Introduction
Ex situ liver surgery is a novel technique
for the treatment of complicated liver tumors that are unresectable
by conventional methods. Since it was first described by Pichlmayr
et al (1) in 1988, ex
situ surgery has increased the survival expectancy of a number
of patients with unresectable liver tumors. Liver
autotransplantation, which has developed from precise liver
resection and liver transplantation, has expanded the surgical
indications for primary and secondary liver tumors or neoplasms
involving the inferior vena cava (IVC) (2).
Hepatoid adenocarcinoma (HAC) is a rare and specific
type of extrahepatic adenocarcinoma that morphologically mimics
hepatocellular carcinoma (HCC). The most common features of HAC are
elevated levels of serum α-fetoprotein (AFP) and carcinoembryonic
antigen (CEA), as well as tissue immunoreactivity for AFP. HAC is
most commonly detected in the stomach (3). Since the tumor frequently occurs with
liver and lymph node metastases, the prognosis is poor (4). It is difficult to differentiate the
hepatic metastasis of HAC from HCC due to their similar
clinicopathological characteristics. The treatment of HAC is more
difficult when the tumor has metastasized to the liver, and
surgical treatment of the metastases is rarely undertaken. In a
previous study, we reported the case of a patient with metastatic
splenic α-fetoprotein-producing adenocarcinoma who had undergone
splenectomy (5). The present study
reports the case of the same patient who underwent a ex vivo
liver resection and partial liver autotransplantation for the
treatment of hepatic metastasis from HAC. Written informed consent
was obtained from the patient.
Case report
Patient
In January 2004, a 56-year-old male was admitted to
Zhongshan Hospital of Xiamen University (Xiamen, China) due to
epigastralgia and melena, with no relevant past medical history. A
physical examination revealed no abnormalities. The serum was
negative for α-fetoprotein, carcinoembryonic antigen, cancer
antigen (CA)125, CA199, CA153 and CA724 and positive for hepatitis
B surface antigen. Endoscopic examination revealed an infiltrating
ulcerative tumor with unclear boundaries, which indicated a
diagnosis of advanced gastric cancer of Borrmann type III. A
radical gastrectomy was performed in January 2004.
Post-operatively, the patient received chemotherapy with three 28
day cycles of 5-fluorouracil (500 mg/m2, days 1–5) +
cisplatin (15–20 mg/m2, days 1–5) + leucovorin (200–400
mg/m2, days 1–2). In April 2009, the patient was once
again admitted to Zhongshan Hospital of Xiamen University due to a
splenic neoplasm detected on computed tomography (CT) scans. The
serum AFP value was 732 ng/ml (normal range, 0–25 ng/ml) and the
CEA level was 1,049 ng/ml (normal range, 0–3.4 ng/ml). No local
recurrence of the tumor was found on endoscopy. A splenectomy was
subsequently performed. Histopathological examination indicated
splenic metastasis from HAC; the immunohistochemical stain was
positive for AFP. Intraperitoneal chemotherapy with fluorouracil
(1,000 mg/m2)was administered for 5 days
post-operatively. However, in July 2009, a positron emission
tomography scan showed liver metastases. The patient underwent
transcatheter arterial chemoembolization (TACE) and radiofrequency
ablation, but the levels of AFP and CEA progressively increased,
predicting a poor outcome. The patient received targeted therapy
with sorafenib (0.4 g, twice daily) for two weeks, but could not
tolerate further treatment due to adverse reactions. The serum AFP
and CEA levels rose to 557 and 609 ng/ml, respectively, in March
2012. An enhanced CT scan revealed multiple metastases in the left
and caudate lobes of the liver, with compression of the portal vein
and IVC (Fig. 1).
Pre-operative serum liver function tests were
normal. The indocyanine green clearance test (ICG15) measurement of
hepatic reserve function was 5.6% (normal value, <10%).
Reconstruction of the liver with CT scan images using a
three-dimensional imaging technique, which we developed (6), is illustrated in Fig. 2.
