Introduction
Lung cancer is the most common type of cancer and
the leading cause of cancer-associated mortality in the world,
becoming a major public health problem globally. Lung cancer
accounts for 13% (1.6 million) of the total number of cancer cases
and 18% (1.4 million) of mortalities in 2008 (1). In recent years, the incidence of lung
cancer in Asia has increased rapidly; however, the etiological
factors of the disease remain unclear. Lung cancer is known to be a
complex disease; however, tobacco smoking, family history, diet and
susceptible gene mutations appear to be involved in its development
(1,2).
Glutathione S-transferases (GSTs) are phase II
enzymes that are key in the detoxification of numerous carcinogens
(3). GSTs mediate the conjugation
of electrophilic compounds to glutathione, resulting in
detoxification of specific environmental carcinogens and pesticides
and the inactivation of polycyclic aromatic hydrocarbons,
facilitating their excretion from the body (4,5). GSTs
are classified into at least four genetically distinct groups: α,
μ, π and θ. Theoretically, individuals lacking a specific GST
enzyme may be at a particularly high risk of developing cancer, if
exposed to certain genotoxicants (4,6). Among
the potential mutations, the most widely known are the deletions of
the GST θ1 (GSTT1) or GST μ1 (GSTM1)
genes (null variants), which result in no enzymatic functional
activity (7,8). Deficiency in GSTT1 isoenzyme
activity may predispose individuals to the effects of electrophilic
carcinogens. Previous studies have proposed that GSTT1
deficiencies may be associated with an increased susceptibility to
lung cancer (9–12); however, other studies contradict
this proposal (13–15). Therefore, numerous molecular
epidemiological studies have been performed to investigate the
potential association between the GSTT1 gene polymorphism
and lung cancer susceptibility, particularly in East Asian
populations (15–17); however, the conclusions are
inconsistent and occasionally contradictory. A previous study has
indicated that the frequency of the GSTT1 null genotype is
higher in Asia compared with other populations (18). Thus, the present study performed a
meta-analysis, including 26 eligible case-control or prospective
studies (15–17,19–41),
aiming to investigate the effect of the GSTT1 polymorphism
on the risk of lung cancer in the East Asian populations.
Materials and methods
Search strategy
Eligible studies were identified by performing a
literature search on the PubMed and China National Knowledge
Infrastructure (CNKI) databases (from inception to March 20, 2014)
using the following keywords: ‘Glutathione S-transferase T1’,
‘GSTT1’, ‘polymorphism’, ‘lung cancer’ and the combined phrases. In
addition, the references of all the relevant articles and reviews
were searched for additional eligible studies. Furthermore, the
full text of each potentially relevant paper was scrutinized to
ensure that the following inclusion criteria were met: i) The
studies had an observational (case-control or prospective) study
design; ii) the authors offered sufficient data for estimating odds
ratios (ORs) and their 95% confidence intervals (CIs); and, iii)
the patients and controls of all the studies were East Asian
(including Chinese, Japanese, South Korean, Mongolian and North
Korean). In cases where multiple studies reporting on the same
population data met the inclusion criteria, the study with the
largest sample size was selected for subsequent analysis.
Data extraction
In order to minimize bias, the following data were
extracted from all the eligible studies by two researchers
independently: First author’s surname, year of publication,
country, source of the controls (population- or hospital-based
studies), histological type of cancer (adenocarcinoma, squamous
cell carcinoma or small-cell carcinoma), smoking status and the
different genotypes in the cases and controls. In accordance with
the definition used in the majority of previous studies, carriers
with at least one GSTT1 allele were defined as the ‘present’
genotype group, whereas individuals carrying no GSTT1
alleles were classified as the ‘null’ genotype group.
Quality assessment
The quality of the studies included in the present
analysis were assessed using an adapted 10-point Newcastle-Ottawa
assessment scale (NOS) (42). The
quality of each study was assessed by two independent reviewers
based on three broad factors: Selection (maximum score, 4),
comparability (maximum score, 2) and exposure (maximum score, 4).
Thus, a total quality score ranging between 0 (lowest score) and 10
(highest score) was obtained by adding all the scores. A total
score of seven or greater indicated that the study was of
high-quality.
