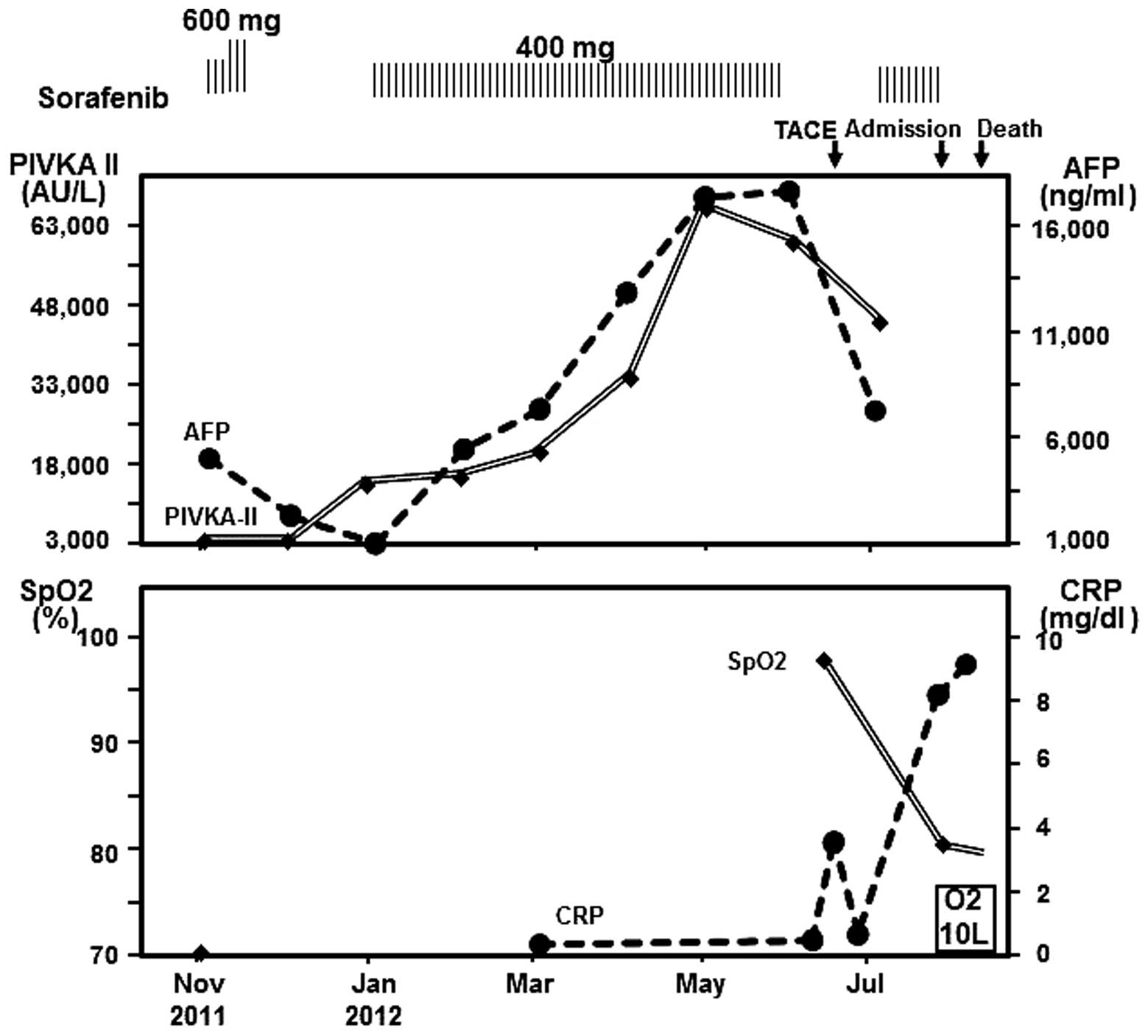
hydrochloride. Fig. 1 shows the
clinical course following the administration of sorafenib. Due to
the patient’s general state of health and Child-Pugh class B (score
7), palliative treatment with sorafenib (400 mg daily) was
initiated in November 2011. After one week, the dosage was
increased to 600 mg/day. Two weeks following initiation, the
administration of sorafenib was discontinued due to hand-foot-skin
reaction, and was resumed at a dose of 400 mg/day four weeks later.
