Introduction
The number of breast cancer patients is increasing,
with >1.6×106 cases diagnosed annually in China
(1). Anthracycline-based
chemotherapy, alone or in association with taxane administration,
is considered to be a standard post-operative adjuvant treatment
strategy for early-stage breast cancer (2). However, anthracycline resistance is an
important problem that is often encountered during the course of
adjuvant chemotherapy.
Akt, also known as protein kinase B, is a
serine/threonine protein kinase that is activated by a range of
stimuli via growth factor receptors, in a phosphoinositide-3-OH
kinase (PI3K)-dependent manner (3).
The phosphorylation of Akt has been demonstrated to promote growth
factor-mediated cell growth, proliferation, migration and survival
(4–6); however, it remains unclear whether the
activation of Akt is associated with the poor prognosis of breast
cancer patients. The results of a study conducted by Andre et
al (7), which assessed
phosphorylated Akt (pAkt) expression in 823 patients with
early-stage breast cancer, indicated that the overexpression of
pAkt was not correlated with patient prognosis. Furthermore, data
collected from 252 breast cancer patients by Tokunaga et al
(8) indicated that no association
exists between the expression of pAkt and the disease-free survival
(DFS) time of patients; however, it was identified that pAkt
expression appears to predict a poor prognosis in patients treated
with endocrine therapy. Additionally, Schmitz et al
(9) collected tissue samples from
99 breast cancer patients without lymph node metastasis and
determined that the expression of pAkt was associated with a
shorter DFS time. The results of the aforementioned studies
demonstrate discrepancies, thus, the present study proposes that
the expression of pAkt may produce different effects in patients
with different clinical characteristics. Furthermore, the
expression of pAkt has been specifically associated with the in
vitro resistance to doxorubicin and paclitaxel in breast cancer
(10–12). It has been proposed that
Akt/mammalian target of rapamycin (mTOR) pathway inhibition may
sensitize breast cancer cells to doxorubicin (13,14);
however, few clinical studies have investigated the association
between pAkt expression and the resistance to doxorubicin in breast
cancer patients.
Extracellular-regulated kinase (Erk) is a member of
the mitogen-activated protein kinase (MAPK) signal-transducing
family, which consists of three key cascades, termed Raf-1, Erk and
p38 MAPK, with Erk being the most relevant factor in breast cancer
(15). The role of Erk1 and Erk2
has been extensively studied in vitro; the two proteins
demonstrate high structural and functional similarity, therefore,
they are often referred to as Erk1/2. The intracellular activity of
the MAPK pathway involves complex interactions between the PI3K/Akt
cascades, which leads to proliferative and apoptotic activities
being generated via competing mechanisms (16); however, it remains unclear whether
augmented phosphorylated Erk1/2 (pErk1/2) activity is a prognostic
factor for the outcome of breast cancer. Milde-Langosch et
al (17) reported that high
pErk1 expression was an independent indicator of a long
recurrence-free survival time; however, conversely and in parallel
to this study, other studies reported that increased MAPK signaling
was associated with a shorter disease-free survival time (18,19).
Furthermore, the expression of pErk1/2 was demonstrated to induce
doxorubicin and paclitaxel resistance in breast cancer cells
(15,16) By contrast, the association between
pErk1/2 expression and the resistance to anthracycline in breast
cancer patients remains unclear. For example, Eralp et al
(20) reported that the expression
of MAPK was associated with anthracycline resistance in
triple-negative breast cancer patients, however, only 13 (11.9%)
patients exhibited anthracycline-resistant disease.
Thus, the aim of the present study was to determine
the clinical significance of pAkt and pErk1/2 protein expression in
early-stage breast cancer patients treated with anthracycline-based
adjuvant chemotherapy. Additionally, a series of hierarchical
clustering analyses were conducted to determine the predictive
value of pAkt and pErk1/2 for the prognosis of breast cancer
patients with varying clinical characteristics.
