Introduction
Sclerosing epithelioid fibrosarcoma (SEF) is a rare
tumor affecting the deep soft tissues that was originally described
by Meis-Kindblom et al in 1995 (1). The most commonly affected sites are the
lower extremities, limb girdles and trunk, followed by the upper
extremities, the head and neck, and the abdominal inguinal region
(2,3).
The tumor may also arise in the bones, neural system, cecum,
ovaries and kidneys (4–8). SEF is an unusual variant of fibrosarcoma
that is formed from epithelioid cells arranged in strands and nests
in a background of a highly sclerotic collagenous stroma. Although
SEFs exhibit an indistinctive appearance and low mitotic activity,
they are capable of distant metastasis and local recurrence. The
current treatment options for SEF are resection, radiation and
chemotherapy (3). Radiofrequency (RF)
ablation and percutaneous permanent iodine-125 implantation have
been used to treat numerous types of solid tumor, including
hepatocellular carcinoma and lung cancers (9–14),
however, at present, the efficacy of such modalities for the
treatment of SEF remains unclear.
The present study describes a rare case of giant
recurrent SEF arising from the chest wall that was accompanied by
acute bleeding, and in addition, discusses the current therapeutic
approaches, including RF ablation and percutaneous permanent
iodine-125 implantation. Written informed consent was obtained from
the patient.
Case report
A 70-year-old male presented to Beijing Chao-Yang
Hospital Affiliated to Capital Medical University (Beijing,
China)for RF ablation of a giant recurrent SEF of the chest wall,
which was accompanied by acute bleeding. The patient had previously
undergone several courses of radiotherapy, in addition to one
surgical wedge resection. A diagnosis of SEF had been previously
confirmed following pathological examination of surgical samples;
histologically, the lesions were characterized by the proliferation
of uniform, small to moderately-sized, slightly angulated, round to
ovoid epithelioid cells with sparse, clear cytoplasm arranged in
distinct cords and strands, embedded in dense collagenous stroma.
The tumor had recurred at the original site 7 months prior to
presentation, and had been gradually increasing in size. According
to the patient, the tumor had increased in size by two-fold during
the previous 2 months. The medical history was notable for
hypertension, chronic cardiac dysfunction and benign prostatic
hypotrophy. Medications included 75 mg/day aspirin, which was
discontinued 1 day prior to admission, 20 mg/day fluvastatin, 80
mg/day valsartan and hydrochlorothiazide, 10 mg morphine sulfate
