Introduction
Solid pseudopapillary tumor (SPT) is a rare,
nonfunctional neoplasm of the pancreas that occurs most frequently
in young females with a mean age of 25 years (90% of all cases
occur in female patients) (1). The
first case was reported by Frantz in 1959 (2). The tumor may occur anywhere in the
pancreas, however, is most frequently identified in the pancreatic
body and tail. Histopathologically, SPT is classically defined as a
large and encapsulated mass composed of a mixture of cystic and
solid areas. Intratumoral hemorrhage is frequent, and
calcifications have been reported in ≤30% of cases (3–5).
Although SPT is considered to be an indolent lesion with low
malignant potential and a favorable prognosis following surgical
resection, a number of cases of locally infiltrating and metastatic
varieties, and post-surgical recurrences have been reported
(1). In 1996, the World Health
Organization renamed the tumor as SPT and reclassified it as a
low-grade malignant tumor (6). The
incidence of SPT is low, accounting for 1–2% of exocrine pancreatic
tumors and 5% of cystic pancreatic neoplasms.
The current study reports a case of SPT with
multiple liver metastases and local recurrence following distal
pancreatectomy for the original lesion; the patient was treated
with hepatic arterial infusion (HAI) chemotherapy, systemic
chemotherapy, transarterial embolization (TAE), and surgical
resection. Written informed consent was obtained from the
patient.
Case report