Materials and methods
Patient selection and histology
A retrospective analysis was performed on 256
patients with histologically confirmed breast cancer who were
treated at The First Hospital of China Medical University
(Shenyang, China) or The Tumor Hospital of Anshan City (Anshan,
China). The present study was approved by the Human Ethics Review
Committee of the First Hospital of China Medical University, and
informed consent was obtained from all patients in accordance with
the Declaration of Helsinki and its later revisions. All patients
underwent a mastectomy or breast conserving surgery between
December 1999 and December 2008. No patients exhibited evidence of
distant metastases at the time of surgery and all patients were
administered with systemic adjuvant chemotherapy after surgery. The
study group included 174 (68.0%) patients treated with
anthracycline-based chemotherapy for 4–6 cycles every three weeks,
including 5-fluorouracil (600 mg/m2)-epirubicin (75–80
mg/m2)-cyclophosphamide (600 mg/m2) and
epirubicin (75–80 mg/m2)-cyclophosphamide (600
mg/m2) schemes, and 82 (32.0%) patients who received
anthracycline associated with taxane chemotherapy for 6–8 cycles
every three weeks, including epirubicin (80–100
mg/m2)-cyclophosphamide (600 mg/m2) followed
by taxanes (175 mg/m2) and taxanes (175
mg/m2) followed by epirubicin(80–100
mg/m2)-cyclophosphamide (600 mg/m2) schemes.
A total of 142 estrogen receptor (ER)- or progesterone receptor
(PR)-positive patients received endocrine therapy with tamoxifen
(20 mg, daily) for premenopausal patients and aromatase inhibitors
(2.5 mg letrozole and 1 mg anastrozole, daily), for postmenopausal
patients, for at least five years. Three human epidermal growth
factor receptor (HER2)-positive patients received adjuvant
trastuzumab (6 mg/kg) therapy every three weeks, and 12 patients
received logical radiotherapy (total dose, 50 Gy), delivered to the
ipsilateral chest wall, supra- and infra-clavicular lymph node
regions. Routine immunohistochemical (IHC) staining was used to
obtain the data regarding hormone receptor and HER2 status via
analysis of the pathology reports. Additionally, medical reports
were reviewed to retrieve clinical information regarding patient
demographics, treatment details and outcome, and tumor samples were
obtained from the patients during surgery.
Immunohistochemical analysis and
assessment
pAkt and pErk expression was assessed by performing
IHC analysis on a tissue microarray containing two spots of each
primary breast cancer tumor tissue. pAkt immunoreactivity,
specifically the phosphorylation of serine 473, was evaluated using
rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at a dilution of 1:250. For pErk1/2 detection, automatic
immunostaining was performed on the DAKO Autostainer Link 48 (Dako,
Glostrup, Denmark) using the monoclonal antibody phospho-p44/42
MAPK (Thr202/Tyr204), clone E10, at a dilution of 1:250 (Santa Cruz
Biotechnology, Inc.). Each slide was deparaffinized in xylene and
rehydrated in graded alcohol to Tris-buffered saline [50 mM Tris
and 150 mM NaCl (pH 7.4)]. Antigen retrieval was performed by
microwaving at 95°C for 20 min in 20 mM Tris, 10 mM citrate and 13
mM EDTA (pH 7.8). Subsequent to blocking the endogenous peroxidase
activity and applying the primary antibody, the slides were
incubated with biotinylated goat anti-mouse immunoglobulins (Fuzhou
Maixin Biological Technology Ltd., Fujian, China) and streptavidin
conjugated to horseradish peroxidase (Fuzhou Maixin Biological
Technology Ltd.), with 3,3′-diaminobenzidine (DAB) chromogen
solution (DAB kit; Fuzhou Maixin Biological Technology Ltd.) and a
substrate buffer containing hydrogen peroxide (Fuzhou Maixin
Biological Technology Ltd.) serving as the substrate system. Tissue
sections were counterstained with hematoxylin, permanently mounted
and independently evaluated by two individuals with no prior
knowledge of the clinical data and pathological parameters. The
scoring system was designed on the basis of tumor grading with
respect to the ratio of stained cells to total tumor cells counted
(1, 1–10%; 2, 11–33%; 3, 34–66%; and 4, 67–100%) and the intensity
of cytoplasmic staining ranged from light to dark brown (0, none;
1, weak; 2, moderate; and 3, strong). By combining the grading and
staining intensity scores, an expression score ranging from 0 to 7
was obtained for pAkt and pErk for each sample. Scores ranging
between 0 and 3 were considered to indicate low protein expression
(negative) and a score between 4 and 7 indicated a high protein
expression level (positive).
