Introduction
A malignant tumor of the ovary with trophoblastic
differentiation may be a gestational or non-gestational
choriocarcinoma and may be a primary tumor or a metastasis from
other organs. Predominantly, non-gestational choriocarcinoma occurs
as a component of a mixed germ cell tumor (1,2). Pure
non-gestational choriocarcinoma is an extremely rare primary germ
cell neoplasm, with reports of only a few cases at the time of the
present case (3,4). Pure choriocarcinoma has been defined as
a tumor that does not include other germ cell tumor elements
(1). It is necessary to distinguish
between cases of non-gestational or gestational choriocarcinoma of
the ovary, as cases of non-gestational choriocarcinoma have a
poorer prognosis compared with gestational cases, and the treatment
regimen for the two diseases differs. In general, the
non-gestational type has only been clinically diagnosed in patients
who were sexually immature, unable to conceive or had never had
sexual intercourse (5). In the
absence of clinical information, it is difficult to differentiate
the two diseases morphologically. The current study reports a rare
case of non-gestational pure choriocarcinoma in a postmenarcheal
young female and describes details of the tumor, including the
clinicopathological findings. Furthermore, a differential diagnosis
between non-gestational and gestational choriocarcinoma of the
ovary, based on immunohistochemical differences, is discussed.
Consent was obtained from the family of the patient.
Case report
In June 2004, a 10-year-old Japanese female
presented to Dokkyo University School Hospital (Tochigi, Japan)
with abdominal pain and a mass. The patient had experienced
diarrhea alternating with constipation and low-grade fever for the
previous two months. Furthermore, weight loss of 8 kg had been
exhibited over the previous three months, with decreased food
intake. The patient experienced the first menarche six months prior
to presenting, and the periods of menstruation were irregular. The
medical history and the family history were non-contributory.
Upon physical examination, an uneven, firm mass (20
cm in diameter) was palpable in the abdomen. The remaining systemic
examination was unremarkable. The serum β-human chorionic
gonadotropin (hCG) and serum α-fetoprotein levels were 6,600 ng/ml
(normal, <0.1 ng/ml) and <5 ng/ml (normal, <10 ng/ml),
respectively. Magnetic resonance imaging showed the pelvic mass,
which exhibited high intensity with a septal structure on
T1-weighted images and irregular high intensity on T2-weighted
images, with peripheral enhancement for
gadolinium-diethylenetriamine pentaacetic acid.
The patient was diagnosed as having an ovarian tumor
and underwent a celiotomy. Intraoperatively, bloody ascitic fluid
was observed in the peritoneal cavity, together with a dark red
fudge-like and fragile 18×15×10-cm mass, weighing 1,110 g and
involving the right ovary. A small number of tumor nodules were
disseminated in the Douglas pouch. The intraperitoneal fudge-like
mass was macroscopically resected by right
salpingo-oophorectomy.