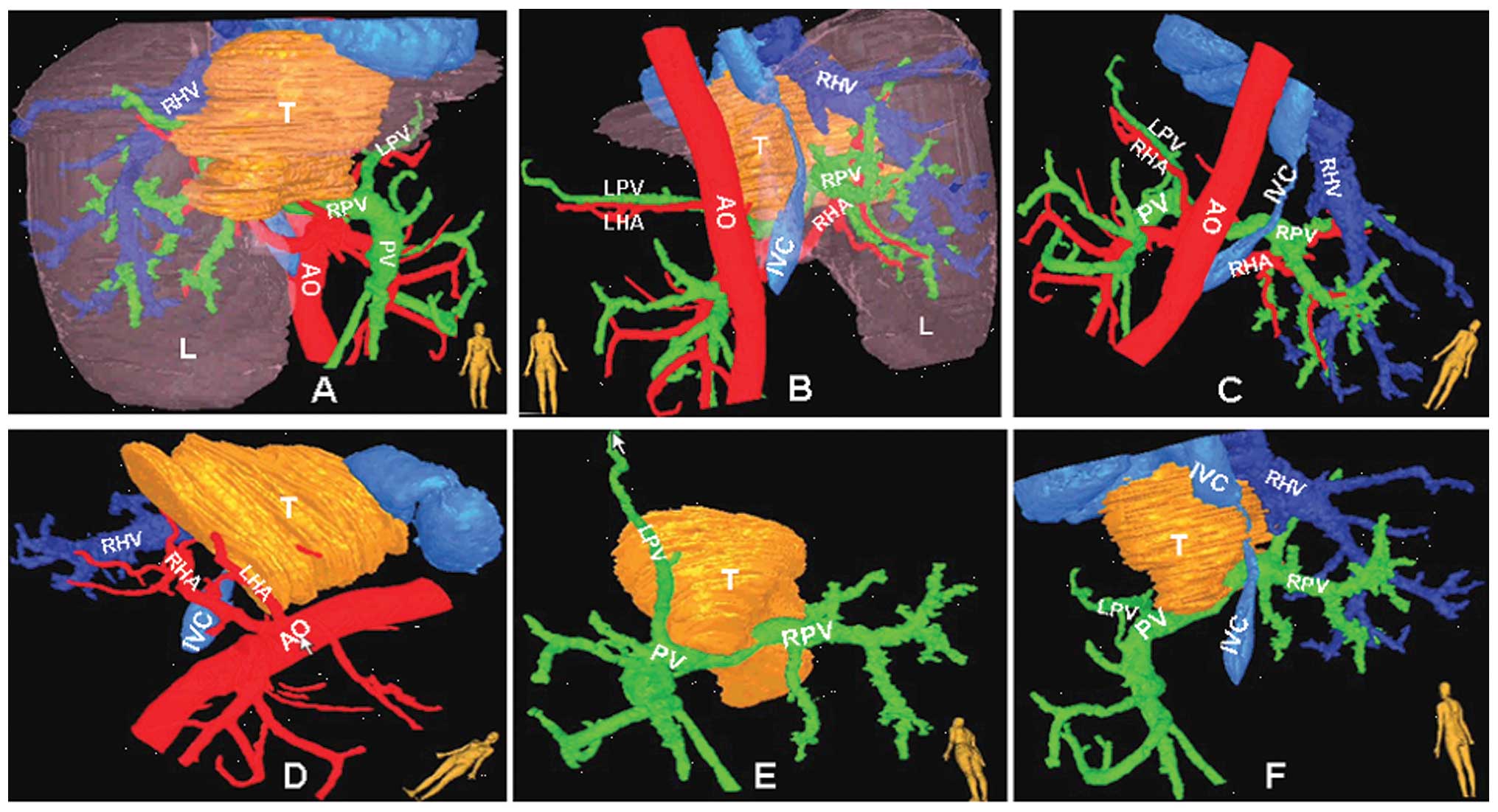 | Figure 2.Three-dimensional imaging of the liver
illustrates the association of the tumor with the vasculature,
using the software we developed. Tumor (yellow), hepatic artery
(red), hepatic vein and IVC (blue), portal vein (green). IVC,
inferior vena cava; T, tumor; L, liver; PV, portal vein; RPV, right
portal vein; LPV, left portal vein; AO, aorta; HA, hepatic artery;
RHA, right hepatic artery; LHA, left hepatic artery. |
Surgery
Intraoperative evaluation
A liver examination by ultrasonography was performed
to confirm the location and size of the lesions. A 13×10×10-cm,
irregular lesion was found in the left and caudate lobes of the
liver. The retrohepatic IVC was fully involved. The right hepatic
vein was free of tumor. Skeletonization of the hepatoduodenal
ligament was performed. The left hepatic artery originated from the
celiac axis and supplied the tumor, and the right hepatic artery
originated from the superior mesenteric artery.