Statistical analysis
χ2 and Fisher’s exact tests were used to
detect the association between pAkt and pErk1/2 protein expression
and the clinical characteristics of the current patients. The DFS
time was defined as the time between the date of surgery and the
date on which the first of the following events occurred:
Locoregional recurrence, distant metastasis, diagnosis of a second
primary tumor or cancer-related mortality. The overall survival
(OS) time was defined as the period of time between the date of
surgery and the date of mortality from any cause. Furthermore, the
predictive value of pAkt and pErk1/2 staining for the DFS and OS
times were summarized using the Kaplan-Meier method, and compared
between different arms using a stratified log-rank test. The
relative risk of reducing DFS was determined using Cox proportional
hazard regression with multivariate analyses. All analyses were
conducted using SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) and two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The median age of the cohort was 50 years (range,
26–71 years) and the majority (n=220; 85.9%) of patients exhibited
invasive ductal carcinoma. The databases used in the present study
were locked on August 1, 2010. After a median follow-up period of
52.5 months (range, 19–127 months), 192 patients (75.0%) were alive
with no evidence of disease, 64 (25.0%) patients had relapsed and
34 (13.3%) had succumbed due to disease progression. Within 12
months of adjuvant chemotherapy, 32 (12.5%) patients developed
recurrence, thus, these patients were defined as having
chemotherapy-resistant disease. Detailed patient characteristics
are indicated in Table I.
 | Table ICorrelation between pAkt and pErk1/2
expression, and the clinicopathological parameters in breast cancer
patients. |
Table I
Correlation between pAkt and pErk1/2
expression, and the clinicopathological parameters in breast cancer
patients.
| | pAkt expression | pErk1/2
expression |
|---|
| |
|
|
|---|
| Parameter | Patients, n | −, n (%) | +, n (%) | P-valuea | −, n (%) | +, n (%) | P-valuea |
|---|
| Age, years | | | | 0.054 | | | 1.000 |
| ≤50 | 132 | 73 (55.3) | 59 (44.7) | | 88 (66.7) | 44 (33.3) | |
| >50 | 124 | 84 (67.7) | 40 (32.3) | | 82 (66.1) | 42 (33.9) | |
| Tumor size, cm | | | | 0.886 | | | 0.460 |
| ≤2 | 70 | 42 (60.0) | 28 (40.0) | | 44 (62.9) | 26 (37.1) | |
| >2 | 184 | 113 (61.4) | 71 (38.6) | | 125 (67.9) | 59 (32.1) | |
| Lymph node
involvement | | | | 0.020b | | | 0.286 |
| 0 | 111 | 77 (69.4) | 34 (30.6) | | 78 (70.3) | 33 (29.7) | |