After five months, sorafenib treatment was discontinued again due
to the patient being treated with TACE, and subsequently resumed at
400 mg/day after four weeks. At 19 days following the treatment
resumption, the patient developed progressive dyspnea and fever,
with worsening general weakness, and presented to the emergency
department of Kansai Medical University Takii Hospital with
dyspnea, cough and fever. Analysis of the vital signs showed a
normal blood pressure of 124/65 mmHg (normal range, 100–129/60–80
mmHg), respiratory rate of 20 breaths/min (normal range, 12–15
breaths/min), pulse of 120 beats/min (normal range, 60–85
beats/min), and body temperature of 37.5°C (normal range,
35.0–37.0°C). Respiratory crackles were audible in the bilateral
lower lung fields; the patient was anemic and icteric. The air
pulse oximetric saturation was 81% (normal limit, >92%);
arterial blood gas analysis showed a PaO2 of 62.5 mmHg
(normal range, 80–100 mmHg); PaCO2 of 28.5 mmHg (normal
range, 35–45mmHg) and pH 7.39 (normal range, 7.35–7.45), despite
oxygen supplementation. Laboratory studies showed marked
leukocytosis with a white blood cell count of 7,300 cells/μl
(normal range, 3,500–8,500 cells/μl), a neutrophil level of 6,607
cells/μl (normal range, 1,470–6,545 cells/μl) and an elevated
C-reactive protein level of 8.05 mg/dl (normal limit, <0.3
mg/dl); elevated aspartate transaminase concentration of 237 IU/l
(normal range, 13–35 IU/l), and alanine transaminase concentration
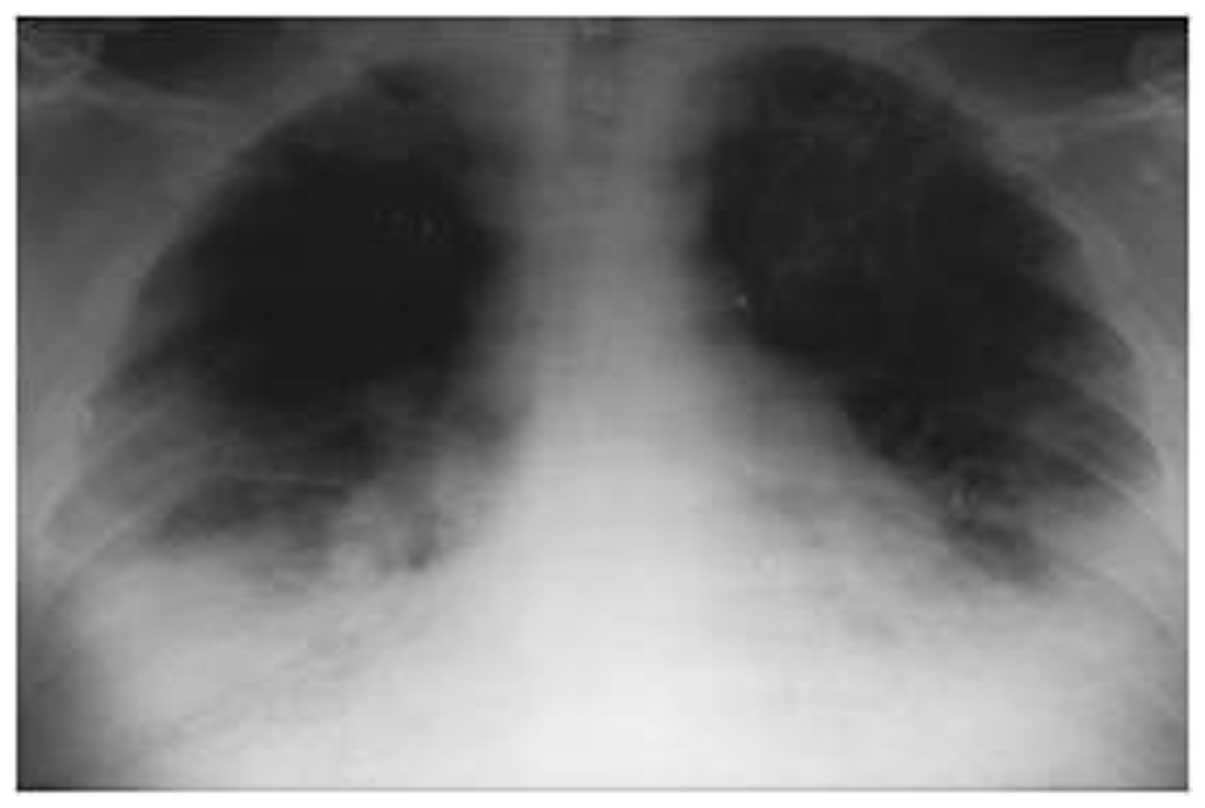
of 89 IU/l (normal range, 5–35 IU/l) (Table I). Chest X-ray radiography revealed
heart enlargement and bilateral pleural effusion, leading to a
diagnosis of acute heart failure (Fig.