Total liver resection
The distal segment of the common bile duct was
invaded by the tumor, so it was removed following transection of
the common duct at the level of cystic duct entry. The hepatic
ligaments were freed to expose the IVC and second porta hepatis,
revealing that the retrohepatic IVC was three-fourths encircled by
the lesion. Once all the vessels had been transected, the liver was
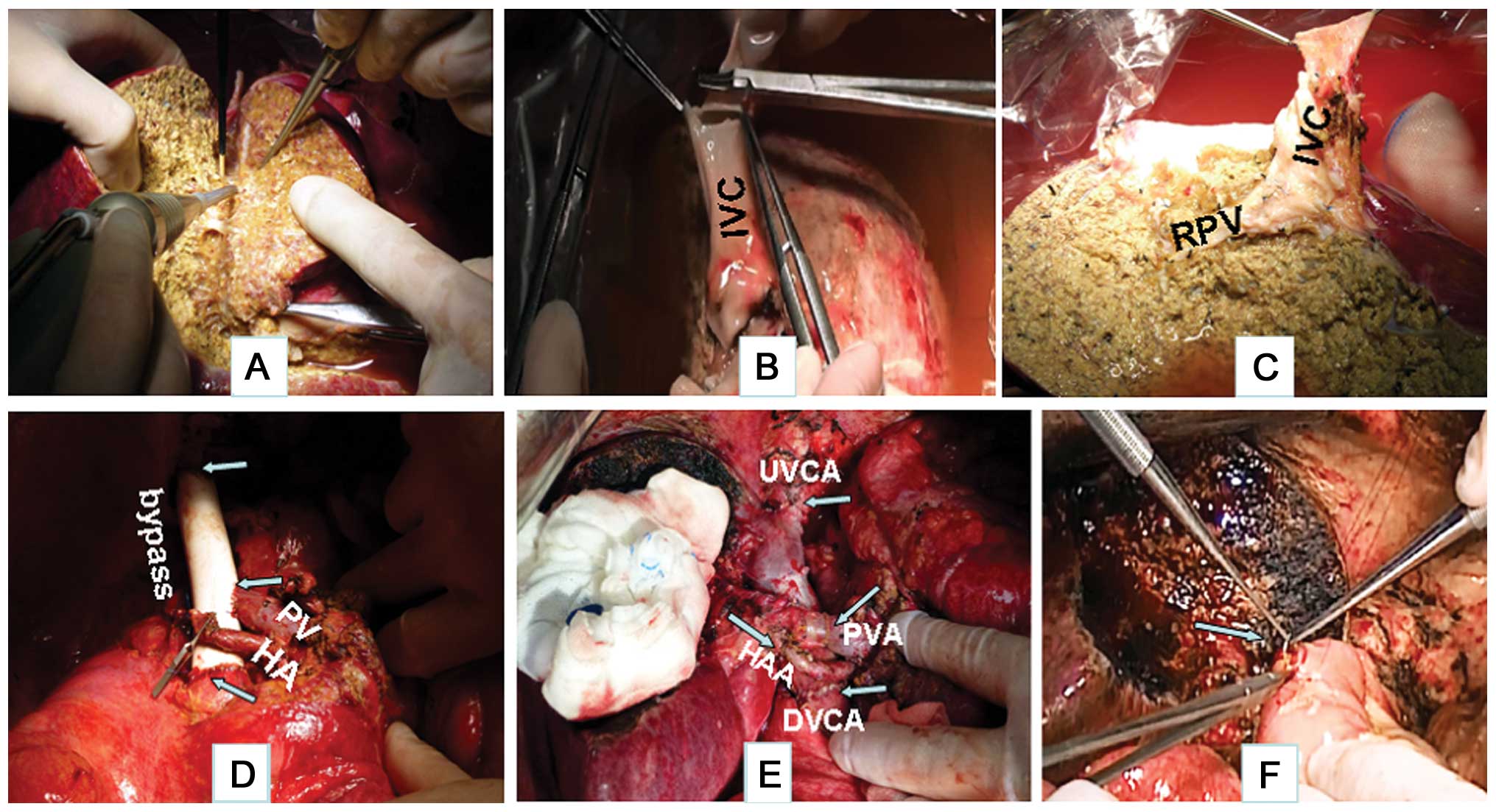
removed and placed in an ice bath.