| ≥1 | 143 | 78 (54.5) | 65 (45.5) | | 91 (63.6) | 52 (36.4) | |
| Histological
grade | | | | 0.038b | | | 0.624 |
| I | 18 | 14 (77.8) | 4 (22.2) | | 11 (61.1) | 7 (38.9) | |
| II | 146 | 80 (54.8) | 66 (45.2) | | 96 (65.8) | 50 (34.2) | |
| III | 63 | 44 (69.8) | 19 (30.2) | | 45 (71.4) | 18 (28.6) | |
| ER | | | | 0.365 | | | 0.592 |
| 0 | 146 | 93 (63.7) | 53 (36.3) | | 95 (65.1) | 51 (34.9) | |
| 1–3 | 109 | 63 (57.8) | 46 (42.2) | | 75 (68.8) | 34 (31.2) | |
| PR | | | | 0.519 | | | 0.894 |
| 0 | 139 | 88 (63.3) | 51 (36.7) | | 92 (66.2) | 47 (33.8) | |
| 1–3 | 116 | 68 (58.6) | 48 (41.4) | | 78 (67.2) | 38 (32.8) | |
| HER2 | | | | 0.414 | | | 0.676 |
| 0–1 | 156 | 93 (59.6) | 63 (40.4) | | 105 (67.3) | 51 (32.7) | |
| 2–4 | 90 | 59 (65.6) | 31 (34.4) | | 58 (64.4) | 32 (35.6) | |
| Chemotherapy
resistance | | | | 0.564 | | | 0.016a |
| Yes | 32 | 18 (56.2) | 14 (43.8) | | 15 (46.9) | 17 (53.1) | |
| No | 224 | 139 (62.1) | 85 (37.9) | | 155 (69.2) | 69 (30.8) | |
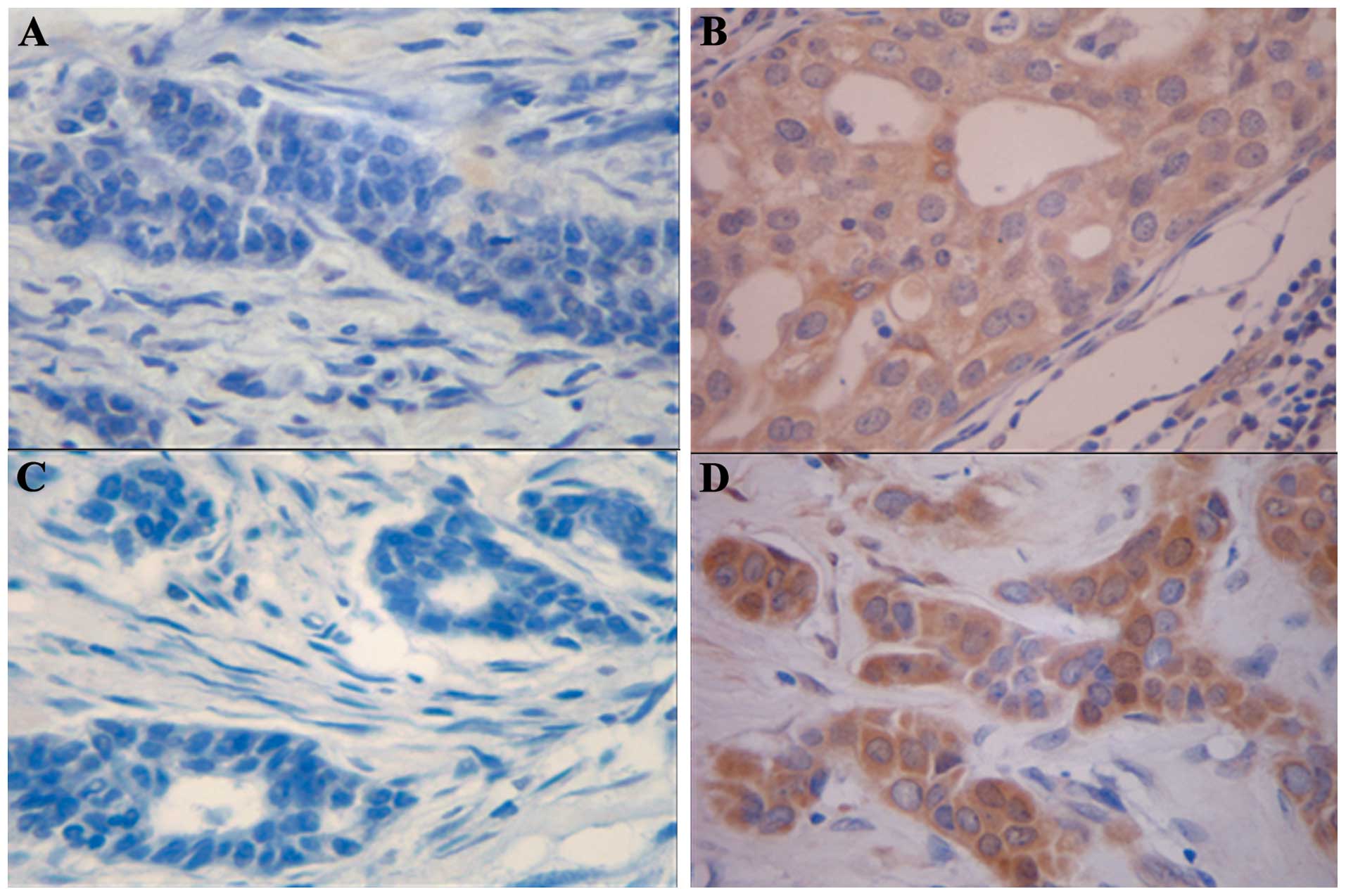
pAkt and pErk1/2 expression patterns in
breast cancer
pAkt and pErk1/2 protein expression levels were
evaluated in 256 breast cancer tissue samples of patients with
early-stage breast cancer. Prominent cytoplasmic and partial
nuclear pAkt and pErk1/2 immunoreactivity was observed in the tumor
cells. In total, 99 cases (38.7%) were classified as positive for
pAkt expression (Fig. 1A and B) and
86 cases (33.6%) were classified as positive for pErk1/2 expression
(Fig. 1C and D); thus, a
significant association was observed between the expression of pAkt
and pErk1/2 (Spearman rank-correlation coefficient, r=0.284;
P<0.001). The expression of pAkt was significantly correlated
with the occurrence of axillary lymph node metastases (P=0.020) and
with the histological grade (P=0.038). However, no statistically
significant correlation was identified between pAkt expression and
chemotherapy resistance (P=0.313), tumor size, patient age, or ER,
PR and HER2 status. Furthermore, no correlation was identified
between pErk1/2 expression and a number of patient clinical
characteristics, including age, tumor size, pathology, lymph node
metastases, tissue grade, and ER, PR and HER2 status. However, a
statistically significant difference in positive staining for
pErk1/2 was observed between chemotherapy-resistant and
chemotherapy-sensitive tumors (53.1 vs. 30.8%; P=0.016). The
associations between pAkt/pErk1/2 expression and the conventional
clinical characteristics of the patients are shown in Table I.