A 33-year-old female was admitted to the Department
of Gastroenterological Surgery, Ishikawa Prefectural Central
Hospital (Kanazawa, Japan) in December 2006 with abdominal pain and
vomiting. A cystic lesion of 10 cm in diameter was detected in the
tail of the pancreas as well as multiple liver tumors using
abdominal computed tomography (CT) (Fig. 1). In January 2007, distal
pancreatectomy was performed at Ishikawa Prefectural Central
Hospital. The pancreatic tumor was diagnosed as SPT on
postoperative pathological examination, which revealed that the
resected tumors were composed of sheets of bland cells with oval to
round nuclei and a focal pseudopapillary appearance. Furthermore,
immunohistochemical analysis revealed positivity for CD10, CD56,
vimentin, neuron-specific enolase (NSE) and α-antitrypsin. The
multiple liver tumors were hypothesized to be SPT metastases.
Following surgery, systemic chemotherapy with gemcitabine (GEM) and
S-1, an oral fluoropyrimidine derivative, was administered.
However, the liver metastases gradually enlarged, and the patient
was referred to the Department of Gastroenterologic Surgery at the
Graduate School of Medicine (Kanazawa, Japan) for hepatic arterial
infusion (HAI) chemotherapy in September 2011.
Physical examination identified palpable hard masses
of >10 cm in diameter around the umbilical area and right lower
quadrant. Abdominal contrast-enhanced CT revealed multiple
heterogeneous solid and cystic tumors in the liver, and a large