Histidine-tryptophan-ketoglutarate solution at 4°C was infused via
the portal vein and hepatic artery (Fig.
3B).
Temporary portacaval shunt
The IVC was quickly replaced with a 20-mm ringed
prosthetic graft, and the portal vein was anastomosed to the graft
to reconstitute blood flow (Fig.
3D).
Ex vivo liver resection
The Cavitron Ultrasonic Surgical Aspirator (CUSA;
Sonoca 300; Söring GmbH, Quickborn, Germany) was used to resect the
hepatic tissues, and bipolar coagulation was used for disconnecting
the small vessels. The big vessels and bile ducts were sutured with
5-0 Prolene. The liver, including the left and caudate lobes, was
resected. Perforations of the IVC caused by stripping the tumor
from it were sutured with 6-0 Prolene (Fig. 3A and C).
Liver reimplantation
The remaining liver was reimplanted following
perfusion via the portal vein with 2% albumin in Ringer's lactate
solution. The suprahepatic and infrahepatic IVC were reconnected by
end-to-end anastomosis with 6-0 Prolene. The main portal vein was
anastomosed to the right portal vein with 7-0 Prolene. The right
hepatic artery was reconnected by end-to-end anastomosis with 7-0
Prolene. Following reconstruction of all the vessels, hemostasis
was complete. The bile duct was reconstructed through a roux-en-Y
hepatojejunostomy (Fig. 3E and
F).
Results
Surgical summary
The surgery lasted 9 h, with an anhepatic period of
4 h. Blood loss during the surgery was 1,500 ml, and four units of
packed red cells and 400 ml of fresh-frozen plasma were
administered. The patient was cared for in the intensive care unit
for three days after the surgery, with no complications
post-operatively. The alanine aminotransferase level rose to 620
µ/l and returned to normal levels seven days later. The patient was
discharged on post-operative day 21.
Pathological examination
Gross liver metastasis from the hepatoid
adenocarcinoma is illustrated in Fig.
4A. The majority of the tumor was composed of scattered large
pleomorphic or multinucleated giant cells. The cells were markedly
atypical, with eosinophilic cytoplasm and round nuclei,
occasionally exhibiting marked nucleoli (Fig. 4B). The tumor cells were arranged in a
tubular pattern (Fig. 4C) and nuclear
division was evident (Fig. 4D).