Statistical analysis
The association between lung cancer risk and the
GSTT1 polymorphism was estimated for each study using crude
ORs with 95% CIs. The pooled ORs were evaluated for null vs.
present genotypes and a χ2-based Q-statistical test was
performed to assess the heterogeneity between the studies (43). P<0.05 was considered to indicate
a statistically significant heterogeneity. In the case of
significant heterogeneity, a random-effect model, as described by
DerSimonian and Laird (44), was
used to calculate pooled estimates. Otherwise, a fixed-effect model
was used, as described by the Mantel-Haenszel method (45). These two models provided similar
results when heterogeneity between the studies was absent. The
potential publication bias was evaluated using the funnel plot and
the linear regression asymmetry test, as previously described by
Egger et al (46). P<0.05
was considered to indicate a statistical significant difference.
All the statistical analyses were performed using the Statistical
Analysis System (version 9.1.3; SAS Institute, Cary, NC, USA) and
Review Manager software (version 5.2; The Cochrane Collaboration,
Oxford, UK), with two-sided P-values.
Results
Eligible studies
Fig. 1 indicates the
process of study selection and exclusion. A total of 103 abstracts
were identified in PubMed and CNKI using the aforementioned key
words. Following careful review of the titles and abstracts, 32
relevant studies describing the association between GSTT1
polymorphisms and lung cancer in the East Asian populations were
selected. However, after obtaining and reading the full articles,
four studies were excluded since they were review articles and two
studies were excluded since they presented no data of interest or
only raw data. Thus, a total of 26 eligible studies, including
7,415 lung cancer cases and 6,084 controls, were selected for
inclusion in the present meta-analysis. The major characteristics
of these studies are presented in Table
I. The sample size range was 107–5,632 samples, while 10
studies used hospital-based control sources, 16 studies were
hospital-based and two studies did not specify. In addition,
sub-analyses by histological type (adenocarcinoma, 7; squamous cell
carcinoma, 7; small-cell carcinoma, 3) and smoking state (smokers,
8; non-smokers, 8) were conducted. The methodological quality of
the studies included is presented in Table I and the Newcastle-Ottawa assessment
scale score range was 4–9, with a mean score of 6.7.
 | Table ICharacteristics of studies included
in the present meta-analysis. |
Table I
Characteristics of studies included
in the present meta-analysis.
| | | | Genotypic
distribution | | | | |
|---|
| | | |
| | | | |
|---|
| | | | Case | Control | Newcastle-Ottawa
scale |
|---|
| | | |
|
|
|
|---|
| Reference | Year | Country | Controls | Null | Present | Null | Present | Selectiona |
Comparabilityb | Assessmenta | Total |
|---|
| Piao et al
(17) | 2013 | Korean | HB | 2070 | 1863 | 858 | 841 | 3 | 2 | 2 | 7 |
| Wang et al