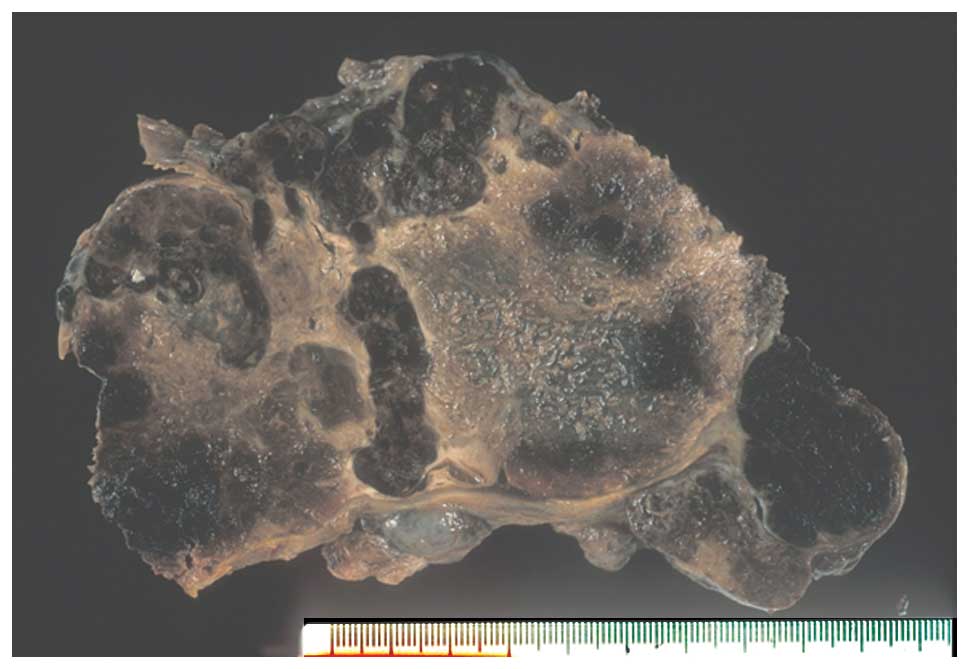
Upon gross inspection, the mass predominantly
consisted of hemorrhagic coagulation necrosis, however, viable
areas were observed in certain regions of the tumor (Fig. 1). Histological specimens were randomly
prepared from 80 sections taken from various sites, followed by
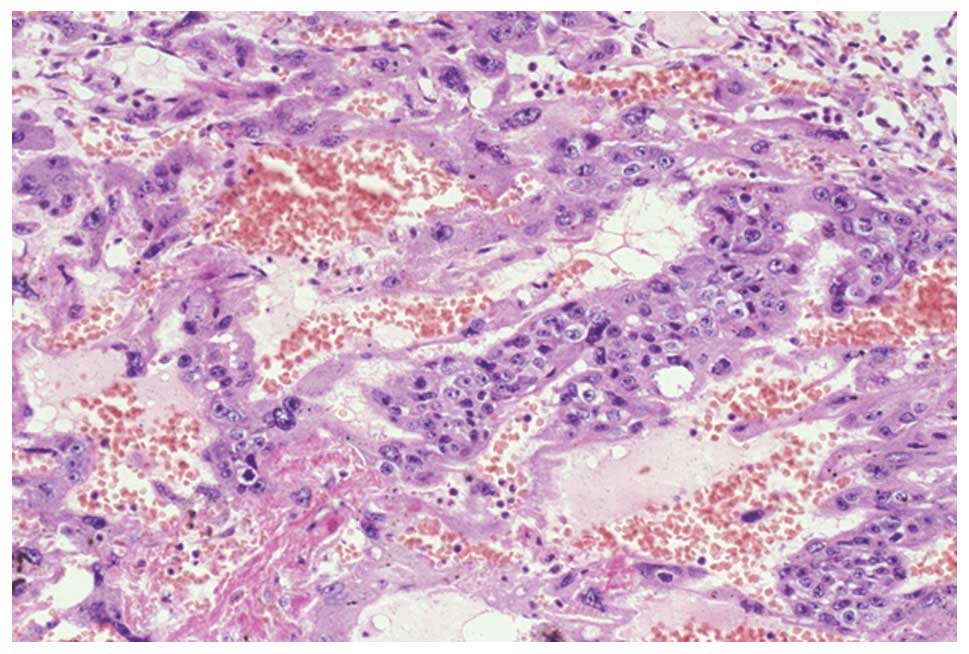
routine staining and immunohistochemistry. Microscopically, viable
tumor cells were present in the periphery of the mass and were
aggregated with one another. The tumor cells were characterized by
an intimate admixture of two cell types, mononuclear and
multinuclear giant cells, which occasionally exhibited a plexiform
pattern. The mononuclear cells were medium in size, polygonal or
round, with clear or amphophilic cytoplasm, and exhibited a
well-defined cell border. The nuclei of these cells were round and
hyperchromatic, with conspicuous nucleoli. Numerous mitotic cells
were observed. These findings indicated cytotrophoblastic cells.
