Introduction
Human glioma is the most common primary tumor in the
central nervous system and is characterized by a high proliferative
and invasive ability (1). Standard
therapies for glioma, including surgery, radiation and
chemotherapy, are only effective in treating patients with a
high-grade condition. Numerous glioma patients have already
developed metastasis at the onset of clinical symptoms (2). The mechanism of glioma tumorigenesis
remains unclear, and the molecular determinants of the
aggressiveness of glioma have been the subject of numerous studies,
but these investigations have not yet reached full fruition
(3–6).
Therefore, there is an acknowledged requirement for novel
approaches based on increased understanding of the biological and
molecular nature of these tumors.
microRNAs (miRNAs) are short non-coding,
single-stranded RNA molecules that are 22–25 nucleotides in length
and negatively regulate gene expression by post-transcriptional
silencing of target messenger RNAs (mRNAs) through complementary
binding (7,8). An increasing number of studies have
indicated that miRNA plays an important role in the development of
various cancers, including glioma, and miRNA has been associated
with tumor suppressor and oncogenic activities (9,10). Out of
these miRNA molecules, miRNA 218 (miR-218) has been revealed to be
downregulated in human glioblastoma multiforme (GBM) specimens
compared with the adjacent brain tissue that is devoid of tumor
cells (11–14). Accumulated data have demonstrated that
the upregulation of miR-218 is able to inhibit tumor cell invasion
and proliferation in glioma cells by altering the expression of
multiple target genes (14–17).
In the present study, the expression of miR-218 was
upregulated by transient transfection of the human glioma U251,
U87, SNB19 and LN229 cells with miR-218 mimics. This was performed
with the aim of affecting the cyclin dependent kinase (CDK)6/cyclin
D1/p21Cip1/Waf1 pathway and demonstrating that miR-218
inactivates the CDK6/cyclin D1/p21Cip1/Waf1 pathway by
directly targeting the 3′-untranslated region (UTR) of CDK6.
Furthermore, it was not only found that the stable expression of
miR-218 inhibited proliferation in vitro and suppressed
tumorigenicity of glioma cells in vivo, but it was also
found that the expression of CDK6 and cyclin D1 in xenograft tumor
tissues was significantly decreased, in contrast to the expression
of p21Cip1/Waf1, which was significantly increased. In
summary, the present results suggest that miR-218 inhibits the
proliferation of glioma cells through the inactivation of the
CDK6/cyclin D1/p21Cip1/Waf1 signaling pathway.
Materials and methods
Clinical samples
Tumor specimens were obtained from patients that
underwent positive debulking surgery in the Neurosurgery Department
of The First Affiliated Hospital of Soochow University (Suzhou,
China) between 2011 and 2013. The diagnosed gliomas were reviewed
on histological slides by an experiential neuropathologist,
according to the 2007 World Health Organization classification
(18), resulting in 20 glioma samples
being classified as grades I and II, 20 as grade III and 20 as
grade IV. In total, 10 normal brain tissue samples were obtained
from the internal decompression of patients with cerebral injury.
For the use of these clinical materials for research purposes,
patient consent and approval from the Ethics Committee of Taizhou
People's Hospital (Taizhou, China) and The First Affiliated
Hospital of Soochow University was obtained prior to the present
study.
Cell lines and transfection
Primary normal human astrocytes (NHAs) were
purchased from Sciencell Research Laboratories (Carlsbad, CA, USA).
The glioma U251, U87, SNB19 and LN229 cell lines were obtained from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Beijing, China). The cells were maintained in RPMI-l640
medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine
serum, 50 units/ml penicillin G and 250 µg/ml streptomycin
(Invitrogen) in a humidified atmosphere containing 5%
CO2 at 37°C. Transfections with miR-218 were performed
in serum-free medium 24 h subsequent to plating, using
Lipofectamine 2000 (Invitrogen). After 6 h, the cells were placed
in complete medium and maintained at 37°C in a 5% CO2
atmosphere.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted from
cultured cells or fresh glioma cancer tissues using TRIzol reagent
(Invitrogen), according to the manufacturer's instructions. The
expression of miR-218 was quantified using the miRNA-specific TaqMan
miRNA Assay kit (Applied Biosystems Life Technologies, Foster City,
CA, USA). U6 small nuclear RNA was used as an internal control. The
mRNA expression of CDK6, cyclin D1 and p21Cip1/Waf1 was
analyzed by qPCR using the SYBR-Green method (7500 ABI; Applied
Biosystems Life Technologies). The protocols were performed
according to the manufacturer's instructions and the results were
normalized to the expression of GAPDH. The primers used were as
follows: CDK6 forward, 5′-CTGAAT GCTCTTGCTCCTTT-3′ and reverse,
5′-AAAGTTTTGGTG GTCCTTGA-3′; cyclin D1 forward, 5′-TCCTCTCCAAAA
TGCCAGAG-3′ and reverse, 5′-GGCGGATTGGAAATG AACTT-3′; p21 forward,
5′-CGATGCCAACCTCCTCAA CGA-3′ and reverse,
5′-TCGCAGACCTCCAGCATCCA-3′; and GAPDH forward,
5′-TCGGAGTCAACGGATTTGG-3′ and reverse,
5′-CATGGGTGGAATCATATTGGA-3′.