2). Sorafenib treatment was discontinued on admission to Kansai
Medical University Takii Hospital as oral administration was
difficult. The patient developed rapidly worsening dyspnea and
hypoxia in spite of therapy with diuretic treatment and providing
oxygen, and the patient succumbed to the disease three days
following admission. The patient had declined mechanical
ventilation. The autopsy was conducted with the consent of the
family.
 | Table ILaboratory data on admission. |
Table I
Laboratory data on admission.
| Marker | Measurement | Range |
|---|
| Hematology |
| WBC | 7,300/μl | |
| Neutro | 90.5% | |
| Lympho | 4.5% | |
| Mono | 4.0% | |
| Eosino | 0.5% | |
| Baso | 0.5% | |
| RBC |
350×104/μl | ↓ |
| Hb | 8.6g/dl | ↓ |
| Ht | 28.1% | ↓ |
| Plt |
8.9×104/μl | ↓ |
| Coagulation |
| PT | 34% | ↓ |
| INR | 1.79 | |
| Biochemistry |
| AST | 237 U/l | ↑ |
| ALT | 89 U/l | ↑ |
| T-Bil | 2.6 mg/dl | ↑ |
| D-Bil | 1.5 mg/dl | ↑ |
| ALP | 428 U/l | ↑ |
| γ-GTP | 17 U/l | |
| LDH | 1,119 U/l | ↑ |
| TP | 6.6 g/dl | |
| Alb | 2.1 g/dl | ↓ |
| BUN | 25 mg/dl | ↑ |
| Creatine | 0.92 mg/dl | |
| CRP | 8.050 mg/dl | ↑ |
| NH3 | 48 μg/ml | |
| Tumor markers |
| AFP | 6,139.0 ng/ml | ↑ |
| AFP-L3 | 65.1% | ↑ |
| PIVKA-II | 27,800 AU/l | ↑ |
| Blood gas
analysis |
| pH | 7.394 | |
| pCO2 | 28.5 mgHg | ↓ |
| pO2 | 62.5 mgHg | ↓ |
|
HCO3− | 17.0 mEq/l | ↓ |
Autopsy findings
Gross findings of the autopsied liver revealed
cirrhosis, and multiple nodular lesions, with the largest measuring
4 cm in diameter, were homogeneously yellow-white to green.
Histologically, the lesions were composed of HCC and intrahepatic
cholangiocarcinoma (ICC) elements. A histological diagnosis of