Overall prognostic value of pAkt and
pErk1/2 expression in breast cancer patients
After a median follow-up period of 52.5 months, the
data indicated that the expression of pAkt was not significantly
associated with the DFS or OS times of the patients (P=0.245 and
P=0.528, respectively; Fig. 2A and
B). The expression of pErk1/2 was significantly associated with
a shorter DFS time (P=0.049, Fig.
2C), however, no significant difference was identified in the
OS time (P=0.848; Fig. 2D). By
contrast, pAkt- or pErk1/2-positive tumors were significantly
associated with a decreased DFS time compared with pAkt- and
pErk1/2-negative tumors (P=0.028; Fig.
2E), however, no significant difference was observed in the OS
time (P=0.171; Fig. 2F).
Subsequently, Cox regression with multivariate analysis was
performed to determine the independent prognostic value of
different variables in association with DFS time, indicating that
the following factors significantly contribute to a decrease in
DFS: Positive lymph node metastasis (P=0.001), ER/PR-negative
tumors (P=0.030) and the coexpression of pAkt and pErk1/2 (P=0.032)
(Table II).
 | Table IIResults of multivariate Cox
regression analysis to determine the independent prognostic value
of different variables in association with DFS time. |
Table II
Results of multivariate Cox
regression analysis to determine the independent prognostic value
of different variables in association with DFS time.
| Covariate | Relative risk | 95% CI | P-value |
|---|
| Age, years (>50
vs. <50) | 1.013 | 0.603–1.703 | 0.961 |
| Tumor size, cm
(>2 vs. ≤2) | 1.118 | 0.587–2.130 | 0.733 |
| Lymph node
involvement (positive vs. negative) | 3.079 | 1.612–5.880 | 0.001a |
| Hormone status
(positive vs. negative) | 0.563 | 0.335–0.947 | 0.030a |
| HER2 status
(positive vs. negative) | 0.927 | 0.539–1.598 | 0.785 |
| Coexpression of
pAkt and pErk1/2 (positive vs. negative) | 1.817 | 1.015–3.139 | 0.032a |
Predictive value of pAkt and pErk1/2
expression for the DFS time of patients in various subgroups
Recent studies have indicated that breast cancer
patients with specific tumor subtypes may be more resistant to
therapy and therefore exhibit decreased DFS times (21–24).
Thus, the present study examined the effect of pAkt and pErk1/2
expression on the following tumor subgroups: HER2-positive;
HER2-negative; ER- or PR-positive; ER- and PR-negative; lymph
node-positive; and lymph node-negative subgroups. In the
HER2-positive subgroup, the DFS time of the pAkt-negative patients
was significantly longer than that of the pAkt-positive patients
(P=0.002); additionally, a significant association was identified
between pErk1/2 expression and decreased DFS time (P=0.002). pAkt
and pErk1/2 expression was not significantly associated with the
DFS time of the patients in the HER2-negative (P=0.623 and P=0.563,
respectively), and ER- and PR-positive (P=0.726 and P=0.223,
respectively) subgroups. In the ER- and PR-negative subgroup, no
significant association was observed between the expression of
pErk1/2 and the DFS time of the patients (P=0.118); however, the
DFS time of the pAkt-negative patients was significantly longer
than that of the pAkt-positive patients (P=0.034). Furthermore,
pAkt and pErk1/2 expression was not significantly associated with
the DFS time of the patients in the subgroup with lymph node
metastases (P=0.778 and P=0.345, respectively) or the lymph
node-negative subgroup (P=0.245 and P=0.123, respectively)
(Table III).
 | Table IIIUnivariate analysis of pAkt and
pErk1/2 for predictive DFS values in different subgroups. |
Table III
Univariate analysis of pAkt and
pErk1/2 for predictive DFS values in different subgroups.