Western blotting
Cells were lysed using 1% nonidet P-40 lysis buffer
48 h subsequent to exposure of the cells to LY294002 or vehicle
treatment. Homogenates were clarified by centrifugation at 20,000 ×
g for 15 min at 4°C, and the protein concentrations were determined
using a bicinchoninic acid protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). SDS-PAGE was performed on 40 µg of
protein from each sample. The gels were then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) and incubated with polyclonal rabbit anti-human CDK6
(dilution, 1:200; cat. no. sc-177; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), polyclonal mouse anti-human cyclin D1 (dilution,
1:200; cat. no. sc-29287; Santa Cruz Biotechnology, Inc.) and
polyclonal mouse anti-human p21Cip1/Waf1 (dilution,
1:100; cat. no. sc-44271; Santa Cruz Biotechnology, Inc.) primary
antibodies, which was followed by incubation with horseradish
peroxidase-conjugated monoclonal goat anti-rabbit or anti-mouse IgG
secondary antibodies (dilution, 1:1,000; cat. no's. W10804 and
W10815; Zymed Life Technologies, Carlsbad, CA, USA). The membranes
were stripped and reprobed with a primary polyclonal mouse
anti-human GAPDH antibody. The protein bands were quantitated by
densitometry using the gel analysis ImageJ software (National
Institutes of Health, Bethesda, MA, USA). The values were
normalized to the expression of GAPDH (dilution, 1:1,000; cat. no.
sc-48167; Santa Cruz Biotechnology, Inc.).
Luciferase assay
The CDK6 3′-UTR sequence that was predicted to
interact with miR-218 and a mutated sequence containing the
predicted target sites were synthesized and inserted into the
XbaI and FseI sites of a pGL3 control vector
(Promega, Madison, WI, USA). These constructs were termed
pGL3-CDK6-3′UTR and pGL3-CDK6-3′UTR-mut. For the reporter assay,
the U87 cells were plated onto 24-well plates and transfected with
pGL3-CDK6-3′UTR or pGL3-CDK6-3′UTR-mut, and P-miR-218 or
P-miR-control vectors using FuGENE HD (Promega, Madison, WI, USA).
A Renilla luciferase vector, pRL-SV50 (Promega), was
cotransfected to normalize the differences in transfection
efficiency. Subsequent to transfection for 48 h, the cells were
harvested and assayed using the Dual-Luciferase Reporter Assay
system (Promega) according to the manufacturer's instructions.
Transfection was repeated three times in triplicate.
Proliferation assay
The proliferative ability of cells was measured by
using the cell counting kit-8 (CCK-8) at 24, 48 and 72 h
post-transfection, according to the manufacturer's protocol.
Briefly, 10 µl of CCK-8 solution was added to each well. Following
incubation at 37°C for 4 h in 5% CO2, the absorbance of
each well at a wavelength of 490 nm was detected using a microplate
reader (Multiskan Spectrum; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Clonogenicity assay
The stably-transfected glioma cells were seeded into
six-well plates and cultured in cell culture medium for two weeks
to allow colony formation. The culture medium was changed every
third day. The colonies were then fixed in 100% methanol and stained
with crystal violet solution. Subsequently, the number of
macroscopically observable colonies was recorded.