(27) | 2012 | China | PB | 90 | 119 | 100 | 156 | 3 | 2 | 2 | 7 |
| Liang et al
(41) | 2012 | China | HB | 45 | 23 | 34 | 36 | 2 | 0 | 2 | 4 |
| Kiyohara et
al (15) | 2012 | Japan | PB | 217 | 245 | 164 | 215 | 3 | 2 | 4 | 9 |
| Liu (34) | 2011 | China | HB | 57 | 43 | 56 | 79 | 3 | 0 | 3 | 6 |
| Tamaki et al
(26) | 2011 | Japan | PB | 97 | 95 | 104 | 99 | 3 | 1 | 3 | 7 |
| Fang (33) | 2011 | China | HB | 45 | 23 | 34 | 36 | 3 | 0 | 2 | 5 |
| Du (31) | 2011 | China | HB | 57 | 68 | 56 | 69 | 3 | 1 | 2 | 6 |
| Bai (29) | 2011 | China | HB | 56 | 72 | 96 | 118 | 3 | 1 | 2 | 6 |
| Fan et al
(39) | 2010 | China | HB | 38 | 20 | 29 | 31 | 3 | 2 | 2 | 7 |
| Qi et al
(37) | 2008 | China | HB | 17 | 36 | 27 | 45 | 3 | 2 | 2 | 7 |
| Yang et al
(25) | 2007 | Korea | HB | 168 | 148 | 166 | 179 | 3 | 2 | 2 | 7 |
| Li (30) | 2007 | China | HB | 17 | 25 | 48 | 55 | 3 | 1 | 2 | 6 |
| Lee et al
(23) | 2006 | Korea | HB | 80 | 89 | 107 | 89 | 2 | 2 | 2 | 6 |
| He and Tang
(35) | 2006 | China | PB | 33 | 28 | 29 | 17 | 4 | 0 | 3 | 7 |
| Chen et al
(20) | 2006 | China | PB | 59 | 38 | 85 | 112 | 3 | 0 | 2 | 5 |
| Yuan et al
(28) | 2005 | China | HB | 82 | 68 | 58 | 94 | 3 | 1 | 2 | 6 |
| Cao et al
(40) | 2004 | China | HB | 69 | 35 | 87 | 118 | 2 | 1 | 2 | 5 |
| Chan-Yeung et
al (19) | 2004 | China | PB | 143 | 86 | 102 | 95 | 4 | 2 | 2 | 8 |
| Wang et al
(5) | 2003 | China | NR | 53 | 59 | 54 | 65 | 2 | 1 | 3 | 6 |
| Wang (32) | 2003 | China | HB | 44 | 33 | 54 | 53 | 3 | 0 | 3 | 6 |
| Zhang (38) | 2002 | China | PB | 74 | 87 | 72 | 93 | 3 | 2 | 3 | 8 |
| London et al
(24) | 2000 | China | PB | 134 | 98 | 426 | 284 | 4 | 2 | 3 | 9 |
| Lan et al
(22) | 2000 | China | PB | 73 | 49 | 64 | 58 | 3 | 2 | 3 | 8 |
| Kiyohara et
al (22) | 2000 | Japan | NR | 47 | 14 | 39 | 12 | 3 | 2 | 4 | 9 |
| Lan et al
(37) | 1991 | China | PB | 52 | 34 | 52 | 34 | 4 | 2 | 2 | 8 |
Meta-analysis
As indicated in Table
II, the pooled ORs were performed for the GSTT1 null vs.
present genotype individuals. The results indicated that
individuals with the GSTT1 null genotype were significantly
associated with an increased risk of developing lung cancer
compared with those carrying the GSTT1 present genotype in
the East Asian populations (OR, 1.23; 95% CI, 1.09–1.38;
Pheterogeneity=0.003; Fig.
2). When stratified by smoking status, a statistically
increased lung cancer risk was identified in smokers (OR, 1.71; 95%
CI=1.04–2.81; Pheterogeneity=0.002). However, no
statistically significant association was identified in non-smokers
(OR=1.13; 95% CI, 0.87–1.46; Pheterogeneity=0.42).
Furthermore, in subgroup analysis by histological type, no
statistically significant association was identified for all the
stratified analyses. The major results of the meta-analysis and the
heterogeneity test are listed in Table
II.
 | Table IIMain results of pooled ORs in the
meta-analysis. |
Table II
Main results of pooled ORs in the
meta-analysis.
| Null versus
present |
|---|
|
|
|---|
| Studies, n | OR | 95% CI | P-valuea |