intermixed HCC-ICC was determined. The HCC element revealed a
proliferating trabecular pattern with bile production,
corresponding to moderate differentiation as Edmondson’s grade II
(7). The ICC element was
well-differentiated, forming a well-developed gland. The largest
mass was widely necrotic and exhibited fibrotic changes, considered
to be the effect of sorafenib treatment and TACE; the remaining
masses were ICC elements. Metastasis to the lungs, hilar lymph
nodes, and mediastinal lymph nodes was observed. Furthermore,
necrosis was identified, partially due to sorafenib treatment, and
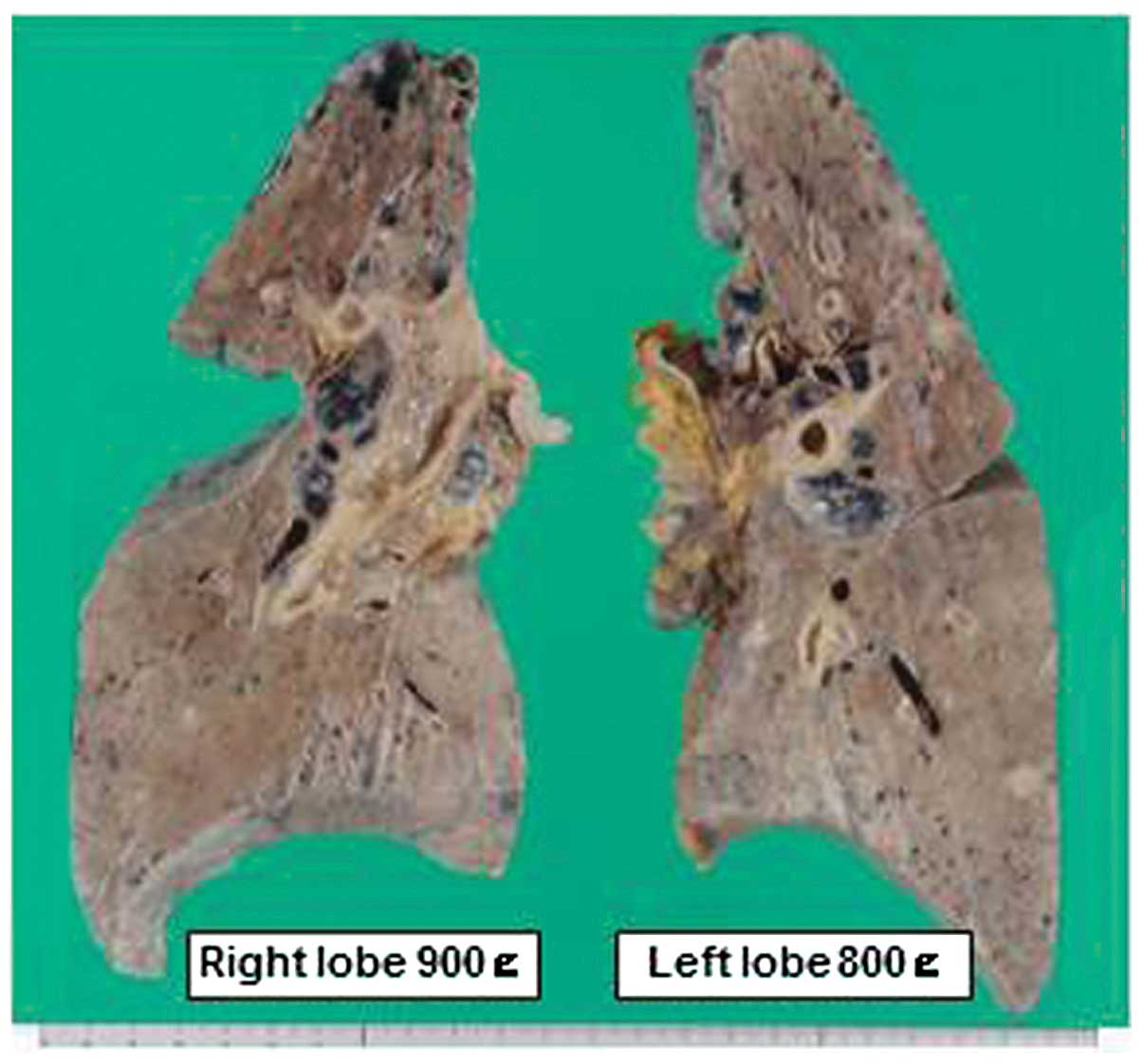
the residual regions showed ICC elements only. On autopsy, the
lungs were swollen, with a combined weight of 1,700 g, and
consolidated with a diffusely glistening spongy cut surface
(Fig. 3). Histologically, the
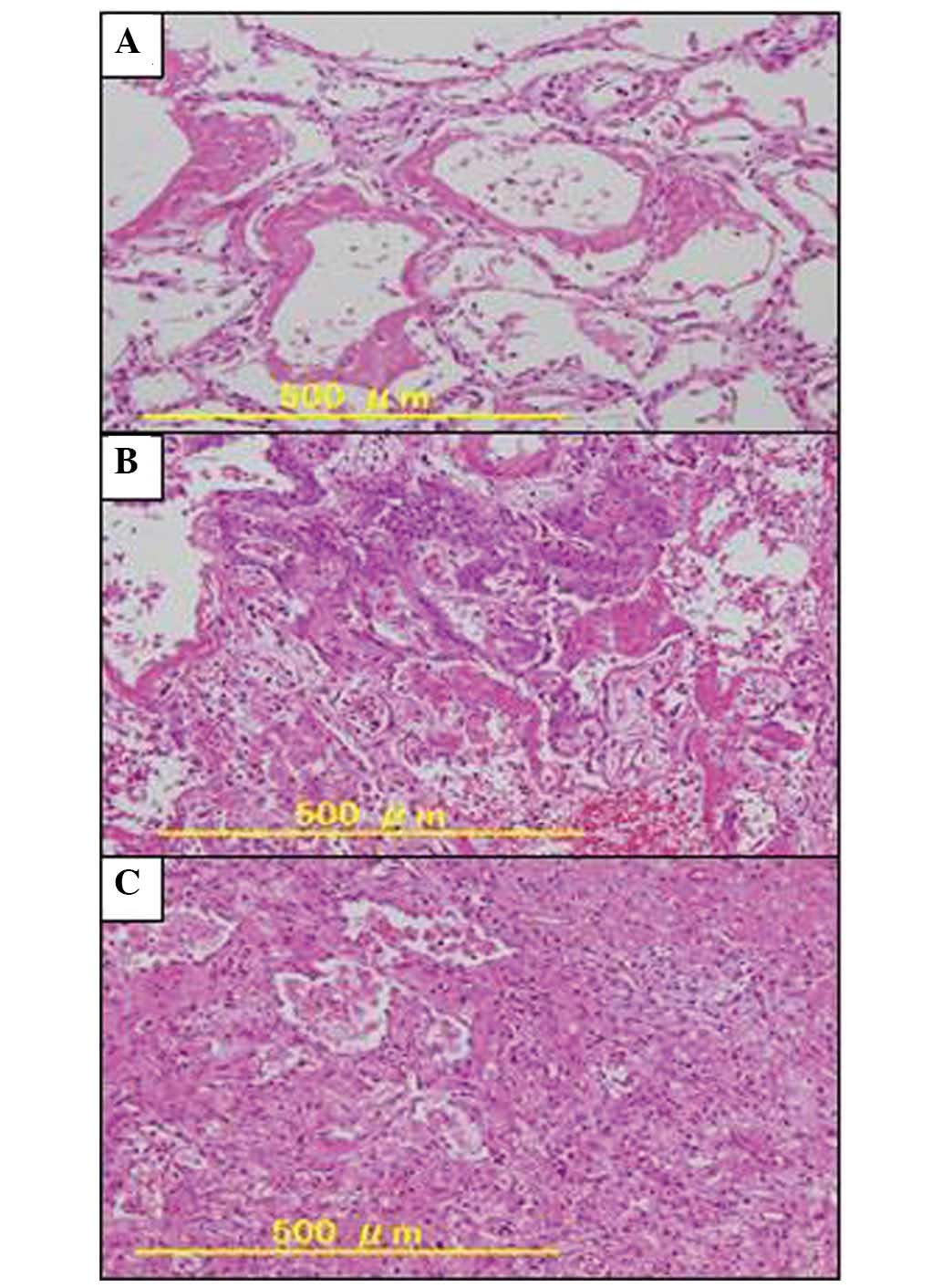
alveoli were obliterated by the hyaline membranes and organization
of exudates with proliferation of fibroblasts, indicating diffuse
alveolar damage (DAD). Notably, the bilateral lungs showed a
diffusely different phases of DAD, with the hyaline membrane
producing an early exudative phase (Fig. 4A), a proliferative phase (Fig. 4B), and late organizing fibrotic
phase (Fig. 4C). The mixed features
of various phases were proposed to correspond to drug-induced DAD.
Honeycombing was not observed. The findings did not indicate other
organisms such as bacteria, cytomegalovirus, Pneumocystis
jirovecii, and fungus. In addition, no vascular changes of
pulmonary hypertension with a plexiform lesion were identified,
however, a number of arterializations of small blood vessels were
revealed. No evidence of recent myocardial infarction or acute