| P-valuea |
|---|
|
|
|---|
| Patient
subgroup | pAkt
expression | pErk1/2
expression |
|---|
| HER2-positive
(n=90) | 0.002b | 0.002b |
| HER2-negative
(n=156) | 0.623 | 0.563 |
| ER- or PR-positive
(n=141) | 0.726 | 0.223 |
| ER- and PR-negative
(n=114) | 0.034b | 0.118 |
| Lymph node-positive
(n=143) | 0.788 | 0.345 |
| Lymph node-negative
(n=111) | 0.245 | 0.123 |
| Anthracycline-based
chemotherapy (n=174) | 0.238 | 0.382 |
| Anthracycline and
taxane chemotherapy (n=82) | 0.743 | 0.030b |
Predictive values of pAkt and pErk1/2
expression to determine the efficacy of anthracycline-based
chemotherapy
In the patients treated with anthracycline-based
chemotherapy, pAkt and pErk1/2 expression was not associated with
DFS of patients (P=0.238 and P=0.382, respectively). In the
patients treated with anthracycline associated with taxane
chemotherapy, no correlation was identified between the expression
of pAkt and the DFS time of the patients (P=0.743), however, the
DFS time of the pErk1/2-negative patients was significantly longer
than that of the pErk1/2-positive patients (P=0.030) (Table III).
Discussion
The present study examined the expression of pAkt
and pErk1/2 in 256 patients with early-stage breast cancer by
performing IHC analysis of the tumor samples. pAkt and pErk1/2
protein expression was identified in 38.7 and 33.6% of the tissue
samples, respectively, and the cytoplasmic and nuclear
immunoreactivity of pAkt and pErk1/2 was observed in the breast
cancer cells. The mechanisms that regulate the induction of Akt
appear to be involved in the activation of Erk1/2 (25), however, no large-scale clinical
study has been conducted analyzing the association between pAkt and
pErk1/2 expression. The results of the present study indicated that
a significant positive correlation exists between the expression of
pAkt and pErk1/2 proteins in breast cancer patients. Furthermore,
pAkt expression was associated with positive nodal status and
histological grade, however, no significant correlation was
identified between pAkt and tumor size, indicating that pAkt may
induce a more malignant phenotype via its role in antiapoptosis and
proliferation. By contrast, no correlation was observed between
pErk1/2 expression and conventional clinical patient
characteristics.
Although the activation of Akt appears to be a
potentially major event in the survival of breast cancer cells, the
present study did not demonstrate a significant association between
pAkt protein expression and the prognosis of patients with
early-stage breast cancer; this is consistent with the results
found by Andre et al (7).
Previous clinical studies regarding the prognostic value of pErk1/2
expression in patients with early-stage breast cancer have produced
discrepancies. For example, the expression of pErk1/2 was
identified to positively correlate with an increased risk of
relapse (18), which is in contrast
to the results determined by Milde-Langosch et al (18), where high expression of pErk1/2 was
significantly associated with a long recurrence-free survival time.
The present study demonstrated that pErk1/2 expression was
associated with a poor prognosis in 256 patients with early-stage
breast cancer. Additionally, to the best of our knowledge, the
present study is the first to report that the coexpression of pAkt
and pErk1/2 are predictive of a shorter DFS time in early-stage
breast cancer patients.
The overexpression of HER2 occurs in ~30% of cases
of human breast cancer and is typically associated with a poor
prognosis (26–28). pAkt and pErk1/2 are important
downstream substrates of HER2 (25–35).
Morse et al (28)
demonstrated that the inhibition of HER2 signaling may decrease
pErk and pAkt expression levels, and reduce breast tumor cell
proliferation. Furthermore, Tokunaga et al (8) reported that HER2-/pAkt-positive tumors
appear to exhibit a trend for a poorer prognosis, and Park et
al (30) indicated that
increased cytoplasmic and nuclear pAkt concentrations may
significantly correlate with HER-2 overexpression. Similarly, the
present study identified that pAkt expression was significantly
associated with decreased DFS time in the HER2-positive patients.
As the phosphorylation of Akt can induce the resistance of breast
cancer to trastuzumab treatment (31,32),
the results of the current study indicate that the treatment of
HER2-positive tumors with pAkt inhibitors may aid in overcoming
trastuzumab resistance. It has previously been reported that a
higher level of pErk1/2 expression predicts a shorter DFS time in
lymph node-positive/HER2-positive patients (33). Similarly, the present study
indicated that the expression of pErk1/2 was associated with a
shorter DFS time in HER2-positive patients with early-stage breast
cancer. Thus, concurrent pAkt and pErk expression may be predictive
of a poor prognosis in HER2-positive patients, and a dual target
inhibitor of pAkt and pErk1/2 may be a potential effective
therapeutic approach for the treatment of HER2-positive breast
cancer, overcoming the resistance of breast cancer patients to
trastuzumab therapy.
