Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent type of cancer worldwide and the fourth most common cause
of cancer-associated mortality (1,2).
Currently, surgical resection and transplantation are the most
effective treatment approaches for HCC (3). However, the recurrence rate within 2
years in patients who have undergone tumor resection remains
>50% (4,5). Uncontrolled tumor metastasis, frequent
intrahepatic spread and extrahepatic metastasis are the primary
causes for the poor prognosis in HCC (6). Therefore, improved understanding of
the molecular mechanisms that underlie HCC invasion and metastasis
is essential for the development of novel therapeutic
strategies.
MicroRNAs (miRNAs) are a class of small non-coding
RNA molecules that negatively regulate the expression of target
genes by mRNA degradation or translational inhibition. Previous
evidence has indicated that the dysregulation of miRNAs may lead to
alterations in diverse biological processes, including
proliferation, differentiation and apoptosis, which are associated
with the development of cancer (7,8).
Several dysregulated miRNAs, including miR-221, miR-21, miR-452,
miR-424 and miR-125b, have been demonstrated to regulate HCC cell
growth, apoptosis, migration and/or invasion (9–13).
However, the role and the underlying molecular mechanisms of
miR-125b in HCC remain largely unknown.
To investigate the possible role of miR-125b in HCC,
the current study investigated miR-125b expression levels in HCC
tissue relative to surrounding healthy tissue. In addition, the
migratory and invasive properties of HCC cells were investigated in
the presence and absence of miR-125b expression.
Materials and methods
Tissue samples, cell lines and cell
transfection
Specimens from HCC and surrounding control tissue
were obtained from 20 patients at Yinzhou People’s Hospital
(Ningbo, China) prior to definitive therapy. The tumor tissues and
adjacent normal tissues were frozen in liquid nitrogen following
resection. Informed consent was obtained from all subjects, and the
study was approved by the review board of the hospital ethics
committee.
Four HCC cell lines (SK-Hep-1, SMMC7721, HepG2 and
Huh7) and a normal liver cell line (L02) were purchased from
American Type Culture Collection (ATCC) and cultured in Dulbecco’s
modified Eagle’s medium (Gibco Life Technologies, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS) with 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C with 5%
CO2.
miR-125b mimics, negative control mimics (NC),
miR-125b inhibitor (anti-miR-125b), negative control inhibitor
(anti-NC) and TAZ short-interfering RNAs (siRNAs) were synthesized
by Shanghai GenePharma Co, Ltd., (Shanghai, China). Transfection
was performed with Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. In brief, for each well, 5 μl mimics, inhibitor or
siRNA (20 μM) was added into 250 μl Opti-MEM medium (Gibco Life
Technologies, Carlsbad, CA, USA), 5 μl of Lipofectamine 2000 into
250 μl Opti-MEM medium, and then mixed mimics, inhibitor or siRNA
with Lipofectamine 2000. The mixture was added to cells and
incubated for 6 h before replacing the medium. Total RNA and
protein were prepared as described below 48 h subsequent to
transfection and were used for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) or western blot analysis.
Plasmid construction and luciferase
reporter assay
This was performed as described in our previous
study (14). In brief, the
wild-type 3′-untranslated (3′UTR) region of TAZ, containing
predicted miR-125b target sites, was amplified by PCR from SK-Hep-1
cell genomic DNA. The primer sequences were as follows: F 5′-GAT
CTG CAG CTC TCC CAG GGG CTG GCT TCA G-3′ and R 5′-GAT CAT ATG GAG
GCA GAA AGG ATG GAG AAG T-3′. The corresponding mutant constructs
were created using the QuikChange site-directed mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA).