Cell cycle assay
The cells were harvested by trypsinization 48 h
subsequent to transfection, washed three times with ice-cold
phosphate-buffered saline (PBS), and fixed with 70% ethanol
overnight at 4°C. The fixed cells were rehydrated in PBS and
subjected to propidium iodide/RNase staining followed by
fluorescence-activated cell sorter scan (FACS) analysis (Becton
Dickinson, Mountain View, CA, USA). The percentage of cells in each
phase of the cell cycle was estimated using PV ELITE software
(Integraph Corporation, Madison, AL, USA).
Animal studies
Stably transfected U87 cells were resuspended in PBS
and implanted into the left flanks of the BALB/c athymic mice, with
1.5×106 cells being administered per flank by
subcutaneous injection. The tumor volumes were determined by
measuring the length (a) in mm, and the width (b) in
mm3, of the mass. The tumor volume (V) was calculated
according to the formula V=ab2/2. The statistical
significance of the difference between the P-miR-218 and
P-miR-control transfected groups was evaluated using Student's
t-test. All procedures that involved animals were performed
according to the guidelines of The First Affiliated Hospital of
Soochow University, Soochow University and Chinese Academy of
Medical Sciences.
Statistical analysis
Experimental data were presented as the mean ±
standard deviation. All statistical analyses were performed using a
two-tailed Student's t-test performed by SPSS software,
version 12.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-218 is downregulated in glioma
tissues
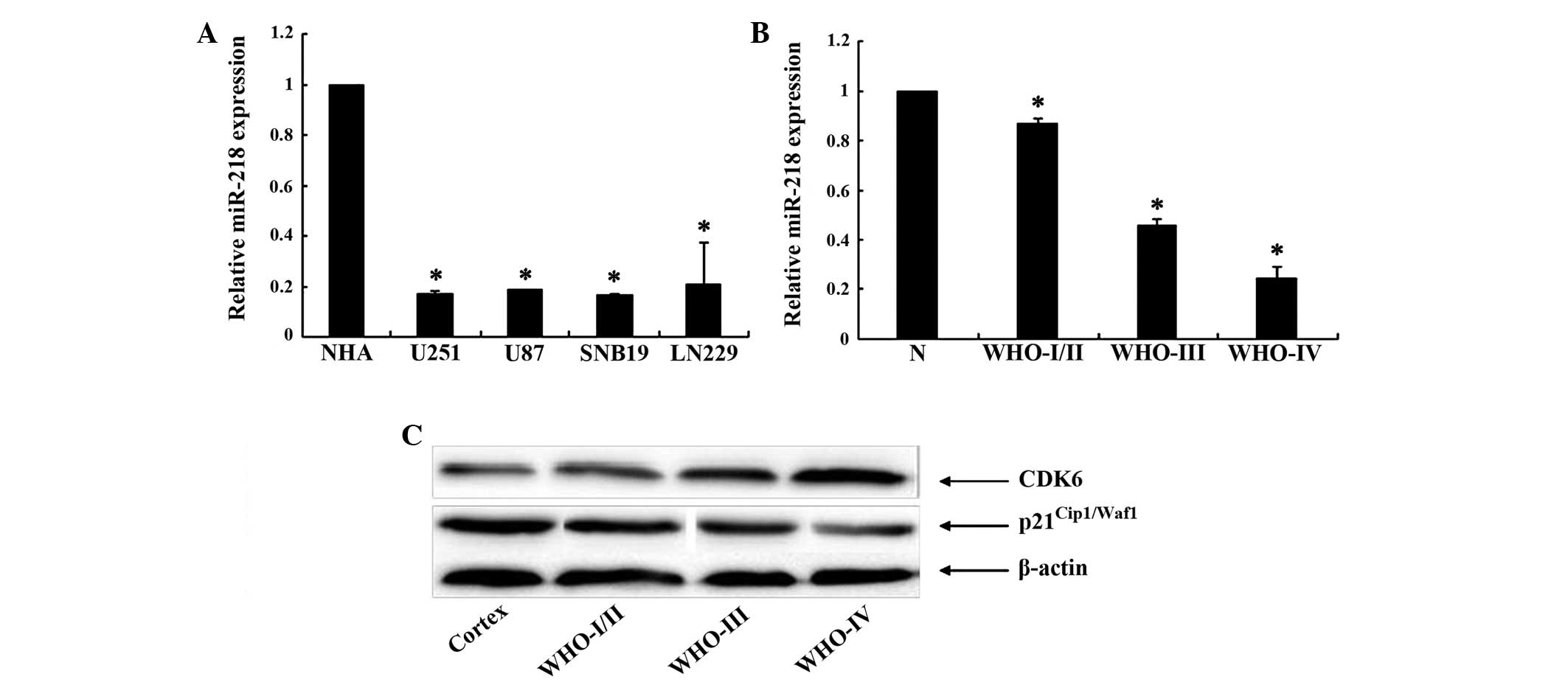
To analyze the expression levels of miR-218, qPCR
was performed to assess miR-218 expression in the glioma U251, U87,
SNB19 and LN229 cell lines. The results revealed that miR-218 was
downregulated in all glioma cell lines compared with the NHAs
(P<0.05). In addition, when miR-218 expression was measured in
10 normal brain and 60 glioma tissue samples, the expression level
of miR-218 was observed to be significantly decreased in glioma
tissues, particularly in grade III/IV tissues (Fig. 1A and B). This result demonstrated that
miR-218 expression decreases markedly from normal brain tissue to
low-grade to GBM tissue. Overall, the present results indicate that
miR-218 was downregulated in glioma cell lines and glioma
tissues.