Conversely, the multinuclear giant cells varied in shape and size,
and were irregularly-shaped cells with abundant dense amphophilic
or vacuolated cytoplasm, and multiple hyperchromatic nuclei without
mitosis. These multinuclear cells were regarded as
syncytiotrophoblasts (Fig. 2). No
evidence of other germ cell elements or chorionic stroma was
observed. Immunohistochemically, although positive cytoplasmic
staining was observed in the syncytiotrophoblasts for β-hCG (rabbit
polyclonal antibody; Nichirei, Tokyo, Japan), no reaction was
observed in the cytotrophoblasts. Additionally, positive staining
was observed for epithelial markers, such as cytokeratin AE1/AE3
(AE1+AE3; Dako, Carpinteria, CA, USA) and epithelial membrane
antigen (E29; Dako) in the two cell types. Furthermore, the cells
were also histochemically positive for the β2-microglobulin (BMG)
antibody (rabbit polyclonal antibody; Dako) (Fig. 3).
One week after surgery, the patient received three
cyles, each lasting three weeks, of chemotherapy: cisplatin (33
mg/m2, days 1–3), etoposide (167 mg/m2, days
1–3) and bleomycin (15 U/m2, day 1) (PEB). The
post-therapeutic period was favorable, with no major complications.
The serum hCG level returned to normal (0.1 ng/ml) five months
after the PEB chemotherapy. Furthermore, the patient is currently
disease-free without evidence of recurrence or metastasis
subsequent to 62 months of follow-up.
Discussion
Two forms of ovarian choriocarcinoma, gestational
and non-gestational, have been reported. Gestational
choriocarcinoma of the ovary may be the result of metastasis from a
uterine trophoblastic disease, or, rarely, it may follow an ovarian
pregnancy. The non-gestational type is a primary germ cell neoplasm
differentiating along the extraembryonic chorionic tissue. The
majority of the tumors occur in combination with other germ cell
neoplasms. Pure choriocarcinoma of the ovary has been defined as a
tumor that occurs in the absence of all other germ cell tumors. No
additional components of germ cell tumors were found in association
with the present tumor. Furthermore, the α-fetoprotein levels were
normal. Pure ovarian choriocarcinoma is extremely rare (1,2), and based
on an extensive search of the English literature, it can be
observed that only a few cases of this type of choriocarcinoma have
been previously described (Table I)
(3,4).
This tumor most commonly occurs in young individuals, and ~50% of
cases are identified at a relatively early stage. In addition, in
~66% of patients chemotherapy improves prognosis.
 | Table I.Clinicopathological characteristics of
32 cases of non-gestational pure ovarian choriocarcinoma. |
Table I.
Clinicopathological characteristics of
32 cases of non-gestational pure ovarian choriocarcinoma.
| Variable | Value |
|---|
| Mean age, years | 13.8±4.2 |
| Duration of abdominal
pain, weeks | 3–16 |
| Side, % |
|
|
Right | 68 |
| Left | 32 |
| Stage, % |
|
| I | 49 |
| II | 17 |
| III | 17 |
| IV | 17 |
| Surgery, % |
|
|
UO/SO | 71 |
|
AH+BSO | 20 |
| BSO | 4 |
| hCG, mU/ml | 0.034–200,000 |
| Pregnancy test,
n | 5 |
| Treatment, n |
|
| Bone
marrow transplant | 2 |
|
Chemotherapy |
|
|
Methotrexate-based | 11 |
|
Vinblastin,
bleomycin and cisplatin | 3 |
|
Cisplatin,
etoposide and bleomycin | 3 |
| Outcome, % |
|
|
Succumbed | 35 |
|
Alive | 65 |
The present case exhibited the required pathological
characteristics, as described in the previously reported cases, to
determine a diagnosis of pure choriocarcinoma (1). No other carcinomatous or germ cell tumor
elements were identified in addition to the trophoblastic cells.