The wild-type and mutant 3′UTR fragments were
subcloned into the pGL3-control vector (Promega Corporation,
Madison, WI, USA) immediately downstream of the stop codon of the
luciferase gene. The DNA fragment encoding the TAZ protein was
amplified by PCR from SK-Hep-1 cell cDNA, and cloned into a
pCMV-Myc expression vector (Clontech Laboratories, Mountain View,
CA, USA). The primer sequences were as follows: F 5′-GCT GAA TTC
GAC CTA GAG GCG CCC CAC AGG C-3′ and R 5′-CTG CTC GAG TCT GTG CGG
GCC AAG AAT CCA G-3′. For luciferase assays, the reporter plasmid
was co-transfected with a control Renilla luciferase vector
(Promega Corporation) into SK-Hep-1 cells in the presence of either
miR-125b or NC. After 48 h, cells were harvested and the luciferase
activity was measured using the Dual-Luciferase Reporter Assay
System (Promega Corporation).
RNA extraction and RT-qPCR
RNA extraction and RT-qPCR was performed as
described in our previous study (14). In brief, total RNA was extracted
from the cultured cells and the HCC tissue specimens using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. The expression level of mature
miR-125b was measured by TaqMan miRNA assays (Applied Biosystems
Life Technologies, Foster City, CA, USA) according to
manufacturer’s instructions and normalized against U6 small nuclear
RNA levels. TAZ expression was measured by SYBR green qPCR assay
(Takara Biotechnology Co., Ltd., Dalian, China) and GAPDH was used
as the endogenous control.
Western blot analysis
Western blotting was performed as described in our
previous study (14). In brief,
protein extracts from cells were prepared using a modified RIPA
buffer with 0.5% sodium dodecyl sulfate (SDS) in the presence of
Complete Mini protease inhibitor cocktail (Roche Diagnostics GmbH,
Mannheim, Germany). Polyacrylamide gel electrophoresis in 10% SDS
gels with low voltage (60 V) for separating gel; use higher voltage
(140 V) for stacking gel., tank-based transfer to Immobilon
Hybond-C membranes (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) and immunodetection were performed with standard techniques.
Antibodies were used in western blot analysis in accordance with
the manufacturer’s instructions. In brief, the membrane was
incubated with mouse anti-human TAZ monoclonal antibody (catalog
no. H00006901-M12; Novus Biologicals, Littleton, CO, USA) and mouse
anti-human β actin monoclonal antibody (catalog no. sc-47778,
Beijing Zhongshan Biotechnology; Beijing, China) at 1:1500 dilution
at 37°C for 2 h, and then with peroxidise-conjugated goat
anti-mouse IgG (catalog no. ZB-2305, Beijing Zhongshan
Biotechnology) at 1:2000 at room temperature for 1 h. Signals were