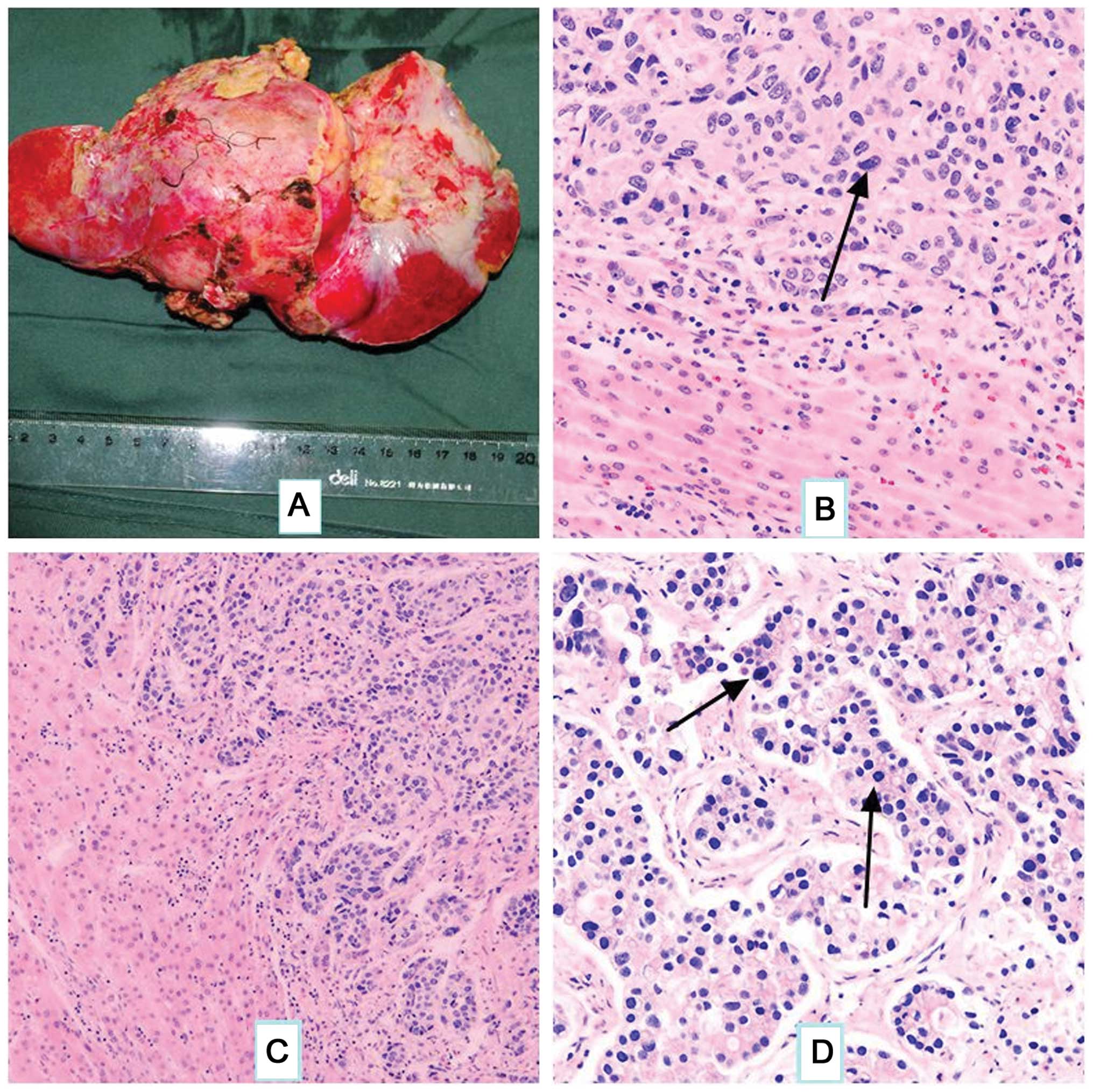 | Figure 4.(A) Gross illustration of hepatic
metastases. (B-D) Histopathological features of the tumor. (B)
Tumor cells are arranged in nests, which are similar to those found
in primary liver cancer tissue. 1–2 large and marked nucleoli
(arrow) are present in the center of the cells (stain, hematoxylin
and eosin; magnification, ×200). (C) The tumor cells are arranged
in a tubular pattern, similar to those found in hepatocellular
carcinoma, and numerous blood sinuses are evident (stain,
hematoxylin and eosin; magnification, ×100). (D) Tumor cells are
arranged with cancer adenoids, with scattered multinucleated giant
cells (arrows). Nuclear division can be easily observed (stain,
hematoxylin and eosin; magnification, ×200). |
Follow-up
The patient remains alive 20 months
post-operatively, with normal AFP and CEA levels, and no evidence
of recurrent disease.
Discussion
Bourreille et al (7) first reported an AFP-producing gastric
tumor in 1970. Ishikura et al (8) proposed the term HAC of the stomach for
this type of primary gastric carcinoma in 1985. The stomach is the
most common site of origin of HAC (9), but HAC has been reported in other
organs, including the bladder, lungs, pancreas, colon, gall
bladder, ovaries and uterus. The reported incidence of HAC is
1.3–15% of all gastric carcinomas (10). As HAC is relatively rare, the
pre-operative diagnostic rate is low, and the diagnosis is usually
made through post-operative pathological examination.