tumor of 15 cm in diameter was identified in the left subphrenic
area, indicating local recurrence following distal pancreatectomy
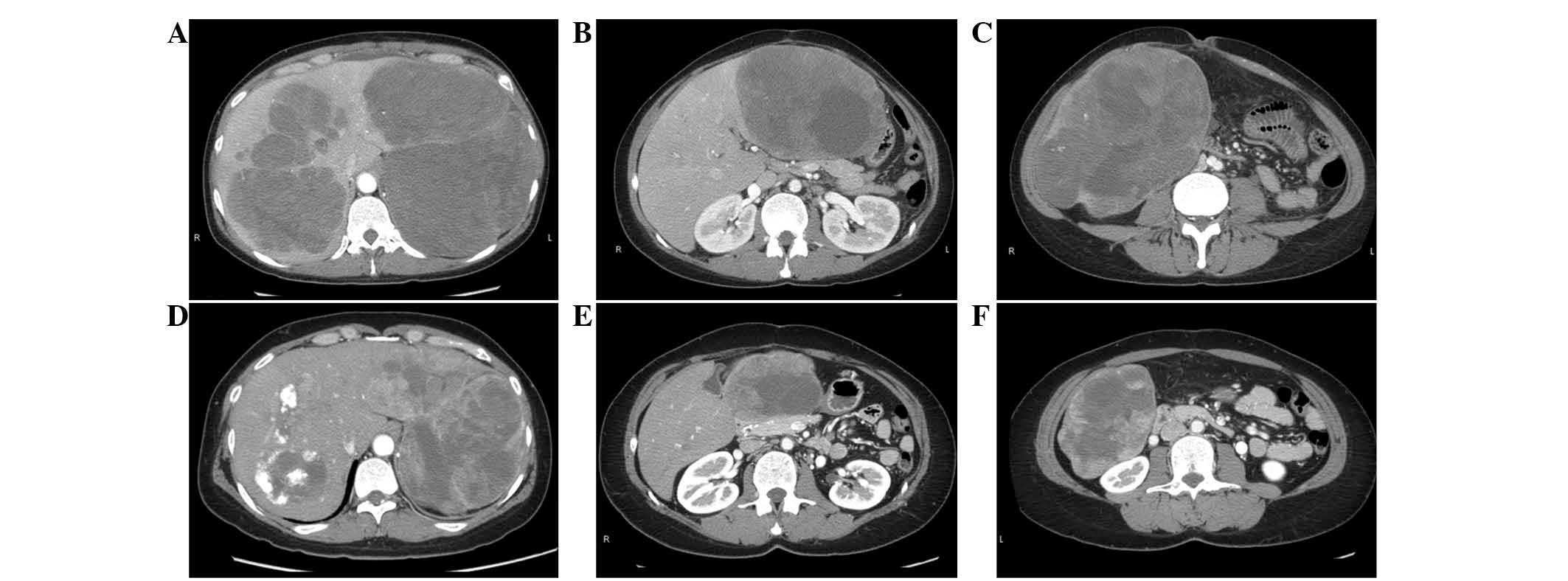
(Fig. 2A). The lateral segment of
the liver was infiltrated with tumors (Fig. 2B), and the 15-cm diameter tumor had
been growing suspended from the posterior segment of the liver
(Fig. 2C). Additionally, large
tumors were identified in the right subphrenic area in the
anteroposterior segment of the liver. Complete surgical resection
of the tumors was considered to be impossible, as the tumors were
located in close proximity to the major Glisson sheath (Fig. 2A). Examination with
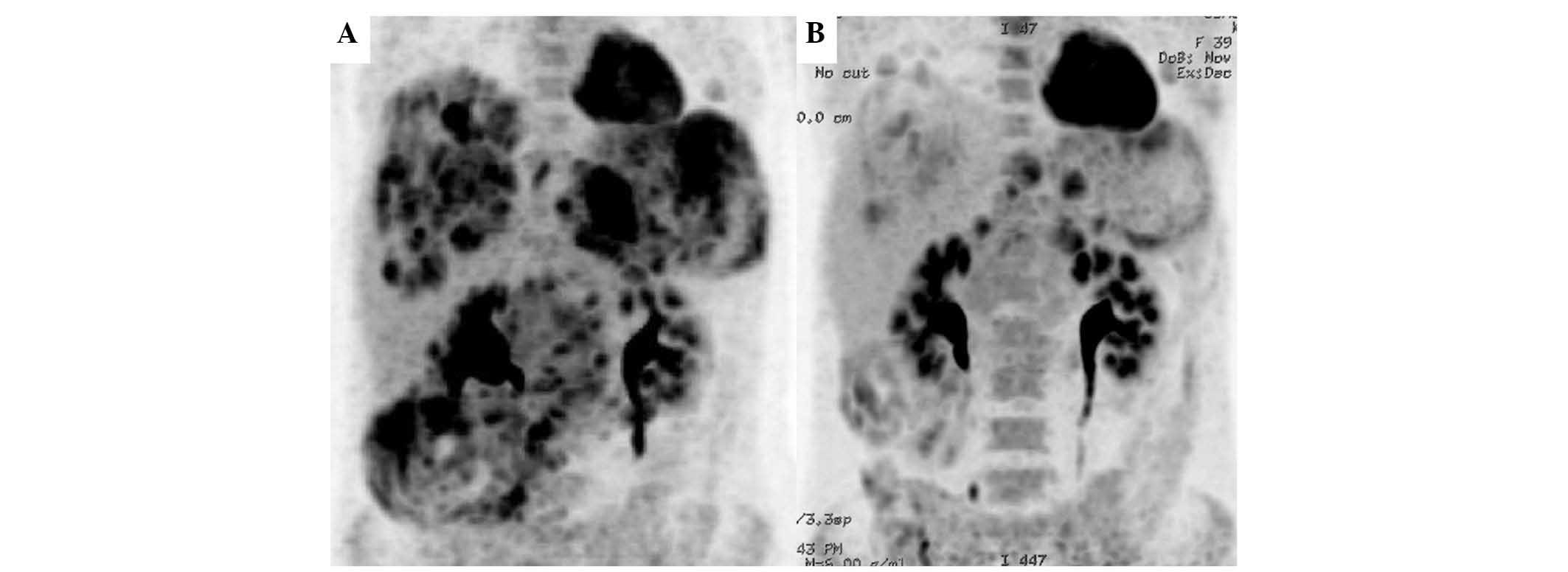
18-fluorodeoxyglucose positron emission tomography (FDG-PET)
revealed all of the tumors to have high FDG uptake, with maximum
standardized uptake values (SUV max) of 7.7–8.8 (Fig. 3A).
As it was impossible to treat the subphrenic local
recurrent tumor with HAI chemotherapy, systemic chemotherapy was
used in combination. In October 2011, oral S-1 (80
mg/m2) and HAI with GEM (1,000 mg/standard liver volume)
were initiated as described in a previous report (7). Following 18 cycles of HAI
chemotherapy, the tumors exhibited a 26.3% reduction in size
(Fig. 2D–F). Although this result
was determined to be stable disease according to the Response
Evaluation Criteria in Solid Tumors guidelines (8), FDG-PET examination revealed obvious
reduction of tumoral FDG uptake, and the SUV max was 5.4 in the