visualized with SuperSignal West Pico Chemiluminescent substrate
(Thermo Fisher Scientific, Inc., Rockford, IL, USA) by exposure to
films.
Wound healing and invasion assays
Cell migration was assessed by wound healing assays.
Cells (2×105 cells/well) were seeded in six-well plates
and cultured to 100% confluence. Wounds were generated in the cell
monolayer using a plastic pipette tip. The cells were then rinsed
with phosphate-buffered saline and cultured for a further 48 h. The
spread of wound closure was observed and images were captured using
a confocal laser scanning microscope (Olympus; Tokyo, Japan) as
described previously (15). For
invasion assays, 2×105 cells were added into the upper
chamber of the insert (6.5 mm in diameter, 8 μm pore size; Corning
Life Sciences, New York, NY, USA) pre-coated with Matrigel (ECM
gel, Sigma-Aldrich, St. Louis, MO, USA). Cells were plated in
medium without serum (Gibco Life Technologies), and medium
containing 10% FBS in the lower chamber served as a
chemoattractant. Following 24 h hours of incubation, the cells that
did not invade through the pores were carefully wiped out with
cotton wool, and the filters were fixed by treatment with 95%
ethanol for 30 min and stained with 0.2% crystal violet solution
(Beyotime; Shanghai, China) for 30 min. Invasive cells adhering to
the undersurface of the filter were counted (5 fields/chamber; 0.24
mm2/field) using an inverted microscope as described in
our previous study (14), and each
experiment was repeated three times.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Data from
three independent experiments are expressed as the mean ± standard
deviation. Differences were assessed by two-tailed Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miR-125b is reduced in HCC
tissues and cell lines
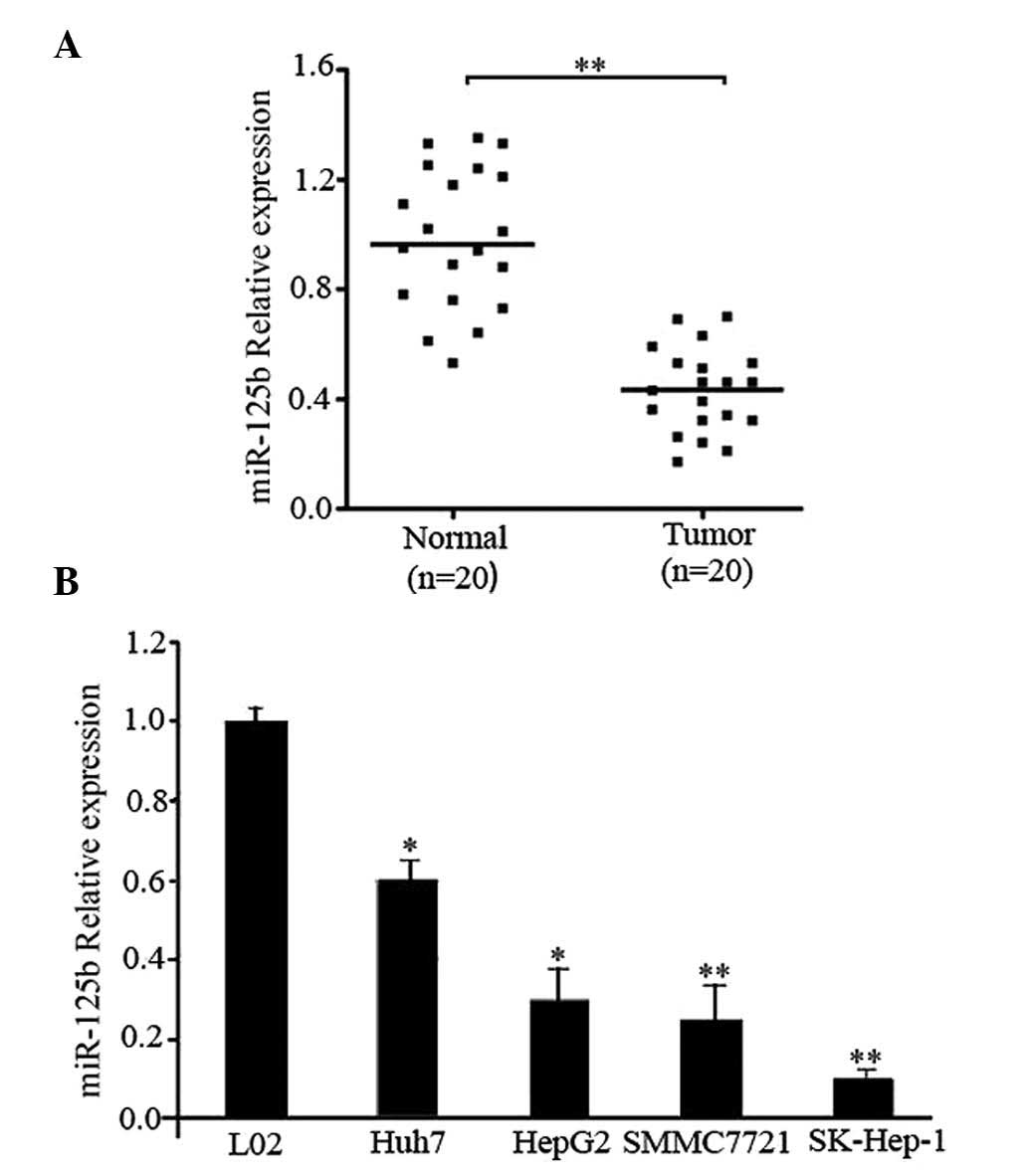
In order to study the expression of miR-125b and its
significance in HCC carcinogenesis, expression levels of miR-125b
were measured in 20 pairs of HCC tissue samples and their
corresponding control liver tissues using RT-qPCR. The results
indicated that miR-125b expression was significantly reduced in HCC
tissues compared with the normal tissues (P<0.01; Fig. 1A). In addition, the expression of
miR-125b in the four HCC cell lines was determined. As presented in
Fig. 1B, the relative expression
levels of miR-125b in the four HCC cell lines were significantly
reduced compared with that of the healthy liver cell line, L02
(Huh7 and HepG2, P<0.05 vs. L02 cells; SMMC7721 and SK-Hep-1,
P<0.01 vs. L02 cells). These results suggest that the
downregulation of miR-125b may be involved in HCC
carcinogenesis.