|---|
| Total | 26 | 1.23 | 1.09–1.38 | 0.003 |
| Histological
type |
| SCLC | 3 | 1.17 | 0.69–1.98 | 0.97 |
| SCC | 7 | 1.33 | 0.83–2.15 | 0.001 |
| AC | 7 | 1.24 | 0.98–1.57 | 0.10 |
| Smoking status |
| Smoker | 8 | 1.71 | 1.04–2.81 | 0.002 |
| Non-smoker | 8 | 1.13 | 0.87–1.46 | 0.42 |
Publication bias
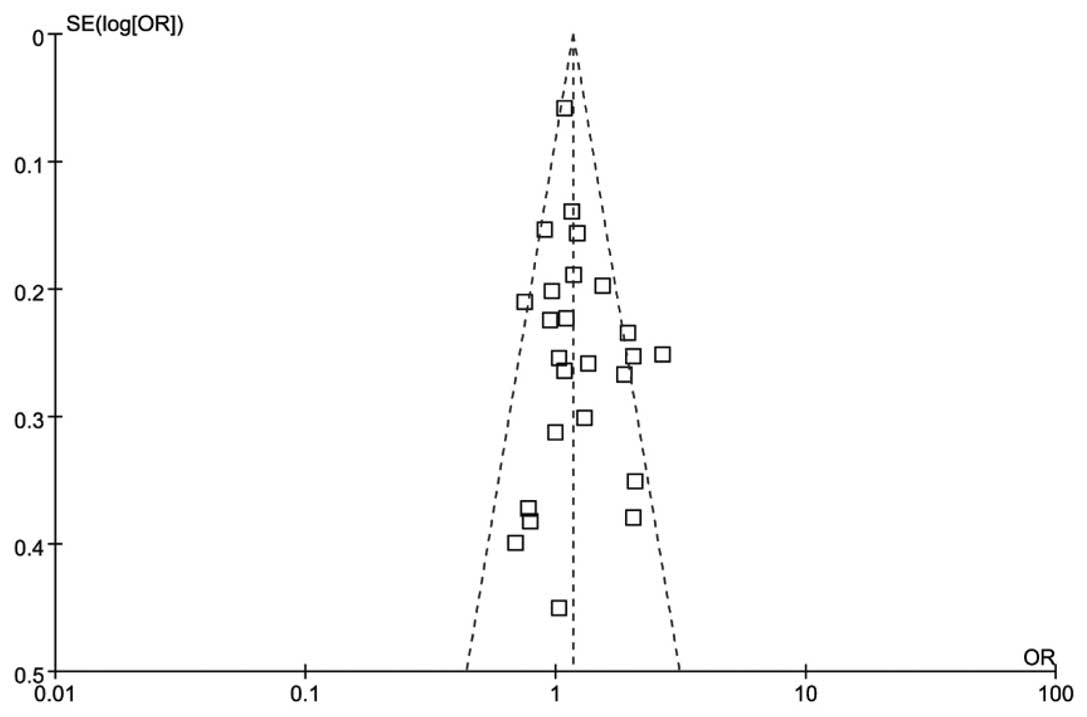
As demonstrated in Fig.
3, the shapes of the funnel plots appeared symmetrical in the
overall populations, indicating the absence of publication bias.
Furthermore, the results of the Egger’s test provided statistical
evidence for the funnel plot asymmetry (t=−1.57; P=0.1265).
Discussion
The results of the current meta-analysis indicated
that GSTT1 polymorphism was associated with an increased
risk of developing lung cancer in the East Asian populations.
Furthermore, in subgroups of smoking status, increased lung cancer
susceptibility was identified in the smoking population.
The GSTT1 gene is located on chromosome
22q11.2 (47). Individuals carrying
homozygous deletions in the GSTT1 genes may present an
impaired ability of metabolically eliminating carcinogenic
compounds and may, therefore, be at an increased risk of developing
lung cancer (6). Since Deakin et
al (48) first investigated the
GSTT1 polymorphism in lung cancer, a number of studies have
been performed to evaluate the association between this homozygous
deletion and the risk of developing lung cancer (4,49,50).
Certain studies have indicated that this polymorphism in the
GSTT1 gene is a risk factor for lung cancer; however, this
is inconsistent with the findings of other studies (51–53).
Additionally, a previous study demonstrated that individuals
harboring the null deletion of the GSTT1 gene had a 2.4-fold
higher risk of developing lung cancer (54). Furthermore, in a previous
meta-analysis focused on the Asian population, the results were
predominantly positive compared with other ethnic groups (55). The disparity in lung cancer
susceptibility among different ethnicities with the GSTT1
null genotype is consistent with a number of previous studies
indicating that the frequency of the GSTT1 deletion varies
among different populations (56,57).
In particular, the prevalence of the GSTT1 null genotype is
lower among Caucasians (10–20%) compared with Asians (50–60%)
(58). Thus, ethnicity may be an
important factor influencing the GSTT1 gene sensitivity to
lung cancer. To the best of our knowledge, the current study is the
first to investigate and determine that the GSTT1 null
genotype is a risk factor of lung cancer in the East Asian
populations.
Tobacco smoking is known to be one of the major risk
factors of lung cancer (56).
Tobacco contains a variety of carcinogens, including polycyclic
aromatic hydrocarbons, N-nitrosamines and aromatic heterocyclic
amines, that are transported through metabolic pathways by the
GSTT1 protein (59). A number of
studies have identified a higher risk of developing lung cancer in
smokers carrying the GSTT1 null genotype (52,60,61).