CDK6 is upregulated and
p21Cip1/Waf1 is downregulated in glioma tissues
The expression levels of CDK6 and
p21Cip1/Waf1 were identified in glioma tissues using
western blot analysis, and it was observed that CDK6 was expressed
at extremely low levels in normal brain tissue, but at extremely
high levels in human glioma tissues. In addition, with the
progression of malignant glioma, the expression of CDK6 is
gradually increased. By contrast, p21Cip1/Waf1 was
expressed at very high levels in normal brain tissue, but at very
low levels in human glioma tissues (Fig.
1C).
Upregulation of miR-218 regulates
expression of CDK6/cyclin D1/p21Cip1/Waf1 in glioma U87
cells
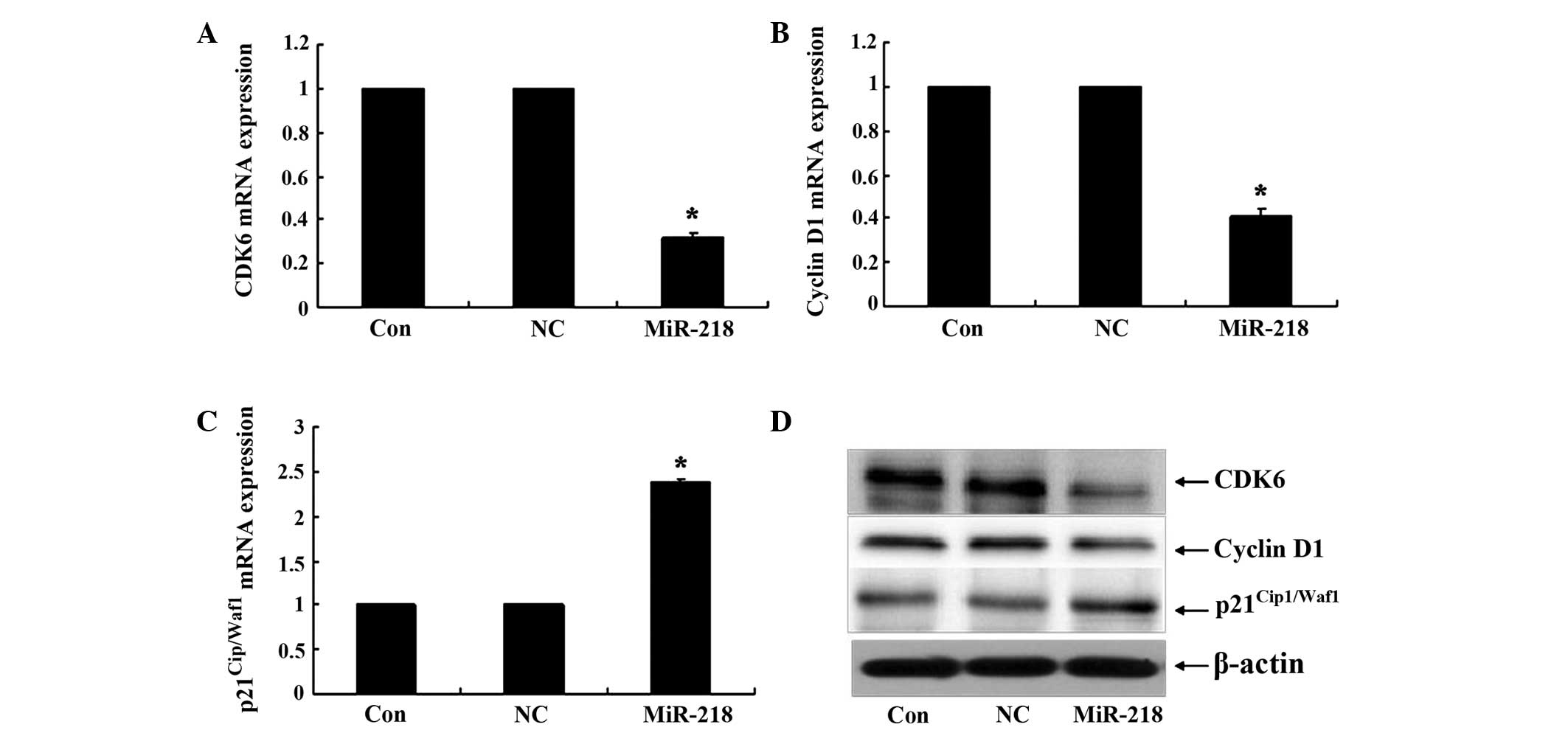
In the present study, the possibility that cell
cycle regulators may be modulated by miR-218 was investigated.