Previous studies have identified a specific
association between the expression of pAkt and pErk1/2, and the
proliferation and chemoresistance of breast cancer cells in
vitro. Additionally, a number of studies have reported that
anthracycline induces secondary Akt activation, which may
contribute to the resistance of breast cancer cells to
anthracycline in vitro (10–12).
Inhibitors of the PI3K/Akt/mTOR pathways, such as rapamycin
analogs, may be used as a therapeutic approach to modulate
anthracycline-induced Akt activation. However, similar to the
results found by Andre et al (7), pAkt expression did not appear to be
predictive for anthracycline efficacy in the present study and was
not associated with anthracycline resistance. Furthermore, the
Raf/MAPK kinase/Erk pathway has been demonstrated to induce
resistance to doxorubicin and paclitaxel via ectopic activation of
Raf in breast cancer cells (15,16).
Similarly, the present study confirmed that a significant
correlation exists between pErk1/2 and chemotherapy-resistance;
this result is in accordance with a study conducted by Eralp et
al (20), which determined that
pErk1/2 expression was associated with anthracycline resistance in
triple-negative breast cancer patients. Additionally, the present
study indicated that pErk1/2 expression was a poor predictor for
the efficacy of adjuvant anthracycline-based chemotherapy,
particularly for patients treated with anthracycline associated
with taxane chemotherapy. Thus, the inhibition of pErk1/2 may be an
effective therapeutic approach to modulate anthracycline resistance
in breast cancer.
Acknowledgements
The abstract was presented at the 2011 ASCO Annual
Meeting June 3-June 7 2011 in Chicago, IL and published as abstract
no. e21026 in J Clin Oncol 20: 2011. The present study was
supported by grants from the National Natural Science Foundation of
China (grant no. 81172535), the Science and Technology Foundation
of Liaoning Province (grant no. 2010225032) and the General Project
of Liaoning Department of Education (grant no. L2010698).
References
|
1
|
Fan L, Strasser-Weippl K, Li JJ, et al:
Breast cancer in China. Lancet Oncol. 15:e279–e289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: Meeting highlights: updated
international expert consensus on the primary therapy of early
breast cancer. J Clin Oncol. 21:3357–3365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amiri A, Noei F, Jeganathan S, Kulkarni G,
Pinke DE and Lee JM: eEF1A2 activates AKT and stimulates
AKT-dependent actin remodeling, invasion and migration. Oncogene.
26:3027–3040. 2007. View Article : Google Scholar
|
|
6
|
Srinivasan S, Koduru S, Kumar R, et al:
Diosgenin targets AKT-mediated prosurvival signaling in human
breast cancer cells. Int J Cancer. 125:961–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andre F, Nahta R, Conforti R, et al:
Expression patterns and predictive value of phosphorylated AKT in
early-stage breast cancer. Ann Oncol. 19:315–320. 2008. View Article : Google Scholar
|
|
8
|
Tokunaga E, Kimura Y, Oki E, et al: Akt is
frequently activated in HER2/neu-positive breast cancers and
associated with poor prognosis among hormone-treated patients. Int
J Cancer. 118:284–289. 2006. View Article : Google Scholar
|
|
9
|
Schmitz KJ, Otterbach F, Callies R, et al:
Prognostic relevance of activated AKT kinase in node-negative
breast cancer: a clinicopathological study of 99 cases. Mod Pathol.
17:15–21. 2004. View Article : Google Scholar
|
|
10
|
Liang K, Lu Y, Li X, Zeng X, Glazer RI,
Mills GB and Fan Z: Differential roles of
phosphoinositide-dependent protein kinase-1 and akt1 expression and
phosphorylation in breast cancer cell resistance to paclitaxel,
doxorubicin and gemcitabine. Mol Pharmacol. 70:1045–1052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knuefermann C, Lu Y, Liu B, et al:
HER2/PI-3k/AKT activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Lu Y, Liang K, Liu B and Fan Z:
Differential responses to doxorubicin-induced phosphorylation and
activation of AKT in human breast cancer cells. Breast Cancer Res.