Similarly, the present study identified that the interaction
between the GSTT1 gene and tobacco consumption has an effect
on the development lung cancer. Furthermore, it has been reported
that not all populations were equally susceptible to
tobacco-associated carcinogens (62). The frequency of the GSTT1
null genotype was lower in Caucasians compared with Asians,
strengthening the hypothesis that polymorphisms in enzymes that
metabolize tobacco carcinogens may have a strong association with
ethnicity (63).
A number of previous studies have evaluated the
effects of gene-gene and gene-environment interactions in lung
cancer development. Chen et al (20) indicated that individuals carrying
the GSTT1 null genotype combined with other GST mutants may
have an enhanced risk of developing lung cancer compared with
individuals carrying any mutant alone. For instance, specific
studies indicated that a daily diet of fruit and vegetables
containing isothiocyanates may reduce the risk of lung cancer among
carriers of at least one functional GSTT1 allele (26,64,65).
Brennan et al (65)
demonstrated that the protective effect of
isothiocyanate-containing vegetables was most apparent in
individuals with low values of circulating GST enzymes due to the
presence of null GSTT1 alleles. In addition, the results are
in accordance with various smaller studies of lung cancer, breast
cancer and colorectal adenomas that demonstrated a protective
effect of isothiocyanates in GSTT1 null carriers (65). More comprehensive studies should be
conducted in the future to fully understand the association between
cancer risk and isothiocyanate consumption in GSTT1 null
individuals.
In the current meta-analysis, various limitations
should be acknowledged. Lung cancer is known to be a consequence of
multiple risk factors. For instance, lifestyle, diet, age, gender,
environment and ethnicity may all contribute as the possible risk
factors (48). However, due to the
limited participant data provided by the individual studies, the
present meta-analysis was not able to conduct a more precise
assessment by ruling out those confounding factors. In addition,
misclassification of cigarette consumption may have occurred due to
the vague definition of cigarette consumption in certain studies.
Furthermore, only published studies were included in the current
meta-analysis, which may have biased the results.
In conclusion, the present study indicated that the
GSTT1 null genotype may contribute towards the increased
lung cancer risk in the East Asian populations. Future large and
well-designed epidemiological studies considering the potential
interactions are required to confirm the results of the present
meta-analysis.
References
|
1
|
Dialyna IA, Miyakis S, Georgatou N and
Spandidos DA: Genetic polymorphisms of CYP1A1, GSTM1 and GSTT1
genes and lung cancer risk. Oncol Rep. 10:1829–1835.
2003.PubMed/NCBI
|
|
2
|
Yang X, Qiu MT, Hu JW, et al: GSTT1 null
genotype contributes to lung cancer risk in Asian populations: a
meta-analysis of 23 studies. PLoS One. 8:e621812013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Litwack G, Ketterer B and Arias IM:
Ligandin: a hepatic protein which binds steroids, bilirubin,
carcinogens and a number of exogenous organic anions. Nature.
234:466–467. 1971. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gallegos-Arreola MP, Gómez-Meda BC,
Morgan-Villela G, et al: GSTT1 gene deletion is associated with
lung cancer in Mexican patients. Dis Markers. 19:259–261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Deng Y, Cheng J, Ding J and
Tokudome S: GST genetic polymorphisms and lung adenocarcinoma
susceptibility in a Chinese population. Cancer Lett. 201:185–193.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salagovic J, Kalina I, Stubna J, et al:
Genetic polymorphism of glutathione S-transferases M1 and T1 as a
risk factor in lung and bladder cancers. Neoplasma. 45:312–317.
1998.
|
|
7
|
Zhong S, Howie AF, Ketterer B, et al:
Glutathione S-transferase mu locus: use of genotyping and
phenotyping assays to assess association with lung cancer
susceptibility. Carcinogenesis. 12:1533–1537. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sprenger R, Schlagenhaufer R, Kerb R, et
al: Characterization of the glutathione S-transferase GSTT1
deletion: discrimination of all genotypes by polymerase chain
reaction indicates a trimodular genotype-phenotype correlation.
Pharmacogenetics. 10:557–565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi S, Watanabe J and Kawajiri K: High
susceptibility to lung cancer analyzed in terms of combined
genotypes of P450IA1 and Mu-class glutathione S-transferase genes.
Jpn J Cancer Res. 83:866–870. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirvonen A, Husgafvel-Pursiainen K,
Anttila S and Vainio H: The GSTM1 null genotype as a potential risk
modifier for squamous cell carcinoma of the lung. Carcinogenesis.