sustained-release tablets every 12 h, and 0.4 mg/day
tamsulosin.
At the time of admission, the patient presented with
pallor and a pulse rate of 98 bpm. The patient exhibited a
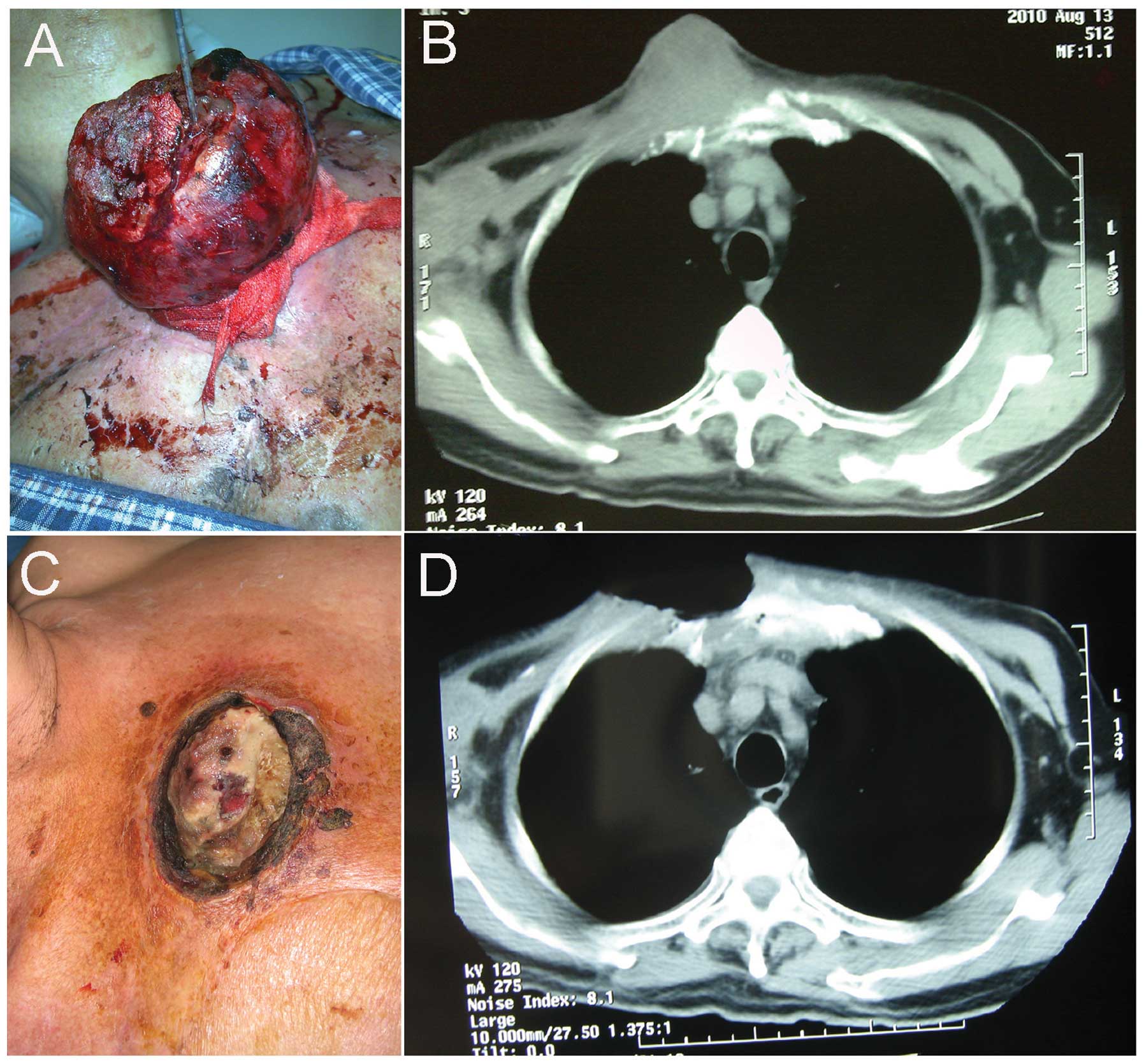
fungating and well-circumscribed mass on the right upper region of
the anterior chest wall, which measured ~21×14×5 cm in size
(Fig. 1A and B). Bleeding was
observed from the ulcerative surface of the tumor. A tamponade
dressing was therefore applied in order to temporarily stop the
bleeding. However, the lesion continued to hemorrhage. The patient
was rehydrated to maintain effective circulation, and urgently
transfused with 600 ml of fresh frozen plasma to improve
coagulation. Chest CT indicated involvement of the sternum and
anterior mediastinum. Laboratory findings revealed a hematocrit
level of 36% (normal range, 40–50%), a hemoglobin level of 10 g/dl
(normal range, 12–16 g/dl), a platelet count of 109,000 (normal
range, 100,000–300,000), a partial thromboplastin time of 33.4 sec
(normal range, 35.0–45.0 sec) and a prothrombin time of 11.8 sec
(normal range, 13.0–17.0 sec). Platelet function investigations
were not performed. The patient remained stable during positioning
and initial CT scanning.
An emergency percutaneous RF ablation, guided by a
Synergy Plus CT scanner (GE Yokogawa Medical Systems Ltd., Tokyo,
Japan), was performed in a CT suite. Local anesthesia was selected,
as the patient was not suitable for general anesthesia. The patient
was placed in a supine position on the CT table, and the RF
procedures were performed using a 15-gauge multitined electrode
(Starburst XL; RITA Medical Systems, Inc., Manchester, GA, USA).
The RF generator (RITA 1500, RITA Medical Systems, Inc.) was used
according to the manufacturer's instructions. Multiple-spot RF
ablation was first performed in order to achieve hemostasis and
reduce and sclerify the lesion. The ablated tumor was then resected
in a block-by-block, superficial-to-deep manner, and the outer
region of the tumor was completely eliminated (Fig. 1C and D). Hemostasis was achieved by
the cessation of bleeding, a decrease of the pulse rate and an
increase in the hemoglobin level. In order to further control the
growth of the residual tumor that had involved the mediastinum,
percutaneous iodine-125 implantation under CT guidance was
subsequently performed, every 30–40 days (Fig. 2). In total, five percutaneous
iodine-125 implantations were performed in order to retard tumor
growth. However, the tumor recurred again 6 months after treatment.
The patient refused any further treatment and was discharged.