It is difficult to differentiate HCC resulting from
hepatic metastases from HAC. One previous study (11) reported that the tumors have similar
enhancement on CT and magnetic resonance imaging, so it is not easy
to differentiate them on the basis of imaging findings. HAC is a
relatively rare disease, while liver cirrhosis and chronic
hepatitis infection can usually be found among patients with HCC,
therefore, the differential diagnosis should be based on the
medical history, dynamic image studies and liver biopsy. The
proportion of HAC with a positive CEA stain is >75%, so high
levels of serum AFP and CEA together should prompt suspicion of
HAC. The patient in the present study had a history of hepatitis B
infection and an elevated serum AFP level pre-operatively,
therefore, the mass could have been misdiagnosed as HCC. However,
the patient also had a history of HAC, had undergone a previous
splenic metastasis tumor resection and presented with significant
increases in serum CEA level; accordingly, a diagnosis of liver
metastasis from HAC was formed.
A number of studies have reported that the prognosis
of HAC is extremely poor even when discovered at an early stage.
Motoyama et al (4) attributed
the poor prognosis of HAC to the frequent occurrence of liver or
lymph node metastases. Metastasis of the liver can occur within a
year post-operation, even if no metastasis is found
pre-operatively. Chang et al (12) reported that the majority of patients
in their series, including three who underwent radical surgery for
early gastric cancer, succumbed to liver metastasis within two
years. Thus, liver metastasis is the main factor affecting the
prognosis of HAC, and the close observation and long-term follow-up
of patients is required.
Holistic therapy based on surgery is the optimal
treatment method for HAC, and surgical treatment for HAC with liver
metastasis may still be valuable. the patient in the present case
patient developed splenic and hepatic metastases five years after
radical resection and chemotherapy for HAC. Metastases of HAC to
the spleen are believed to be unusual, but a splenectomy was
performed on the present patient, as there was no endoscopic
evidence of recurrence of the gastric tumor (5). The hepatic metastatic carcinoma was
rapidly growing, with invasion of the IVC and the second porta
hepatis. The patient's response to TACE was extremely poor. The IVC
was stenotic due to the tumor, so surgery was considered as the
only option for a cure.
In recent years, with a better understanding of the
vascular and segmental anatomy of the liver through the experience
of liver transplantation, the results of liver surgery have rapidly
improved. Liver tumor metastasis to the vena cava does not obviate
the requirement for liver resection (2). Ex vivo liver resection is a novel
surgical technique mainly used for large liver tumors located in
the dorsum of the liver with involvement of the hepatic veins or
the retrohepatic IVC (2). Pichlmayr
et al (1) first reported the
surgery in 1988 and subsequently performed 11 procedures. This
approach has now become recognized and is promoted worldwide. Ex
vivo liver resection has increased the resectability rate in
patients with advanced tumors, provided a partial solution for
bleeding and long-term blocking in certain liver tumor resections,
and avoided the requirement for liver transplantation in specific
patients. The technique can also be applied to cases of hilar
cholangiocarcinoma and complex liver trauma. The present patient
presented with liver metastases invading the hepatic vein and
retrohepatic IVC, so the tumor was unresectable with conventional
surgery. For achieving R0 resection, ex vivo liver resection
appears to be suitable, as it provides enough time for the
completion of complex resections and vascular reconstructions in a
bloodless environment, thereby reducing the risk of bleeding.
Complete pre-operative assessments of liver function and reserve
are essential. A computer-aided pre-operative planning system for
liver surgery based on CT images has been used to define the
association between the tumor and blood vessels (13), and a simulation resection can be used
to evaluate the resection range and residual liver volume.