14:1479–1481. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexandrie AK, Sundberg MI, Seidegård J,
et al: Genetic susceptibility to lung cancer with special emphasis
on CYP1A1 and GSTM1: a study on host factors in relation to age at
onset, gender and histological cancer types. Carcinogenesis.
15:1785–1790. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Houlston RS: Glutathione S-transferase M1
status and lung cancer risk: a meta-analysis. Cancer Epidemiol
Biomarkers Prev. 8:675–682. 1999.
|
|
13
|
London SJ, Daly AK, Cooper J, et al:
Polymorphism of glutathione S-transferase M1 and lung cancer risk
among African-Americans and Caucasians in Los Angeles County,
California. J Natl Cancer Inst. 87:1246–1253. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saarikoski ST, Voho A, Reinikainen M, et
al: Combined effect of polymorphic GST genes on individual
susceptibility to lung cancer. Int J Cancer. 77:516–521. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiyohara C, Horiuchi T, Takayama K and
Nakanishi Y: Genetic polymorphisms involved in carcinogen
metabolism and DNA repair and lung cancer risk in a Japanese
population. J Thorac Oncol. 7:954–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tamaki Y, Arai T, Sugimura H, et al:
Association between cancer risk and drug-metabolizing enzyme gene
(CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms
in cases of lung cancer in Japan. Drug Metab Pharmacokinet.
26:516–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piao JM, Shin MH, Kim HN, et al:
Glutathione-S-transferase (GSTM1, GSTT1) null phenotypes and risk
of lung cancer in a Korean population. Asian Pac J Cancer Prev.
14:7165–7169. 2013. View Article : Google Scholar
|
|
18
|
Shaikh RS, Amir M, Masood AI, et al:
Frequency distribution of GSTM1 and GSTT1 null allele in Pakistani
population and risk of disease incidence. Environ Toxicol
Pharmacol. 30:76–79. 2010. View Article : Google Scholar
|
|
19
|
Chan-Yeung M, Tan-Un KC, Ip MS, et al:
Lung cancer susceptibility and polymorphisms of
glutathione-S-transferase genes in Hong Kong. Lung Cancer.
45:155–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HC, Cao YF, Hu WX, et al: Genetic
polymorphisms of phase II metabolic enzymes and lung cancer
susceptibility in a population of Central South China. Dis Markers.
22:141–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiyohara C, Yamamura KI, Nakanishi Y, et
al: Polymorphism in GSTM1, GSTT1 and GSTP1 and susceptibility to
lung cancer in a Japanese population. Asian Pac J Cancer Prev.
1:293–298. 2000.
|
|
22
|
Lan Q, He X, Costa DJ, et al: Indoor coal
combustion emissions, GSTM1 and GSTT1 genotypes and lung cancer
risk: a case-control study in Xuan Wei, China. Cancer Epidemiol
Biomarkers Prev. 9:605–608. 2000.PubMed/NCBI
|
|
23
|
Lee KM, Kang D, Lee SJ, et al: Interactive
effect of genetic polymorphism of glutathione S-transferase M1 and
smoking on squamous cell lung cancer risk in Korea. Oncol Rep.
16:1035–1039. 2006.PubMed/NCBI
|
|
24
|
London SJ, Yuan JM, Chung FL, et al:
Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms,
and lung-cancer risk: a prospective study of men in Shanghai,
China. Lancet. 356:724–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang M, Choi Y, Hwangbo B, et al: Combined
effects of genetic polymorphisms in six selected genes on lung
cancer susceptibility. Lung Cancer. 57:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Deng Y, Cheng J, et al: GST
genetic polymorphisms and lung adenocarcinoma susceptibility in a
Chinese population. Cancer Lett. 201:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang N and Wu Y, Zhou X and Wu Y:
Association between genetic polymorphism of metabolizing enzymes
and DNA repairing enzymes and the susceptibility of lung cancer in
Henan population. Wei Sheng Yan Jiu. 41:251–256. 2012.(In Chinese).
PubMed/NCBI
|
|
28
|
Yuan T, Zhou Q, Zhu W, et al: Relationship
between genetic polymorphism of GSTT1 gene and inherent
susceptibility to lung cancer in Han population in Sichuan, China.