Discussion
At present, the optimal treatment strategy for SEF
remains controversial. Surgical resection is the mainstay of
therapy for SEF. However, SEF is an aggressive tumor that is prone
to repeated local recurrence if not widely excised. It has been
reported that >50% of SEF patients experience persistent disease
or local recurrence. Furthermore, the metastatic rate of SEF is
between 43 and 86%. In total, approximately one-third of SEF
patients are alive with the disease, and the mortality rate is
between 25 and 57% (2). Therefore,
the first and most important step in the course of surgery is the
wide excision of the tumor in order to guarantee the clearance of
all microscopic tumors. The recommended margin of resection for
smaller tumors is between 3 and 5 cm, whereas for larger tumors,
this should be extended to between 7 and 8 cm. In addition,
grafting is often necessary (15).
Pre- or post-operative radiation therapy is considered to be an
important treatment for SEF. However, not all SEFs are sensitive to
irradiation. It has been previously suggested that radiation
therapy should only be administered in cases where surgery is
impossible, and/or where there may be the potential to slow the
progress of the disease (15).
Chemotherapy is less commonly administered due to the general
insensitivity of SEF to this treatment.
Treatment for recurrent SEF is even more
challenging. In the present study, the recurrent tumor arose from a
deep site in the chest wall of a male patient. Although the tumor
was well-circumscribed from the outside, distinct
clinicopathological characteristics were also observed. The
prominent characteristic was that the patient suffered from an
emergent bleeding of the tumor that was difficult to control. The
second characteristic was that the patient was 70 years old and
presented with multiple comorbid diseases, including hypertension
and chronic cardiac dysfunction. Thirdly, CT scans indicated that
the sternum and anterior mediastinum had been involved by the
tumors. Finally, the patient resolutely refused further surgery.
Therefore, secondary surgery was not taken into account in the
present case. Following careful discussion and consideration, it
was decided that RF ablation and percutaneous iodine-125
implantation would be used to treat the patient.
RF ablation is the use of RF energy to thermally
destroy living tissue. The process has gained interest as a
minimally invasive strategy for the management of focal malignant
diseases. Due to the fact that a number of these tumors are not
responsive to curative surgical resection, RF ablation represents a
novel addition to the range of available treatments. The advantages
of RF ablation include real-time imaging guidance, the ability to
remove tumors in patients who are unsuitable for surgical
resection, a reduced risk of morbidity compared with surgical
intervention, and the potential for being performed repeatedly
(16). In addition, RF ablation has
been used as an emergency procedure to control bleeding from
ruptured tumor tissues (17–19). In the present study, not only was the
tumor successfully destroyed, but the hemorrhaging was also
effectively controlled following the use of RF ablation. In order
to further control the growth of the tumor that had involved the
mediastinum, percutaneous iodine-125 implantation was used under CT
guidance. The implantation of radioactive material into tumors was
established in 1901 by Pierre Curie, who used newly-discovered
radium (20,21). Since 1965, iodine-125 seeds have been
used. The long half-life and low energy of iodine-125 seeds have
several radiobiological advantages. Firstly, the radiation hazard
for members of the patient's family and involved personnel is
reduced to insignificant levels. Secondly, the seeds are viable for
long periods of time, and can therefore be ordered at fixed
intervals and stored until use. Finally, the seeds can be applied
as a permanent implant into the tumor (22). Compared with external radiation
therapy, iodine-125 implantation can treat deep-seated tumors with
a much higher total dose, which can be delivered more precisely and
at a low dose rate.
Due to the rarity and complex spectrum of the
tumors, guidelines for the treatment of recurrent SEFs have been
difficult to establish. A multidisciplinary approach for the
management of these patients is therefore advocated. The modality
of treatment should be individually tailored based upon the
analysis of the patient's situation, in addition to the tumor size,
growth rate and location. Regular and careful follow-up
examinations are essential and will lead, in the majority of
patients, to a more suitable modality of treatment, which should
prolong life and improve overall health. In the present case, after
RF ablation and percutaneous iodine-125 implantation treatment, the
patient survived for one year prior to being lost to follow-up.
Despite tumor recurrence six months after therapy, treatment was
considered to be satisfactory. In conclusion, the high local tumor
control rates, minimal invasion and low morbidity suggest that RF
ablation and percutaneous permanent iodine-125 implantation is a
feasible and safe salvage therapy for patients with recurrent SEF
of the chest wall.
Acknowledgements
This study was supported by grants from the Dr
Jieping Wu Medical Foundation (no. 320675012712) and the Program
for Medical Key Discipline of Shijingshan District (no.
20130001).