7:R589–R597. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roudier E, Mistafa O and Stenius U:
Statins induce mammalian target of rapamycin (mTOR)-mediated
inhibition of Akt signaling and sensitize p53-deficient cells to
cytostatic drugs. Mol Cancer Ther. 5:2706–2715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mondesire WH, Jian W, Zhang H, Ensor J,
Hung MC, Mills GB and Meric-Bernstam F: Targeting mammalian target
of rapamycin synergistically enhances chemotherapy-induced
cytotoxicity in breast cancer cells. Clin Cancer Res. 10:7031–7042.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santen RJ, Song RX, McPherson R, Kumar R,
Adam L, Jeng MH and Yue W: The role of mitogen-activated protein
(MAP) kinase in breast cancer. J Steroid Biochem Mol Biol.
80:239–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCubrey JA, Steelman LS, Abrams SL, et
al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in
malignant transformation and drug resistance. Adv Enzyme Regul.
46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milde-Langosch K, Bamberger AM, Rieck G,
Grund D, Hemminger G, Müller V and Löning T: Expression and
prognostic relevance of activated extracellular regulated kinases
(ERK1/2) in breast cancer. Br J Cancer. 92:2206–2215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mueller H, Flury N, Eppenberger-Castori S,
Kueng W, David F and Eppenberger U: Potential prognostic value of
mitogen-activated protein kinase activity for disease-free survival
of primary breast cancer patients. Int J Cancer. 89:384–388. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gee JM, Robertson JF, Ellis IO and
Nicholson RI: Phosphorylation of ERK1/2 mitogen-activated protein
kinase is associated with poor response to anti-hormonal therapy
and decreased patient survival in clinical breast cancer. Int J
Cancer. 95:247–254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eralp Y, Derin D, Ozluk Y, et al: MAPK
overexpression is associated with anthracycline resistance and
increased risk for recurrence in patients with triple-negative
breast cancer. Ann Oncol. 19:669–674. 2008. View Article : Google Scholar
|
|
21
|
Glück S, de Snoo F, Peeters J, et al:
Molecular subtyping of early-stage breast cancer identifies a group
of patients who do not benefit from neoadjuvant chemotherapy.
Breast Cancer Res Treat. 139:759–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glück S, Ross JS, Royce M, et al: TP53
genomics predict higher clinical and pathologic tumor response in
operable early-stage breast cancer treated with
docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat.
132:781–791. 2012. View Article : Google Scholar
|
|
23
|
Rouzier R, Perou CM, Symmans WF, et al:
Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sørlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee ER, Kim JY, Kang YJ, et al: Interplay
between PI3K/Akt and MAPK signaling pathways in DNA-damaging
drug-induced apoptosis. Biochim Biophys Acta. 1763:958–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hudis CA: Trastuzumab - mechanism of
action and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meric-Bernstam F and Hung MC: Advances in
targeting human epidermal growth factor receptor-2 signaling for
cancer therapy. Clin Cancer Res. 12:6326–6330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiessling R, Wei WZ, Herrmann F,
Lindencrona JA, Choudhury A, Kono K and Seliger B: Cellular
immunity to the Her-2/neu protooncogene. Adv Cancer Res.
85:101–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hung MC and Lau YK: Basic science of
HER-2/neu: a review. Semin Oncol. 26(Suppl 12): 51–59.
1999.PubMed/NCBI
|
|
30
|
Park SS and Kim SW: Activated Akt
signaling pathway in invasive ductal carcinoma of the breast:
correlation with HER2 overexpression. Oncol Rep. 18:139–143.
2007.PubMed/NCBI
|
|
31
|
Gori S, Sidoni A, Colozza M, et al: EGFR,
pMAPK, pAkt and PTEN status by immunohistochemistry: correlation
with clinical outcome in HER2-positive metastatic breast cancer
patients treated with trastuzumab. Ann Oncol. 20:648–654. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|
|
33
|
Esteva FJ, Sahin AA, Smith TL, et al:
Prognostic significance of phosphorylated P38 mitogen-activated
protein kinase and HER-2 expression in lymph node positive breast
carcinoma. Cancer. 100:499–506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morse MA, Wei J, Hartman Z, et al:
Synergism from combined immunologic and pharmacologic inhibition of
HER2 in vivo. Int J Cancer. 126:2893–2903. 2010.
|
|
35
|
Johnston SR: Targeting downstream
effectors of epidermal growth factor receptor/HER2 in breast cancer
with either farnesyltransferase inhibitors or mTOR antagonists. Int
J Gynecol Cancer. 16(Suppl 2): 543–548. 2006. View Article : Google Scholar : PubMed/NCBI
|