cardiac decompensation was identified. Due to all of the results, a
clinical diagnosis of fatal interstitial pneumonia associated with
sorafenib treatment was determined.
Discussion
The current study presents an autopsy case involving
a patient with advanced HCC who developed rapidly progressive
interstitial lung disease following resumption of treatment with
sorafenib. In the absence of other etiologies, and due to the
autopsy findings, this patient was considered to have
sorafenib-induced interstitial pneumonia.
Histologically, the autopsied lungs revealed DAD,
which is the morphological precursor to acute interstitial
pneumonia and is characterized by a rapid and fatal clinical
course. DAD manifests clinically as acute respiratory distress
syndrome (ARDS) (8); it may be
observed in sepsis, shock, trauma, severe ARDS, and idiopathic
cases with undetected etiological factors as well as acute
exacerbations of chronic interstitial lung diseases. While diffuse
bilateral opacity is often observed on lung radiology, numerous
cases display deep hypoxemia that requires mechanical ventilation;
the mortality rate is 43–50% (9,10). A
number of drugs have been associated with lung injury with a DAD
pattern. The clinical features of lung toxicity are not specific
(dyspnea, cough, fever, pulmonary infiltrates), and the
differential diagnosis includes infection, relapse of the
underlying disease, pulmonary edema, and changes due to oxygen or
radiation (11). The pathological
findings of drug-related DAD are also nonspecific and the diagnosis
is one of exclusion (12). In the
present case, the clinical history and exclusion of other causative
factors indicate that sorafenib is the cause of lung injury.