miR-125b suppresses HCC cell migration
and invasion in vitro
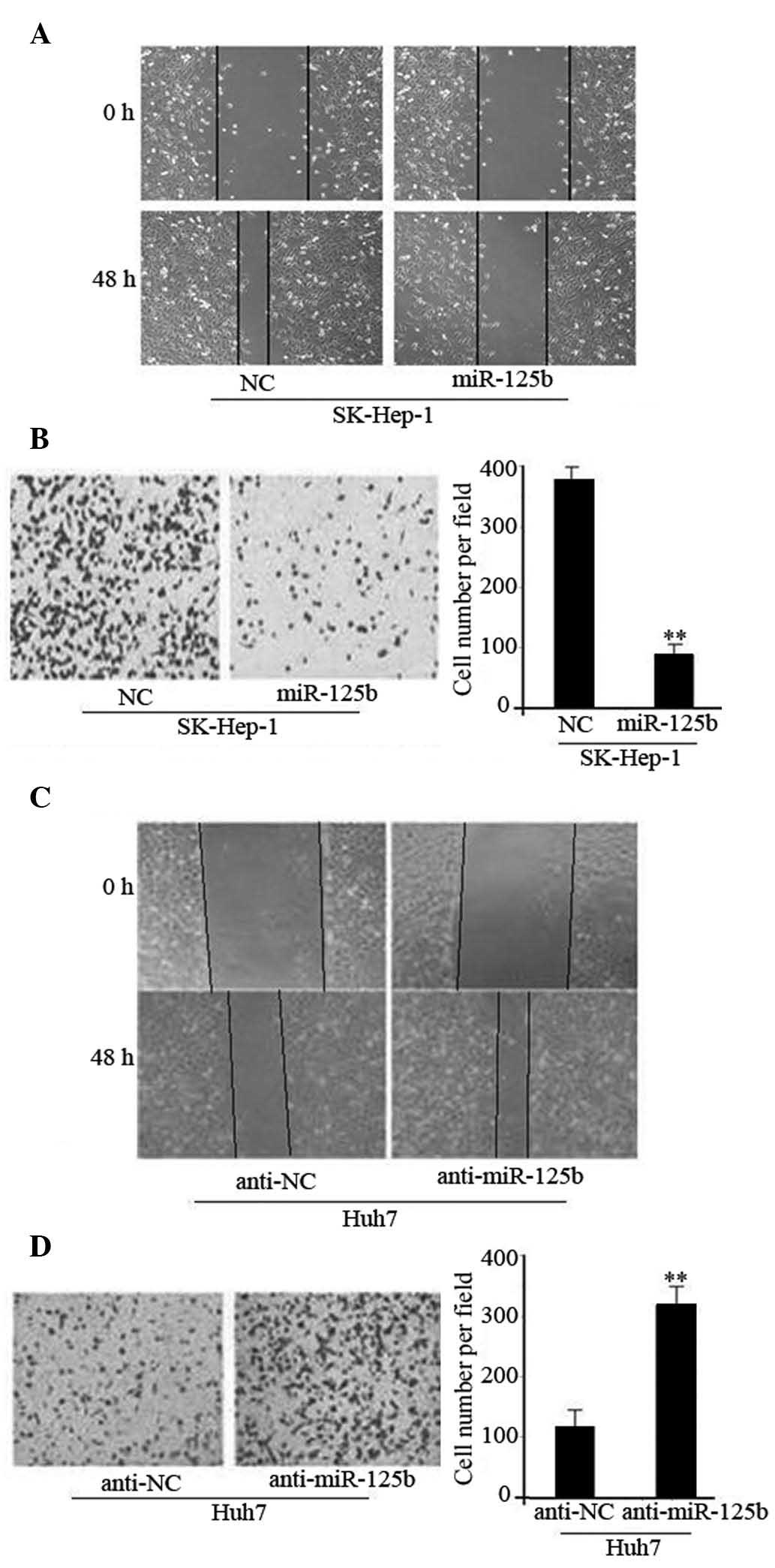
In order to investigate the function of miR-125b in
cell migration and invasion, miR-125b was overexpressed using miRNA
mimics in the SK-Hep-1 HCC cell line; then a wound healing assay
was performed. As presented in Fig.