Furthermore, the histological examination revealed no similar
tumors in other organs. However, it was difficult to determine
morphologically whether the case was a non-gestational or
gestational tumor. The majority of studies have suggested that the
diagnosis of pure non-gestational choriocarcinoma should be limited
to premenarcheal patients (1–3,5).
Differentiation between non-gestational and
gestational choriocarcinoma is required, as the chemotherapy and
prognosis differ for the two tumor types. A study of the tumor
characteristics has been previously performed in vitro by
the genetic analysis of cell lines. Tanaka et al (6) reported a lack of effective messenger RNA
for BMG in gestational human choriocarcinoma cell lines, as well as
the presence of messenger RNA for BMG in non-gestational
choriocarcinoma. Several previous studies have also investigated
the high expression levels of BMG in choriocarcinoma. Kato et
al (7) investigated
choriocarcinoma cells that produced moderate amounts of surface and
secreted BMG. Norman et al (8)
also reported that the serum BMG level was elevated in patients
with choriocarcinoma. The study indicated that BMG may be
clinically used as a serum marker for this disease. In the present
study, BMG was detected in each of the two types of tumor cells
using immunohistochemistry. By contrast, there have been no
previous studies to immunohistochemically confirm the expression of
BMG in the trophoblastic tumor; to the best of our knowledge, the
current case study is the first to present this point. Based on the
immunohistochemical findings and previous studies, a diagnosis of
primary, pure non-gestational choriocarcinoma of the ovary was
suggested. This study indicates that BMG may be clinically used as
a serum marker for non-gestational choriocarcinoma in the future.
However, the cause of the BMG expression in non-gestational
choriocarcinoma remains unclear.
In conclusion, the current study presents a case of
primary pure ovarian choriocarcinoma, which was successfully
treated with surgery and a PEB regimen. Further pathological and
biological techniques for the differentiation of gestational and
non-gestational tumors is required for the efficient treatment of
choriocarcinoma in the future.
References
|
1
|
Russell P and Farnsworth A:
Non-gestational choriocarcinomasSurgical Pathology of the Ovaries.
2nd. Churchill Livingstone; Edinburgh: pp. 263–264. 1997
|
|
2
|
Scully RE, Young RH and Clement PB:
ChoriocarcinomaTumors of the Ovary, Maldeveloped Gonads, Fallopian
Tube, and Broad Ligament. Atlas of Tumor Pathology. 3rd series.
Fascicle. 23. Armed Forces Institute of Pathology; Washington DC:
pp. 258–260. 1998
|
|
3
|
Goswami D, Sharma K, Zutshi V, Tempe A and
Nigam S: Nongestational pure ovarian choriocarcinoma with
contralateral teratoma. Gynecol Oncol. 80:262–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inaba H, Kawasaki H, Hamazaki M, et al: A
case of metastatic ovarian non-gestational choriocarcinoma:
successful treatment with conservative type surgery and
myeloablative chemotherapy. Pediatr Int. 42:383–385. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacobs AJ, Newland JR and Green RK: Pure
choriocarcinoma of the ovary. Obstet Gynecol Surv. 37:603–609.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka K, Nabeshima Y, Takahashi H, et al:
Lack of effective messenger RNA for beta 2-microglobulin in a
gestational human choriocarcinoma cell line (GCH-1). Cancer Res.
41:3639–3641. 1981.PubMed/NCBI
|
|
7
|
Kato M, Ohashi K, Saji F, Wakimoto A and
Tanizawa O: Expression of HLA class I and beta 2-microglobulin on
human choriocarcinoma cell lines: induction of HLA class I by
interferon-gamma. Placenta. 12:217–226. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Norman RJ, Jialal I, Joubert SM and
Green-Thompson RW: Beta-2-microglobulin in trophoblastic disease. S
Afr Med J. 64:90–92. 1983.PubMed/NCBI
|