Zhongguo Fei Ai Za Zhi. 8:107–111. 2005.(In Chinese). PubMed/NCBI
|
|
29
|
Bai TY: The study on the polymorphisms of
GSTM1, GSTM3, GSTT1, GSTP1 genes and susceptibility to lung cancer
in Mongolian population. PhD dissertation. Inner Mongolia Medical
College; Inner Mongolia: pp. 15–18. 2011, (In Chinese).
|
|
30
|
Li YF: A case-control study on the
associations between polymorphism of GSTM1, GSTT1 and
susceptibility to breast cancer and lung cancer. PhD dissertation.
Sichuan University; Sichuan: pp. 26–33. 2007, (In Chinese).
|
|
31
|
Du GB: A study on relationship between
genetic polymorphism of GSTM1 and GSTT1 gene and susceptibility to
lung cancer in the population of northern Sichuan of China. PhD
dissertation. North Sichuan Medical University; Sichuan: pp. 18–25.
2011, (In Chinese).
|
|
32
|
Wang N: The relationship between the
deletion of GSTM1, GSTT1 and susceptibility to lung cancer. PhD
dissertation. Zhengzhou University; Zhengzhou: pp. 18–26. 2003, (In
Chinese).
|
|
33
|
Fan J: Study on the polymorphisms of GSTM1
and GSTT1 genes associated with susceptibility to lung cancer. PhD
dissertation. GuangXi Medical University; GuangXi: pp. 15–24. 2011,
(In Chinese).
|
|
34
|
Liu JN: Study on the relationship between
the genetic polymorphism of GSTT1 gene, smoking and lung cancer
susceptibility. PhD dissertation. Yanbian University; Yanbian: pp.
8–12. 2009, (In Chinese).
|
|
35
|
He DX and Tang Y: The relationship of
GSTT1 polymorphism and chromosome 15 aberration in lung cancer
patients. Zhong Liu Fang Zhi Yan Jiu. 33:308–310. 2006.(In
Chinese).
|
|
36
|
Qi XS, Lv HM, Xia Y, et al: A primary
case-control study on the relationship between genetic
polymorphisms of GSTT1 and lung cancer susceptibility to the people
living in high radon-exposed area. Zhong Guo Zhi Ye Yi Xue.
31:361–367. 2008.(In Chinese).
|
|
37
|
Lan Q, He XZ, Costa D, et al: Glutathione
S-transferase GSTM1 and GSTT1 genotypes and susceptibility to lung
cancer. Wei Sheng Yan Jiu. 28:9–11. 1999.
|
|
38
|
Zhang JK: Genetic polymorphisms of
glutathione S-transferase M1 and T1 gene related with the
susceptibility to lung cancer. PhD dissertation. Jinan University;
Guangzhou: pp. 25–32. 2002, (In Chinese).
|
|
39
|
Fan J, Gan LZ, Liang KC and Liang XM:
Relationship of GSTM1 and GSTT1 genetic polymorphisms with lung
cancer susceptibility in Guangxi Zhuang population. Zhong Liu Xue
Za Zhi. 16:922–925. 2010.(In Chinese).
|
|
40
|
Cao YF, Chen HC, Liu XF, et al: Study on
the relationship between the genetic polymorphisma of GSTM1 and
GSTT1 genes and lung cancer susceptibility in the population of
Hunan province of China. Life Sci Res. 8:126–132. 2004.(In
Chinese).
|
|
41
|
Liang KC, Gan LK, Ruan L, et al:
Correlational research of the relationship between the genetic
polymorphism of GSTM1 and GSTT1 in the Zhuang population and lung
cancer. Acta Med Sinica. 25:813–817. 2012.(In Chinese).
|
|
42
|
Wells GA, Shea B, O’Connell D, et al;
Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale
(NOS) for assessing the quality if nonrandomized studies in
meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Accessed Oct 19, 2009
|
|
43
|
Lau J, Ioannidis JP and Schmid CH:
Quantitative synthesis in systematic reviews. Ann Intern Med.
127:820–826. 1997. View Article : Google Scholar
|
|
44
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
46
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rebbeck TR: Molecular epidemiology of the
human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer
susceptibility. Cancer Epidemiol Biomarkers Prev. 6:733–743.