2A, overexpression of miR-125b leads to the suppression of
tumor cell mobility in the SK-Hep-1 cells compared with the
corresponding controls. Furthermore, Transwell assays indicated
that miR-125b significantly reduced the invasive capacity of
SK-Hep-1 cells (P<0.01; Fig.
2B). By contrast, the wound healing and invasion of Huh7 cells
was increased following the silencing of endogenous miR-125b using
anti-miR-125b (P<0.01; Fig. 2C and
D). Together, these results imply that miR-125b can suppress
HCC cell migration and invasion in vitro.
miR-125b downregulates TAZ by directly
targeting its 3′UTR
To investigate the molecular mechanism of miR-125b,
bioinformatic algorithms (TargetScan 6.2, www.targetscan.org; and PicTar, pictar.mdc-berlin.de)
were used to predict a large number of potential miR-125b target
genes. Among them, TAZ was identified to possess a putative
miR-125b binding site within its 3′UTR (Fig. 3A). To verify whether TAZ is the
direct downstream target of miR-125b, a fragment of TAZ 3′UTR
containing the putative miR-125b binding site was cloned into a
luciferase reporter vector. The luciferase reporter assay indicated
that the upregulation of miR-125b significantly inhibited the
relative luciferase activity of TAZ 3′UTR in SK-Hep-1 cells, but
did not significantly inhibit the mutant TAZ 3′UTR (Fig. 3B). In addition, RT-qPCR and western
blot analysis demonstrated that the overexpression of miR-125b
substantially reduced the expression of TAZ in SK-Hep-1 cells, and
that knockdown of miR-125b increased TAZ expression in Huh7 cells
(Fig. 3C and D). These results
indicate that TAZ is a direct target of miR-125b in HCC cells.
TAZ is involved in miR-125b-induced
suppression of HCC cell invasion
To determine whether TAZ acts as a critical mediator
of miR-125b in HCC cells, a specific siRNA against TAZ was used to
knockdown TAZ expression (siTAZ). As presented in Fig. 4A, si-TAZ significantly reduced the
expression levels of TAZ protein and suppressed SK-Hep-1 cell
invasion (P<0.01). To determine whether forced expression of TAZ
is able to rescue the suppressive effect of miR-125b, SK-Hep-1
cells were co-transfected with miR-125b and TAZ plasmids lacking
the 3′UTR region. The results indicated that forced expression of
TAZ significantly rescued the inhibition of miR-125b-induced cell
invasion (P<0.01; Fig. 4B).
Taken together, these results indicate that miR-125b regulates HCC
invasion at least in part by downregulating TAZ.
Discussion
In the present study, the expression levels of
miR-125b in HCC tissues and cell lines were measured, and the
biological functions and regulatory mechanisms of miR-125b in
tumorigenesis were investigated. miR-125b was downregulated in HCC
tissues and cell lines and was able to inhibit cell invasion via
the regulation of TAZ expression. These findings indicate that
miR-125b is a notable tumor suppressor in HCC.
miR-125b is an miRNA that is expressed in neurons
and astrocytes in the brain (16).