Quantitative PCR analysis revealed that treatment with the miR-218
mimic for 24 h significantly upregulated the mRNA levels of
p21Cip1/Waf1, followed by a decrease in the expression
of CDK6 and cyclin D1 (Fig. 2A–C).
Notably, western blot analysis revealed similar results to the
quantitative PCR analysis by miR-218 overexpression (Fig. 2D). Overall, these results indicated
that miR-218 is able to transcriptionally regulate the expression
of CDK6, cyclin D1 and p21.
CDK6 is a functional downstream target
of miR-218
Using the publicly available algorithms in
TargetScan (Whitehead Institute for Biomedical Research, Cambridge,
MA, USA), CDK6 was theoretically identified as the target gene of
miR-218 (Fig. 3A). A luciferase
reporter assay further confirmed the direct association between
miR-218 and the 3′-UTR of CDK6 mRNA. The luciferase activity for
the wild-type 3′-UTR of CDK6 was significantly inhibited by
cotransfection with miR-218 mimics compared with constructs
containing mutated 3′-UTRs. This demonstrated that CDK6 is a direct
target of miR-218 (Fig. 3B).
Upregulation of miR-218 inhibited cell
proliferation in U87 cells
To determine the effects of miR-218 on glioma cell
proliferation in vitro, cell proliferation and plate
clonogenic assays were used. As shown in Fig. 4A, the cell growth inhibition rate was
evidently increased in the miR-218 mimic group compared with the
control groups at 48 and 72 h post-transfection (P<0.05). In
Fig. 4B and C, stable overexpression
of miR-218 markedly reduced the number of surviving colonies from
the U87 cell line compared with the control and NC groups
(P<0.05). This finding indicates that miR-218 is able to
significantly inhibit the proliferation of glioma cells.
Consequently, a cell cycle analysis was conducted in the U87 cells
transiently transfected with the miR-218 mimics. The results
revealed that the cells transfected with the miR-218 mimics
exhibited an increased accumulation in the
G0-G1 phase with a decreased number of cells
in the S phase (Fig. 4D).
Upregulation of miR-218 suppresses
tumorigenicity of U87 cells in vivo
To substantiate the role of miR-218 in glioma
carcinogenesis, the effects of miR-218 on tumorigenicity of glioma
cells were assessed in vivo. Cells that were stably
transfected with U87-miR-218 mimic or U87-mimic-NC were implanted
into the left flanks of BALB/c athymic mice by subcutaneous
injection. At 28 days post-injection, the mean volumes of tumors
generated from the miR-218 group were significantly smaller compared
with those originating from the NC group (Fig. 5A and B). Western blot analysis of the
glioma xenograft tissues revealed that the expression of CDK6 and
cyclin D1 was decreased in the xenograft tumor tissues derived from
the miR-218 group compared with the NC group. By contrast, the
expression of p21Cip1/Waf1 was significantly increased
(Fig. 5C).