Maintaining a stable hemodynamic status and avoiding
ischemia-reperfusion injury are two prominent considerations for
this surgery. Certain studies recommend the use of veno-venous
bypass during the anhepatic period. The aim of this technique is to
reduce hemodynamic instability, ensure splanchnic decompression,
and minimize venous hypertension and intestinal edema (14). However, the ex situ ex vivo
procedure is associated with complications, including air embolism,
thromboembolic events and mechanical injury due to global capillary
leak, which can lead to severe post-operative water, electrolyte
and acid-base imbalances (15). Thus,
the present study used a vascular prosthesis to reconstitute blood
flow in the portal vein and IVC to ensure the stability of general
circulation, lessen ischemia-reperfusion injury, and avoid
acid-alkali and electrolyte imbalances. Wen et al (16) recommended construction of a temporary
portacaval shunt to improve hemodynamic status, reduce the
requirement for intraoperative blood transfusion and preserve renal
function. The shunt can also permit enough time for the completion
of the ex vivo liver resection. Compared with the
traditional veno-venous bypass technology, this procedure has a
shorter surgical duration and anhepatic phase. Zhang et al
(17) reported three cases of
ex-vivo hepatectomy using this procedure and found no
post-operative hepatic dysfunction resulting from the delayed liver
hemoperfusion; thus, it was suggested that this method is feasible
and safe, with a shortened surgical duration, and less
intraoperative bleeding and reperfusion injuries. Gringeri et
al (18) performed a temporary
portacaval shunt with a stretch of aortic graft to replace the
caval vein in a porcine model; the temporary shunt ensured
hemodynamic stability during the anhepatic phase and lengthened the
portal vein, which facilitated construction of a good
anastomosis.
The amount of time that the liver can safely
tolerate warm ischemia appears to be limited, so hypothermic
perfusion has been considered. The advantage of this technique is
that the low temperature increases the tissue tolerance to
ischemia. Hannoun et al (19)
suggested that hypothermia facilitated the bloodless transection of
liver parenchyma and increased the chance of resectability for
advanced tumors. The histidine-tryptophan-ketoglutarate solution is
better than the University of Wisconsin preservation solution, as
it minimizes the risk of cardiocirculatory complications following
reperfusion (20). The patient in the
present study had a good recovery, without the complications of
renal insufficiency and heart failure. At the hypothermic perfusion
stage, CUSA and bipolar coagulation were used for transection of
the liver parenchyma; thus, the small vessels and bile ducts could
be separated during a bloodless condition to prevent bleeding and
leakage following re-perfusion. Once all the vessels had been
reconstructed, implantation of the remnant liver and anastomosis of
vessels was performed exactly as in living donor transplantation.
The patient presented with hepatic metastases from HAC, so
lymphatic and connective tissue from the hepatoduodenal ligament
were removed, which could have compromised the blood supply of the
bile duct. Consequently, partial resection of the bile duct was
performed and the bile flow was reconstituted with a
hepatic-jejunostomy.
Ex situ ex vivo surgery should only be
performed in specialized centers, where surgeons are familiar with
complex hepatobiliary surgery and liver transplantation. This
technique may be the last resource for otherwise unresectable
malignancy (14), but the associated
morbidity and mortality remain high, and tumor recurrence is a
major problem. Raab et al (21) also recommended that the ex situ
procedure be used only in specialized centers with extensive
experience in both conventional liver surgery and
transplantation.
HAC is prone to liver and lymph node metastasis,
which predicts high malignancy and a poor prognosis, but this does
not mean that there is no chance of treatment with a good
prognosis. Ex situ liver surgery may be the only effective
treatment for patients with liver tumors that cannot be resected by
traditional procedures. The surgery offers a chance for hepatectomy
of the primary and secondary liver tumors, even with hepatic vein
and IVC infiltration within the tumor. The safety and efficacy of
this technique requires verification through further clinical
studies in practice.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81172286, 81372618
and 61327001) and the National Key Sci-Tech Special Project of
China (grant no. 2012ZX10002-011-005c).
References
|
1
|
Pichlmayr R, Bretschneider HJ, Kirchner E,
et al: Ex situ operation on the liver. A new possibility in liver
surgery. Langenbecks Arch Chir. 373:122–126. 1988.[(In German)].