lesion with the highest uptake (Fig.
3B).
While chemotherapy was effective, a complete
resection was not predicted to be successful in this situation, due
to the involvement of the major Glisson sheath. For the right
subphrenic tumor, transcatheter arterial embolization (TAE) was a
viable treatment. However, TAE was likely to cause the other tumors
to rupture due to post-treatment necrosis. Therefore, TAE was
performed for the unresectable right subphrenic liver tumor
occupying the anteroposterior segment, and the other tumors were
surgically resected once the patient’s condition stabilized
post-TAE.
The resected tumors were diagnosed as liver
metastases and a local recurrence of SPT on postoperative
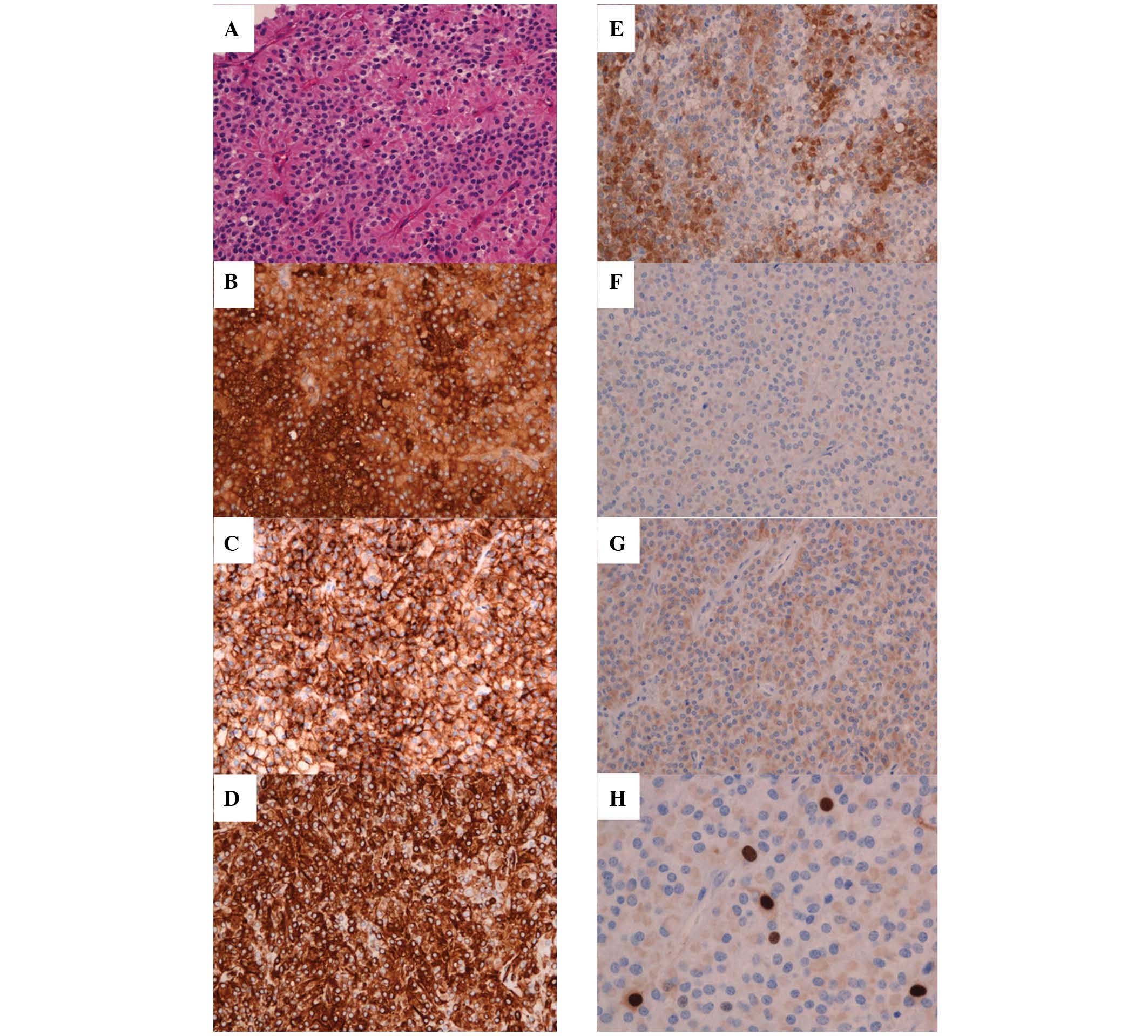
pathological examination. The tumor specimen was a highly vascular
lesion composed of sheets of bland cells with oval to round nuclei,
moderate cytoplasm, ill-defined cell borders, and a focal
pseudopapillary appearance (Fig.
4A). On immunohistochemical analysis, the tumor cells reacted
positively for CD10, CD56, vimentin, NSE, and α-antitrypsin,
however, the Ki-67 index was low (1–2%; Fig. 4B–H). Electron microscopy was
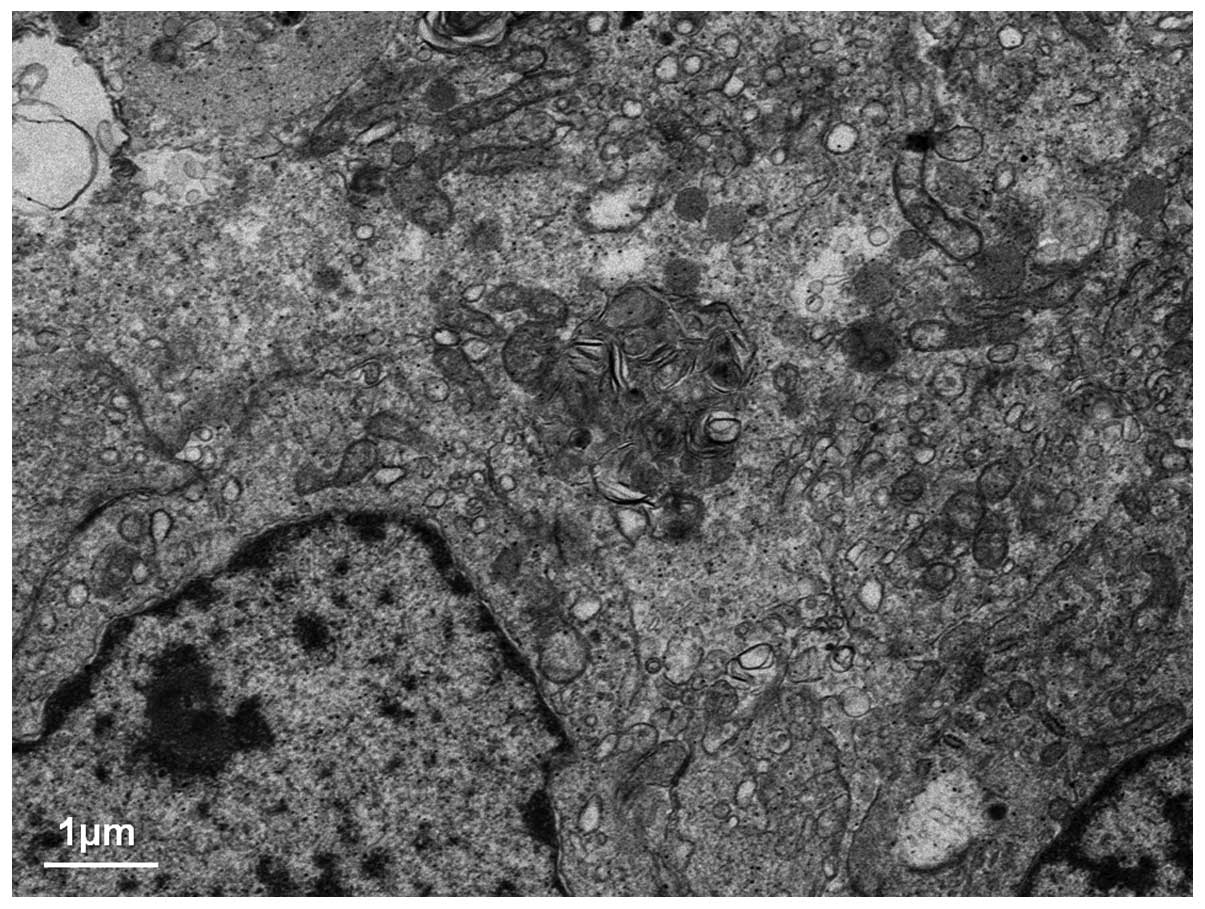
performed to characterize the nature of the cytoplasmic vacuoles
that appeared to be dilated or distended mitochondria; the
remaining fractions appeared to be smooth endoplasmic reticulum,
consistent with a previous report (Fig.
5). In a number of the small vacuoles, a few cristae could