References
|
1
|
Meis-Kindblom JM, Kindblom LG and Enzinger
FM: Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma
simulating carcinoma. Am J Surg Pathol. 19:979–993. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antonescu CR, Rosenblum MK, Pereira P,
Nascimento AG and Woodruff JM: Sclerosing epithelioid fibrosarcoma:
a study of 16 cases and confirmation of a clinicopathologically
distinct tumor. Am J Surg Pathol. 25:699–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ossendorf C, Studer GM, Bode B and Fuchs
B: Sclerosing epithelioid fibrosarcoma: case presentation and a
systematic review. Clin Orthop Relat Res. 466:1485–1491. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frattini JC, Sosa JA, Carmack S and Robert
ME: Sclerosing epithelioid fibrosarcoma of the cecum: a
radiation-associated tumor in a previously unreported site. Arch
Pathol Lab Med. 131:1825–1828. 2007.PubMed/NCBI
|
|
5
|
Grunewald TG, von Luettichau I, Weirich G,
et al: Sclerosing epithelioid fibrosarcoma of the bone: a case
report of high resistance to chemotherapy and a survey of the
literature. Sarcoma. 2010:4316272010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanson IM, Pearson JM, Eyden BP, Slawik S
and Harris M: Evidence of nerve sheath differentiation and high
grade morphology in sclerosing epithelioid fibrosarcoma. J Clin
Pathol. 54:721–723. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe K and Suzuki T: Epithelioid
fibrosarcoma of the ovary. Virchows Arch. 445:410–413. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Argani P, Perlman EJ, Breslow NE, et al:
Clear cell sarcoma of the kidney: a review of 351 cases from the
National Wilms Tumor Study Group Pathology Center. Am J Surg
Pathol. 24:4–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin ZY, Chen J and Deng XF: Treatment of
hepatocellular carcinoma adjacent to large blood vessels using 1.5T
MRI-guided percutaneous radiofrequency ablation combined with
iodine-125 radioactive seed implantation. Eur J Radiol.
81:3079–3083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen K, Chen G, Wang H, et al: Increased
survival in hepatocellular carcinoma with iodine-125 implantation
plus radiofrequency ablation: a prospective randomized controlled
trial. J Hepatol. 61:1304–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiraki T, Gobara H, Iguchi T, Fujiwara H,
Matsui Y and Kanazawa S: Radiofrequency ablation for early-stage
nonsmall cell lung cancer. Biomed Res Int. 2014:1520872014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu L, Zhou L, Xu K and Mu F: Combination
of cryosurgery and Iodine-125 seeds brachytherapy for lung cancer.
J Thorac Dis. 4:504–507. 2012.PubMed/NCBI
|
|
13
|
Yang H, Liu YH, Xu L and Liu LH: Efficacy
of permanent iodine-125 seed implants and gemcitabine chemotherapy
in patients with platinum-resistant recurrent ovarian carcinoma.
Asian Pac J Cancer Prev. 15:9009–9013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang YL, Meng N, Wang JJ, Ran WQ, Yuan
HS, Qu A and Yang RJ: Percutaneous computed
tomography/ultrasonography-guided permanent iodine-125 implantation
as salvage therapy for recurrent squamous cell cancers of head and
neck. Cancer Biol Ther. 9:959–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Staffords ES and Ward GE: Treatment of
fibrosarcoma. Ann Surg. 137:639–644. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lau WY and Lai EC: The current role of
radiofrequency ablation in the management of hepatocellular
carcinoma: a systematic review. Ann Surg. 249:20–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchizaki U, Miyamori H, Kitagawa S and
Kaneko S: Radiofrequency ablation for life-threatening ruptured
hepatocellular carcinoma. J Hepatol. 40:354–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun WB, Ding XM, Ke S, Gao J and Zhang YF:
Repeated radiofrequency ablation as both salvage solution and
curative treatment for spontaneous rupture of giant medial lobe
hepatocellular carcinoma. Chin Med J (Engl). 122:2067–2070.
2009.PubMed/NCBI
|
|
19
|
Manikam J, Mahadeva S, Goh KL and Abdullah
BJ: Percutaneous, non-operative radio frequency ablation for
haemostasis of ruptured hepatocellular carcinoma.
Hepatogastroenterology. 56:227–230. 2009.PubMed/NCBI
|
|
20
|
Grammaticos PC: Pioneers of nuclear
medicine, Madame Curie. Hell J Nucl Med. 7:30–31. 2004.PubMed/NCBI
|
|
21
|
Schwarz SB, Thon N, Nikolajek K, Niyazi M,
Tonn JC, Belka C and Kreth FW: Iodine-125 brachytherapy for brain
tumours - a review. Radiat Oncol. 7:302012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holm HH, Strøyer I, Hansen H and Stadil F:
Ultrasonically guided percutaneous interstitial implantation of
iodine 125 seeds in cancer therapy. Br J Radiol. 54:665–670. 1981.
View Article : Google Scholar : PubMed/NCBI
|