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hemming AW, Mekeel KL, Zendejas I, Kim RD,
Sicklick JK and Reed AI: Resection of the liver and inferior vena
cava for hepatic malignancy. J Am Coll Surg. 217:115–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinoma of the stomach. A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motoyama T, Aizawa K, Watanabe H, Fukase M
and Saito K: alpha-Fetoprotein producing gastric carcinomas: a
comparative study of three different subtypes. Acta Pathol Jpn.
43:654–661. 1993.PubMed/NCBI
|
|
5
|
Deng Z, Yin Z, Chen S, Peng Y, Wang F and
Wang X: Metastatic splenic α-fetoprotein-producing adenocarcinoma:
report of a case. Surg Today. 41:854–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song X, Cheng M, Wang B, Huang S and Huang
X: Computer-aided preoperative planning for liver surgery based on
CT images. Procedia Engineering. 24:133–137. 2011. View Article : Google Scholar
|
|
7
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha feto protein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.[(In French)]. PubMed/NCBI
|
|
8
|
Ishikura H, Fukasawa Y, Ogasawara K,
Natori T, Tsukada Y and Aizawa M: An AFP-producing gastric
carcinoma with features of hepatic differentiation. A case report.
Cancer. 56:840–848. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Cheng Y, Sheng W, et al: Analysis
of clinicopathologic features and prognostic factors in hepatoid
adenocarcinoma of the stomach. Am J Surg Pathol. 34:1465–1471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Plaza JA, Vitellas K and Frankel WL:
Hepatoid adenocarcinoma of the stomach. Ann Diagn Pathol.
8:137–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jo JM, Kim JW, Heo SH, Shin SS, Jeong YY
and Hur YH: Hepatic metastases from hepatoid adenocarcinoma of
stomach mimicking hepatocellular carcinoma. Clin Mol Hepatol.
18:420–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang YC, Nagasue N, Abe S, Taniura H,
Kumar DD and Nakamura T: Comparison between the clinicopathologic
features of AFP-positive and AFP-negative gastric cancers. Am J
Gastroenterol. 87:321–325. 1992.PubMed/NCBI
|
|
13
|
Mönch J, Mühler K, Hansen C, et al: The
LiverSurgeryTrainer: training of computer-based planning in liver
resection surgery. Int J Comput Assist Radiol Surg. 8:809–818.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gruttadauria S, Marsh JW, Bartlett DL,
Gridelli B and Marcos A: Ex situ resection techniques and liver
autotransplantation: last resource for otherwise unresectable
malignancy. Dig Dis Sci. 50:1829–1835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khoury GF, Mann ME, Porot MJ, Abdul-Rasool
IH and Busuttil RW: Air embolism associated with veno-venous bypass
during orthotopic liver transplantation. Anesthesiology.
67:848–851. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen PH, Lin KH, Chen YL, Hsieh CE, Ko CJ
and Kuo SJ: Extracorporeal hepatic resection and
autotransplantation using temporary portocaval shunt provides an
improved solution for conventionally unresectable HCC. Dig Dis Sci.
58:3637–3640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang KM, Hu XW, Dong JH, et al: Ex-situ
liver surgery without veno-venous bypass. World J Gastroenterol.
18:7290–7295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gringeri E, Polacco M, D'Amico FE, et al:
A new liver autotransplantation technique using subnormothermic
machine perfusion for organ preservation in a porcine model.
Transplant Proc. 43:997–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hannoun L, Delrivière L, Gibbs P, Borie D,
Vaillant JC and Delva E: Major extended hepatic resections in
diseased livers using hypothermic protection: preliminary results
from the first 12 patients treated with this new technique. J Am
Coll Surg. 183:597–605. 1996.PubMed/NCBI
|
|
20
|
Hatano E, Tanaka A, Shinohara H, et al:
Superiority of HTK solution to UW solution for tissue oxygenation
in living related liver transplantation. Transplant Proc.
28:1880–1881. 1996.PubMed/NCBI
|
|
21
|
Raab R, Schlitt HJ, Oldhafer KJ,
Bornscheuer A, Lang H and Pichlmayr R: Ex-vivo resection techniques
in tissue-preserving surgery for liver malignancies. Langenbecks
Arch Surg. 385:179–184. 2000. View Article : Google Scholar : PubMed/NCBI
|