The role of miR-125b in malignancies is controversial: miR-125b
acts as a tumor suppressor in breast cancer, ovarian carcinoma and
hepatocellular carcinoma (17–19,
13) and miR-125b expression is
associated with an improved clinical outcome in liver cancer
patients (20). However, in
prostate cancer cells miR-125b has been demonstrated to act as an
oncogene that promotes proliferation and contributes to prostate
cancer pathogenesis (21). miR-125b
has also been reported to negatively regulate the tumor-suppressor
gene p53, and suppress p53-dependent apoptosis in zebrafish and
humans (22). Consistent with
previous findings in HCC (13), the
functional studies presented in the current study indicated that
overexpression of miR-125b significantly suppresses HCC cell
migration and invasion in vitro.
The present study examined the molecular mechanism
by which miR-125b suppresses HCC cell migration and invasion, and
TAZ was identified as a direct target of miR-125b. TAZ, also termed
WW domain containing transcriptional regulator 1 (WWTR1), is a WW
domain-containing transcriptional coactivator that activates
numerous transcriptional factors that serve important roles in the
development of various tissues in mammals (23). TAZ has also been demonstrated to
regulate stem cell differentiation and renewal through modulation
of the transcription factors peroxisome proliferator-activated
receptor-γ (PPARγ) and runt-related transcription factor 2 (Runx2),
and a number of members of the SMAD gene family (24,25).
In a previous study, elevated TAZ expression was observed in
>20% breast cancer samples, particularly in invasive ductal
carcinomas (26), which implicates
TAZ in metastasis and suggests that it may increase the malignancy
of breast cancer. Additionally, Zhou et al (27) reported that TAZ is overexpressed in
non-small-cell lung carcinoma (NSCLC), and knockdown of TAZ
significantly impaired the tumorigenic ability of the NSCLC cells.
To the best of our knowledge, the present study is the first to
demonstrate that knockdown of TAZ mimics the overexpression of
miR-125b in HCC cells by suppressing invasion. Forced expression of
TAZ rescued the suppressive effect of miR-125b in vitro,
suggesting that miR-125b overexpression or siRNA-mediated
downregulation of the target gene TAZ is a potential HCC
therapy.
In conclusion, the present study demonstrated that
miR-125b is significantly downregulated in HCC tissues and cell
lines, and that forced overexpression of miR-125b in HCC cells
suppressed cell invasion and migration partly through the
suppression of TAZ. This finding aids the understanding of the
underlying molecular mechanism of HCC carcinogenesis and provides a
strong rationale to investigate whether miR-125b may act as a
potential biomarker and therapeutic target for HCC in future
studies.
Acknowledgements
This study was supported by Ningbo Municipal Medical
Science and Technique Foundation (grant no. 2013A30), Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY14H160002) and Zhejiang Provincial Medicine and Health Science
Research Foundation of China (grant no. 2014KYB248).
References
|
1
|
Mínguez B and Lachenmayer A: Diagnostic
and prognostic molecular markers in hepatocellular carcinoma. Dis
Markers. 31:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishida N and Goel A: Genetic and
epigenetic signatures in human hepatocellular carcinoma: a
systematic review. Curr Genomics. 12:130–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng KK, Lo CM, Liu CL, Poon RT, Chan SC and
Fan ST: Survival analysis of patients with transplantable recurrent
hepatocellular carcinoma: implications for salvage liver
transplant. Arch Surg. 143:68–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim do Y, Paik YH, Ahn SH, Youn YJ, Choi
JW, Kim JK, Lee KS, Chon CY and Han KH: PIVKA-II is a useful tumor
marker for recurrent hepatocellular carcinoma after surgical
resection. Oncology. 72(Suppl 1): 52–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Zhang JB, Qin Y, Wang W, Wei L, et
al: PROX1 promotes hepatocellular carcinoma metastasis by way of
up-regulating hypoxia-inducible factor 1α expression and protein
stability. Hepatology. 58:692–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahara H, Kay MA, Yasui W and Tahara E:
MicroRNAs in Cancer: the 22nd Hiroshima Cancer Seminar/the 4th
Japanese Association for RNA Interference Joint International
Symposium, 30 August 2012, Grand Prince Hotel Hiroshima. Jpn J Clin
Oncol. 43:579–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan Q, Loya K, Rani B, Möbus S,
Balakrishnan A, et al: MicroRNA-221 overexpression accelerates
hepatocyte proliferation during liver regeneration. Hepatology.