still be identified as mitochondrial, and a gradual transition from
the normal mitochondria to those with attenuation of cristae was
observed as well as loss of matrix.
The postoperative course was uneventful, and the
patient is currently under monthly observation at an outpatient
clinic and receiving adjuvant chemotherapy with oral S-1 (100 mg,
every other day).
Discussion
SPTs of the pancreas are uncommon low-malignant
epithelial tumors that are typically identified in adolescent
females. Metastatic disease is rare and only occurs in around
10–15% of patients (9–11). Previous studies have indicated the
most frequently observed metastatic sites to be the liver and
omentum (12). Resection of the
primary pancreatic tumors or liver metastases has yielded excellent
survival with an overall cure rate of >90% (13–16).
The clinical features of SPT are non-specific and are often caused
by compression from the tumor. Abdominal pain or discomfort is the
most common symptom, followed by back pain, nausea, vomiting,
weight loss, and diarrhea (1). A
number of patients present with jaundice, upper gastrointestinal
bleeding, or other rare symptoms; however, a considerable number of
patients exhibit no symptoms, and SPT is identified incidentally
during physical examination or on ultrasound, CT, or other imaging
examinations (1). Although
resection of the tumor yields a five-year survival rate of 97%,
local recurrence or distant metastases occur in 10–15% of patients
(1).