Discussion
Rapid and unrestrained cell proliferation is a
fundamental component of the malignant phenotype of cancer, not
only for the development and growth of primary tumors, but also for
the colonization of metastatic tumor cells in their target organs
(19). Cell cycle progression
involves sequential activation of CDKs, which possess an
association with corresponding regulatory cyclins that is necessary
for their activation (20). As a
critical modulator of the G1 to S phase transition,
increased expression of cyclin D1 in cancer cells results in an
uncontrolled growth advantage. The cyclin D-CDK4/CDK6 and cyclin
E-CDK2 complexes regulate cell cycle transition from the
G1 to S phase by phosphorylating and inactivating the
retinoblastoma (Rb) protein. However, aberrant activation of the
cyclin/CDK complexes can be partly ascribed to the loss or
inactivation of endogenous CDK inhibitors, including
p15Ink4b, p16Ink4a, p21Cip1/Waf1
and p27Kip1 (21). In
addition, miR-153 overexpression in prostate cancer cells has been
found to increase the expression of the G1/S
transitional promoter cyclin 1 and to decrease the expression of
the cyclin-dependent kinase (CDK) inhibitor p21Cip1
(22). However, the underlying
mechanism that modulates the abundance of the cyclins and CDKs in
glioma has yet to be elucidated.
An increasing number of studies have indicated that
microRNAs are associated with proliferation and apoptosis by
negative regulation of the expression of oncogenes or tumor
suppressor genes (23–25). Numerous studies have demonstrated that
the expression level of miR-218 is frequently downregulated in
several human cancers, including gastric cancer, lung squamous cell
carcinoma, malignant astrocytomas and medulloblastomas, which
suggests that miR-218 may function as a tumor suppressor (26–28).
Previous studies have demonstrated that the ectopic expression of
miR-218 contributes to the inhibition of the proliferation,
invasion and migration of glioma cells, and induces apoptosis by
downregulating the directly targeted gene of miR-218. miR-218
inhibits the expression of target genes that include IKK-β, and
inhibits the expression of NF-κB in a dose-dependent manner.
However, miR-218 also reduces the expression of matrix
metalloproteinase-9 (MMP-9) and inhibits the invasive and migratory
ability of glioma cells (14).
EGFR-coamplified and overexpressed protein (ECOP) has also been
identified as a functional downstream target of the genes targeted
by miR-218. ECOP is able to regulate the transcriptional activity
of NF-κB and is associated with the apoptotic response.
Overexpression of miR-218 restrains the activity of NF-κB through
the target gene ECOP, thus inducing glioma cell apoptosis and
inhibiting the activity, proliferation and tumorigenicity of glioma
cells (15). The expression of
lymphoid enhancer-binding factor 1 (LEF1) and MMP-9 in the
high-grade glioma group was extremely high, while the expression in
the low-grade glioma group was extremely low, and the expression of
LEF1 and MMP-9 was negatively correlated with the expression of
miR-218. Overexpression of miR-218 has been found to inhibit the
Wnt/LEF1 signaling pathways that lead to a reduction in MMP-9
synthesis and inhibit tumor invasion (16). The abnormal expression of miR-218 in
glioma cells decreased, but abnormal increase CDK6 expression, the
expression level of both negatively correlated. Overexpression of
miR-218 in glioma cell line can inhibit CDK6 expression and glioma
cell proliferation and promote its apoptosis (16).
In the present study, the expression levels of
miR-218, CDK6 and p21Cip1/Waf1 were detected in 70
tissue samples obtained from normal brain tissue and low- and
high-grade glioma tissues by RT-qPCR and western blot analysis. It
was found that the expression of miR-218 and
p21Cip1/Waf1 was always inversely associated with the
expression of CDK6. Notably, the protein and mRNA levels of CDK6
and cyclin D1 were significantly decreased and the levels of
p21Cip1/Waf1 were significantly increased subsequent to
the transfection of U87 cells with miR-218 mimics. CDK6 was
identified as a direct functional target of miR-218 using
bioinformatics analysis, and this finding was experimentally
confirmed using a luciferase reporter assay. Therefore, it was found
that miR-218 was involved in the modulation of the CDK6/cyclin
D1/p21Cip1/Waf1 pathway and downregulation of CDK6
expression by directly targeting the 3′-UTR of CDK6. In the gain of
function investigation, the overexpression of miR-218 was found to
inhibit the proliferation of glioma cells proliferation and result
in a G0/G1 phase arrest of the cell cycle
in vitro. miR-218 may also suppress the tumorigenicity of
glioma cells in vivo. The results of western blot analysis
substantiate that the expression of CDK6 and cyclin D1 in xenograft
tumor tissues was significantly decreased. By contrast, the
expression of p21Cip1/Waf1 was significantly increased.