Treatment with a number of molecular-targeted
agents, including gefitinib, erlotinib, imatinib, and bortezomib,
has been associated with pulmonary toxicity (13). However, the underlying mechanisms of
how these molecular-targeted agents induce interstitial pneumonia
remain unknown. The reduction of intrapulmonary vascular
endothelial growth factor (VEGF) levels in the early stages of lung
injury and normalization following recovery in ARDS have been
confirmed in numerous studies (14,15),
as VEGF acts as a growth and anti-apoptotic factor on alveolar
epithelial cells, in addition to its known effects on endothelial
cells (16). Therefore, the
pulmonary toxicity induced by sorafenib treatment may be associated
with its mechanism of antitumor activity, involving the inhibition
of the VEGF signaling pathway.
In previously reported cases, the initial symptoms
of pulmonary toxicity appeared after a limited treatment duration
with sorafenib (one to six weeks) (3–5). By
contrast, the present case showed delayed onset after eight months
of sorafenib treatment. The patient had been treated with sorafenib
for six months with no respiratory symptoms prior to resuming
sorafenib treatment. After 19 days of resuming the treatment,
however, the patient developed acute interstitial pneumonia. A
number of immune-related mechanisms in the interstitial pneumonia
may be induced by sorafenib.
In conclusion, severe respiratory failure with a
histological pattern of DAD may develop following resumption of
treatment with sorafenib. Therefore, physicians must be aware of
interstitial pneumonia as a potential pulmonary toxicity associated
with sorafenib treatment, when a patient resumes treatment with
sorafenib, even after prolonged use.
References
|
1
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43–9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ide S, Soda H, Hakariya T, et al:
Interstitial pneumonia probably associated with sorafenib
treatment: An alert of an adverse event. Lung Cancer. 67:248–250.
2010. View Article : Google Scholar
|
|
4
|
Myung HJ, Jeong SH, Kim JW, et al:
Sorafenib-induced interstitial pneumonitis in a patient with
hepatocellular carcinoma: a case report. Gut Liver. 4:543–546.
2010. View Article : Google Scholar
|
|
5
|
Takeda H, Nishikawa H, Iguchi E, et al:
Sorafenib-induced acute interstitial pneumonia in patients with
advanced hepatocellular carcinoma: report of three cases. Clin J
Gastroenterol. 5:407–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bayer Healthcare Japan. Safety information
for Nexabar™ 200mg tablets. June. 2012, (In Japanese). http://www.nexavar.jp/ja/home/usage-information/servey-record/hcc/.
Accessed March 10, 2014
|
|
7
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Myers JL and Katzenstein AL: Beyond a
consensus classification for idiopathic interstitial pneumonias:
progress and controversies. Histopathology. 54:90–103. 2009.
View Article : Google Scholar
|
|
9
|
Esteban A, Fernández-Segoviano P,
Frutos-Vivar F, et al: Comparison of clinical criteria for the
acute respiratory distress syndrome with autopsy findings. Ann
Intern Med. 141:440–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zambon M and Vincent JL: Mortality rates
for patients with acute lung injury/ARDS have decreased over time.
Chest. 133:1120–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barber NA and Ganti AK: Pulmonary
toxicities from targeted therapies: a review. Target Oncol.
6:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muller NL, White DA, Jiang H, et al:
Diagnosis and management of drug-associated interstitial lung
disease. Br J Cancer. 91(Suppl 2): S24–S30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwaiblmair M, Behr W, Haeckel T, et al:
Drug induced interstitial lung disease. Open Respir Med J. 6:63–74.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abadie Y, Bregeon F, Papazian L, et al:
Decreased VEGF concentration in lung tissue and vascular injury
during ARDS. Eur Respir J. 25:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vasakova M, Sterclova M, Kolesar L, et al:
Bronchoalveolar lavage fluid cellular characteristics, functional
parameters and cytokine and chemokine levels in interstitial lung
diseases. Scand J Immunol. 69:268–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roberts JR, Perkins GD, Fujisawa T, et al:
Vascular endothelial growth factor promotes physical wound repair
and is anti-apoptotic in primary distal lung epithelial and A549
cells. Crit Care Med. 35:2164–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|