The primary morphological differential diagnosis for
SPT is pancreatic neuroendocrine tumor. Traditionally, negative
staining for neuroendocrine markers, in particular chromogranin A,
has been considered crucial to this distinction. However, a recent
study demonstrated aberrant nuclear staining for β-catenin and a
loss of membranous expression of E-cadherin with aberrant nuclear
localization of the cytoplasmic domain in all pancreatic SPT cases
analyzed (17). In this study, the
majority of pancreatic SPTs were also strongly positive for
vimentin (100%), β-catenin nuclear stain (100%), CD10 (96%),
progesterone receptor (79%), CD56 (75%), cytokeratin (28%),
synapthophysin (26%), and chromogranin A (15%) (17). In the present case, CD10, CD56, and
vimentin were strongly positive.
At present, no consistent clinical or histological
criteria has been established to predict the biological behavior of
SPT. Invasion of blood vessels, peritoneal infiltration, invasion
of adjacent structures, a high degree of cellular polymorphism, and
an elevated mitotic rate are characteristics proposed to be
associated with metastases and recurrence. However, the absence of
these features does not preclude malignant behavior. In the present
case, the patient exhibited multiple liver metastases at initial
presentation in the absence of malignant histological behavior.
The current study demonstrates that SPT is an
indolent tumor with an excellent prognosis and that surgical
resection is the mainstay of treatment, even in the presence of
local invasion and extrapancreatic involvement. Indeed, involvement
of the surgical margins (R1) does not appear to be associated with
a poor outcome. In previously reported studies, ≤20% of the cases
have exhibited liver metastases at the time of resection, however,
the overall five-year survival rate remains >95% (11). In the present patient, as the liver
metastases were diffuse, radical excision of these metastases was
not possible. Therefore, combination therapy with TAE and
chemotherapy was selected for treatment of the remnant liver
tumor.
The value of chemotherapy for patients with SPT
remains unknown, however, a number of anecdotal studies have
reported its benefit (13,18–20)
and lack of benefit (9,21,22).
Two cases involving resectable tumors were subjected to
chemotherapy with cisplatin and 5-fluorouracil (5-FU) (19) or GEM (20), but in the latter case, previous
treatment with 5-FU and radiation had failed to decrease the tumor
size. Radiotherapy is occasionally used for the treatment of
unresectable tumors or as an adjuvant treatment following tumor
resection (13). Two cases of
radiosensitivity in unresectable tumors have been reported
(23,24). Kanter et al reported the
advantages of neoadjuvant chemotherapy with GEM for a large SPT
(25). In another report, a patient
who presented with a large SPT arising from the pancreatic body and
tail (with gastric wall infiltration and para-aortic
lymphadenopathy) was treated with GEM and cisplatin. During this
therapy, the tumor regressed by >50%, with disappearance of the
para-aortic lymphadenopathy and posterior gastric wall
infiltration; the patient subsequently underwent full surgical
resection (26).
In the current patient, oral S-1 and HAI with GEM
were administered, which resulted in the reduction of tumor size on
CT and an obvious reduction of FDG uptake on FDG-PET analysis. It
was originally proposed that S-1 and GEM had been invalidated as
treatment options based on their ineffective use at the previous
hospital, however, it is possible that a sufficient amount had not
been administered due to patient complaints and side effects. In
the Department of Gastroenterologic Surgery at the Graduate School
of Medicine, combination treatment with oral S-1 and HAI using GEM
was effective. However, there is a possibility that oral S-1
administration alone was effective in treating the local recurrence
at the left diaphragm, which was not reached by the arterial
infusion of GEM but nonetheless decreased slightly in size.
Therefore, oral S-1 was used for postoperative adjuvant
chemotherapy.
Transarterial tumor embolization and transcatheter
arterial chemoembolization are seldom used to treat SPT or similar
pancreatic neoplasms (21,22,27).
Among previous cases, one patient experienced a significant
reduction in metastases of the right lobe (21), one succumbed to the disease
following the procedure (27), and
one patient’s disease remained unchanged (22). Radiofrequency ablation (RFA) is also
a seldom-used modality (22,28,29).