57:299–310. 2013. View Article : Google Scholar
|
|
10
|
Qiu X, Dong S, Qiao F, Lu S, Song Y, Lao
Y, Li Y, et al: HBx-mediated miR-21 upregulation represses
tumor-suppressor function of PDCD4 in hepatocellular carcinoma.
Oncogene. 32:3296–3305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng Q, Sheng Q, Jiang C, Shu J, et al:
MicroRNA-452 promotes tumorigenesis in hepatocellular carcinoma by
targeting cyclin-dependent kinase inhibitor 1B. Mol Cell Biochem.
389:187–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu L, Ding GF, He C, Sun L, Jiang Y and
Zhu L: MicroRNA-424 is down-regulated in hepatocellular carcinoma
and suppresses cell migration and invasion through c-Myb. PLoS One.
9:e916612014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alpini G, Glaser SS, Zhang JP, Francis H,
Han Y, Gong J, Stokes A, et al: Regulation of placenta growth
factor by microRNA-125b in hepatocellular cancer. J Hepatol.
55:1339–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Wang Y, Song Y, Fu Z and Yu W:
miR-27a regulates cisplatin resistance and metastasis by targeting
RKIP in human lung adenocarcinoma cells. Mol Cancer. 13:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Y, Shen Y, Xue L and Fan H: miR-140
suppresses tumor growth and metastasis of non-small cell lung
cancer by targeting insulin-like growth factor 1 receptor. PLoS
One. 8:e736042013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smirnova L, Gräfe A, Seiler A, Schumacher
S, Nitsch R and Wulczyn FG: Regulation of miRNA expression during
neural cell specification. Eur J Neurosci. 21:1469–1477. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Yan LX, Wu QN, Du ZM, Chen J,
Liao DZ, et al: miR-125b is methylated and functions as a tumor
suppressor by regulating the ETS1 proto-oncogene in human invasion
breast cancer. Cancer Res. 71:3552–3562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, et al: MicroRNA expression profiles in serous ovarian
carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scott GK, Goga A, Bhaumik D, Berger CE,
Sullivan CS and Benz CC: Coordinate suppression of ERBB2 and ERBB3
by enforced expression of microRNA miR-125a or miR-125b. J Biol
Chem. 282:1479–1486. 2007. View Article : Google Scholar
|
|
20
|
Li W, Xie L, He X, Li J, Tu K, Wei L, et
al: Diagnostic and prognostic implications of microRNAs in human
hepatocellular carcinoma. Int J Cancer. 123:1616–1622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu
M, et al: An androgen-regulated miRNA suppresses Bak1 expression
and induces androgen-independent growth of prostate cancer cells.
Proc Natl Acad Sci USA. 104:19983–19988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B,
Korzh V, et al: MicroRNA-125b is a novel negative regulator of p53.
Genes Dev. 23:862–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Degerny C, Xu M and Yang XJ: YAP,
TAZ, and Yorkie: a conserved family of signal-responsive
transcriptional coregulators in animal development and human
disease. Biochem Cell Biol. 87:77–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong JH, Hwang ES, McManus MT, Amsterdam
A, Tian Y, Kalmukova R, et al: TAZ, a transcriptional modulator of
mesenchymal stem cell differentiation. Science. 309:1074–1078.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varelas X, Sakuma R, Samavarchi-Tehrani P,
Peerani R, et al: TAZ controls Smad nucleocytoplasmic shuttling and
regulates human embryonic stem-cell self-renewal. Nat Cell Biol.
10:837–848. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chan SW, Lim CJ, Guo K, Ng CP, Lee I,
Hunziker W, Zeng Q and Hong W: A role for TAZ in migration,
invasion, and tumorigenesis of breast cancer cells. Cancer Res.
68:2592–2598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Z, Hao Y, Liu N, et al: TAZ is a
novel oncogene in non-small cell lung cancer. Oncogene.
30:2181–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|