Overall, the present results indicate that miR-218 inhibits the
proliferation of glioma cells through targeting CDK6 to inhibit
cyclin D1 activity and activate endogenous CDK inhibitors of
p21Cip1/Waf1 activity.
In conclusion, the present study reveals an
association between miR-218-mediated downregulation of glioma cell
proliferation and the inactivation of CDK6/cyclin
D1/p21Cip1/Waf1 signaling. The present results suggest
that miR-218 is critical for the inhibition of proliferation of
glioma cells, and understanding the role of miR-218 may provide
important insights into the treatment of gliomas.
References
|
1
|
Yu X, Zhang W, Ning Q and Luo X:
MicroRNA-34a inhibits human brain glioma cell growth by
down-regulation of Notch1. J Huazhong Univ Sci Technolog Med Sci.
32:370–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou SX, Ding BJ, Li HZ, Wang L, Xia F, Du
F, Liu LJ, Liu YH, Liu XD, Jia JF, et al: Identification of
microRNA-205 as a potential prognostic indicator for human glioma.
J Clin Neurosci. 20:933–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang R, Jiao Z, Li R, et al: p68 RNA
helicase promotes glioma cell proliferation in vitro and in vivo
via direct regulation of NF-κB transcription factor p50. Neuro
Oncol. 14:1116–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng SY, Dong CG, Wu WK, et al: Lentiviral
expression of anti-microRNAs tar geting miR-27a inhibits
proliferation and invasiveness of U87 gliomacells. Mol Med Rep.
6:275–281. 2012.PubMed/NCBI
|
|
6
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long JM and Lahiri DK: Advances in
microRNA experimental approaches to study physiological regulation
of gene products implicated in CNS disorders. Exp Neurol.
235:402–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park S and James CD: ECop
(EGFR-coamplified and overexpressed protein), a novel protein,
regulates NF-kappaB transcriptional activity and associated
apoptotic response in an IkappaBalpha-dependent manner. Oncogene.
24:2495–2502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu B, Hsu PK, Karayiorgou M and Gogos JA:
MicroRNA dysregulation in neuropsychiatric disorders and cognitive
dysfunction. Neurobiol Dis. 46:291–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao SAM, Santosh V and Somasundaram K:
Genome-wide expression profiling identifies deregulated miRNAs in
malignant astrocytoma. Mod Pathol. 23:1404–1417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song L, Huang Q, Chen K, Liu L, Lin C, Dai
T, Yu C, Wu Z and Li J: miR-218 inhibits the invasive ability of
glioma cells by direct downregulation of IKK-β. Biochem Biophys Res
Commun. 402:135–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia H, Yan Y, Hu M, et al: MiR-218
sensitizes glioma cells to apoptosis and inhibits tumorigenicity by
regulating ECOP-mediated suppression of NF-κB activity. Neuro
Oncol. 15:413–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Yan W, Zhang W, et al: MiR-218
reverses high invasiveness of glioblastoma cells by targeting the
oncogenic transcription factor LEF1. Oncol Rep. 28:1013–1021.
2012.PubMed/NCBI
|
|
17
|
Zhang JM, Sun CY, Yu SZ, et al:
Relationship between miR-218 and CDK6 expression and their
biological impact on glioma cell proliferation and apoptosis.
Zhonghua Bing Li Xue Za Zhi. 40:454–459. 2011.[(In Chinese)].
PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai J, Wu J, Zhang H, Fang L, Huang Y,
Yang Y, Zhu X, Li R and Li M: miR-186 downregulation correlates
with poor survival in lung adenocarcinoma, where it interferes with
cell-cycle regulation. Cancer Res. 73:756–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Z, He B, He J and Mao X: Upregulation
of miR-153 promotes cell proliferation via downregulation of the
PTEN tumor suppressor gene in human prostate cancer. Prostate.
73:596–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao B, Tan L, He B, et al: MiRNA-329
targeting E2F1 inhibits cell proliferation in glioma cells. J
Transl Med. 11:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petrocca F, Visone R, Onelli MR, Shah MH,
Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini
M, et al: E2F1-regulated microRNAs impair TGFbeta-dependent
cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell.
13:272–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|