RFA is a safe and effective treatment for multiple unresectable
liver metastases of SPT (29).
However, incomplete RFA may induce dedifferentiation and
epithelial-mesenchymal transition of the tumor (30,31).
Therefore, it is advisable to limit the use of RFA to unresectable
and small lesions.
In the current study, TAE was performed for residual
liver tumors prior to surgical resection. Oral S-1 was subsequently
administered as postoperative chemotherapy, and additional TAE is
planned. In the future, if the central region of the liver
enlarges, surgery for the residual right lobe tumor may become
possible.
In conclusion, this case demonstrates one method of
treatment for SPT: Preoperative chemotherapy with GEM HAI in
combination with oral S-1 and TAE. If complete resection can be
achieved, the majority of patients with SPT have a favorable
prognosis. In patients with unresectable metastases from SPT, it is
crucial to perform systematic multimodal treatment, with a
combination of surgery, chemotherapy and interventional radiology,
to maximize treatment success.
References
|
1
|
Sperti C, Berselli M, Paasquali C,
Pastorelli D and Pedrazzoli S: Aggressive behavior of
solid-pseudopapillary tumor of pancreas in adults: a case report
and review of the literature. World J Gastroenterol. 14:960–965.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frantz VK: Tumor of the pancreas. blumerg
CW: atlas of tumor pathology Washington, DC: US Armed Forces
Institute of Pathology; pp. 32–33. 1959
|
|
3
|
Cantisani KM, Mortele KJ, Levy A, et al:
MR imaging features of solid pseudopapillary tumor of the pancreas
in adult and pediatric patients. AJR Am J Roentogenol. 181:395–401.
2003. View Article : Google Scholar
|
|
4
|
Coleman KM, Doherty MC and Bigler SA:
Solid-pseudopapillary tumor of the pancreas. Radiographics.
23:1644–1648. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vargas-Serrano B, Dominguez-Ferreras E and
Chinchon-Espino D: Four cases of solid pseudopapillary tumor of
pancreas: imaging findings and pathological correlations. Eur J
Radiol. 58:132–139. 1993. View Article : Google Scholar
|
|
6
|
Kloppel G, Solcia E, Longnecker DS, et al:
Histological typing of tumors of the exocrine pancreas. sobin LH:
world health organization international histological classification
of rumors. 2nd ed. Berlin, Heidelberg, New York: Springer; pp.
15–22. 1996
|
|
7
|
Tajima H, Ohta T, Kitagawa H, et al: Pilot
study of hepatic arterial infusion chemotherapy with gemcitabine
and 5-fluorouracil for patients with postoperative liver metastases
from pancreatic cancer. Exp Ther Med. 2:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
9
|
Martin RC, Klimstra DS, Brennan MF and
Conlon KC: Solid pseudopapillary tumor of the pancreas: a surgical
enigma? Ann Surg Oncol. 9:35–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pettinato G, Manivel JC, Ravetto C, et al:
Papillary cystic tumor of the pancreas. a clinicopathologic study
of 20 cases with immunohistochemical, ultrastructual and flow
cytometric observations and a review of the literature. Am J Clin
Pathol. 98:478–488. 1992.PubMed/NCBI
|
|
11
|
Tang LH, Aydin H, Brennaan MF and Klimstra
DS: Clinically aggressive solid pseudopapillary tumor of the
pancreas: a report of two cases with components of undifferentiated
carcinoma and a comparative clinicopathologic analysis of 34
conventional cases. Am J Surg Pathol. 29:512–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao C, Guvendi M, Demenico DR, Kim K,
Thomford NR and Howard JM: Papillary cystic and solid tumors of the
pancreas: a pancreatic embryonic tumor? studies of three cases and
cumulative review of the worlds’ literature. Surgery. 118:821–828.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsunou H and Konishi F: Papillary-cystic
neoplasm of the pancreas. A clinicopathologic study concerning the
tumor aging and malignancy of nine cases. Cancer. 65:283–291. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goh BK, Tan YM, Cheow PC, et al: Solid
pseudopapillary neoplasms of the pancreas: an updated experiences.