1997.PubMed/NCBI
|
|
48
|
Deakin M, Elder J, Hendrickse C, et al:
Glutathione S-transferase GSTT1 genotypes and susceptibility to
cancer: studies of interactions with GSTM1 in lung, oral, gastric
and colorectal cancers. Carcinogenesis. 17:881–884. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sunaga N, Kohno T, Yanagitani N, et al:
Contribution of the NQO1 and GSTT1 polymorphisms to lung
adenocarcinoma susceptibility. Cancer Epidemiol Biomarkers Prev.
11:730–738. 2002.PubMed/NCBI
|
|
50
|
Harms C, Salama SA, Sierra-Torres CH, et
al: Polymorphisms in DNA repair genes, chromosome aberrations and
lung cancer. Environ Mol Mutagen. 44:74–82. 2004. View Article : Google Scholar
|
|
51
|
Stücker I, Hirvonen A, de Waziers I, et
al: Genetic polymorphisms of glutathione S-transferases as
modulators of lung cancer susceptibility. Carcinogenesis.
23:1475–1481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang P, Bamlet WR, Ebbert JO, et al:
Glutathione pathway genes and lung cancer risk in young and old
populations. Carcinogenesis. 25:1935–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cabral RE, Caldeira-de-Araujo A,
Cabral-Neto JB and Costa Carvalho Mda G: Analysis of GSTM1 and
GSTT1 polymorphisms in circulating plasma DNA of lung cancer
patients. Mol Cell Biochem. 338:263–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sreeja L, Syamala V, Hariharan S, et al:
Possible risk modification by CYP1A1, GSTM1 and GSTT1 gene
polymorphisms in lung cancer susceptibility in a South Indian
population. J Hum Genet. 50:618–627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Langevin SM, Ioannidis JP, Vineis P and
Taioli E; Genetic Susceptibility to Environmental Carcinogens group
(GSEC). Assessment of cumulative evidence for the association
between glutathione S-transferase polymorphisms and lung cancer:
application of the Venice interim guidelines. Pharmacogenet
Genomics. 20:586–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Raimondi S, Paracchini V, Autrup H,
Barros-Dios JM, Benhamou S, Boffetta P, et al: Meta- and pooled
analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J
Epidemiol. 164:1027–1042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Y, Yang H, Li L and Wang H:
Glutathione S-transferase T1 gene deletion polymorphism and lung
cancer risk in Chinese population: a meta-analysis. Cancer
Epidemiol. 34:593–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nelson HH, Wiencke JK, Christiani DC, et
al: Ethnic differences in the prevalence of the homozygous deleted
genotype of glutathione S-transferase theta. Carcinogenesis.
16:1243–1245. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhong S, Howie AF, Ketterer B, et al:
Glutathione S-transferase mu locus: use of genotyping and
phenotyping assays to assess association with lung cancer
susceptibility. Carcinogenesis. 12:1533–1537. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Spitz MR, Duphorne CM, Detry MA, et al:
Dietary intake of isothiocyanates: evidence of a joint effect with
glutathione S-transferase polymorphisms in lung cancer risk. Cancer
Epidemiol Biomarkers Prev. 9:1017–1020. 2000.PubMed/NCBI
|
|
61
|
Hou SM, Fält S and Nyberg F: Glutathione
S-transferase T1-null genotype interacts synergistically with heavy
smoking on lung cancer risk. Environ Mol Mutagen. 38:83–86. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Spitz MR, Wei Q, Dong Q, et al: Genetic
susceptibility to lung cancer: the role of DNA damage and repair.
Cancer Epidemiol Biomarkers Prev. 12:689–698. 2003.PubMed/NCBI
|
|
63
|
Honma HN, De Capitani EM, Perroud MW, et
al: Influence of p53 codon 72 exon 4, GSTM1, GSTT1 and
GSTP1*B polymorphisms in lung cancer risk in a Brazilian
population. Lung Cancer. 61:152–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng
P, et al: Dietary isothiocyanates, glutathione S-transferase -M1,
-T1 polymorphisms and lung cancer risk among Chinese women in
Singapore. Cancer Epidemiol Biomarkers Prev. 10:1063–1067.
2001.PubMed/NCBI
|
|
65
|
Brennan P, Hsu CC, Moullan N,
Szeszenia-Dabrowska N, Lissowska J, Zaridze D Jr, et al: Effect of
cruciferous vegetables on lung cancer in patients stratified by
genetic status: a mendelian randomisation approach. Lancet.
366:1558–1560. 2005. View Article : Google Scholar : PubMed/NCBI
|