J Surg Oncol. 95:640–644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda N, Nagakawa T, Ohta T, et al:
Clinicopathological studies on solid and cystic tumors of the
pancreas. Gastroenterol Jpn. 26:497–502. 1991.PubMed/NCBI
|
|
16
|
Salvia R, Bassi C, Festa L, et al:
Clinical and biological behavior of pancreatic solid
pseudopapillary tumors: report on 31 consecutive patients. J Surg
Oncol. 95:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen QN, Johns LA, Gill JA, et al:
Clinical and immunohistochemical features of 34 solid
pseudopapillary tumors of the pancreas. J Gastroenterol Hepatol.
26:267–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimizu M, Matsumoto T, Hirokawa M, Monobe
Y, Iwamoto S, Tsunoda T and Manabe T: Solid-pseudopapillary
carcinoma of the pancreas. Pathol Int. 49:231–234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strauss JF, Hirsch VJ, Rubey CN and
Pollock M: Resection of a solid and papillary epithelial neoplasm
of the pancreas following treatment with CIS-platinum and
5-fluorouracil: a case report. Med Pediatr Oncol. 21:365–367. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maffuz A, Bustamante FT, Silva JA and
Torres-Vargas S: Preoperative gemcitabine for unresectable solid
pseudopapillary tumor of the pancreas. Lancet Oncol. 6:185–186.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuda Y, Imai Y, Kawata S, Nisikawa M,
Miyoshi S, Saito R, Minami Y and Tarui S: Papillary-cystic neoplasm
of the pancreas with multiple hepatic metastases: a case report.
Gastroenterol JPN. 22:379–384. 1987.PubMed/NCBI
|
|
22
|
Kang CM, Kim KS, Choi JS, Kim H, Lee WJ
and Kim BR: Solid pseudopapillary tumor of the pancreas suggesting
malignant potential. Pancreas. 32:276–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fried P, Cooper J, Balthazar E, Fazzini E
and Newall L: A role for radiotherapy in the treatment of solid and
papillary neoplasms of the pancreas. Cancer. 56:2783–2785. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zauls JA, Dragun AE and Sharma AK:
Intensity-modulated radiation therapy for unresectable solid
pseudopapillary tumor of the pancreas. Am J Clin Oncol. 29:639–640.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanter J, Wilson DB and Strasberg S:
Downsizing to resectability of a large solid and cystic papillary
tumor of the pancreas by single-agent chemotherapy. J Pediatr Surg.
44:E23–E25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das G, Bhuyan C, Das BK, et al:
Spleen-preserving distal pancreatectomy following neoadjuvant
chemotherapy for papillary solid and cystic neoplasm of pancreas.
Indian J Gastroenterol. 23:188–189. 2004.PubMed/NCBI
|
|
27
|
Levy P, Bougaran J and Gayet B: Diffuse
peritoneal carcinomas of pseudo-papillary and solid tumor of the
pancreas. role of abdominal injury. Gastroenterol Clin Biol.
21:789–793. 1997.
|
|
28
|
Huang HI, Shih SC, Chang WH, Wang TE, Chen
MJ and Chan YJ: Solid-pseudopapillary tumor of the pancreas:
clinical experience and literature review. World J Gastroenterol.
11:1403–1409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JX, Wu H, Huang JW, Prasoon P and Zeng
Y: Synchronous intraoperative radiofrequency ablation for multiple
liver metastasis and resection of giant solid pseudopapillary
tumors of the pancreas. Chinese Med J. 125:1661–1663. 2012.
|
|
30
|
Tajima H, Ohta T, Okamoto K, et al:
Radiofrequency ablation induces dedifferentiation of hepatocellular
carcinoma. Oncol Lett. 1:91–94. 2010.PubMed/NCBI
|
|
31
|
Tajima H, Ohta T, Shoji Y, et al:
Expression of epithelial-mesenchymal transition markers in locally
recurrent hepatocellular carcinoma after radiofrequency ablation.
Exp Ther Med. 1:347–350. 2010. View Article : Google Scholar
|