Introduction
Ovarian cancer is one of the most commonly diagnosed
cancer types of the female genital tract (1). Its incidence is highest in developed
countries, although the risk of ovarian cancer may be increased by
high parity and the use of oral contraceptives (2). Epithelial ovarian cancer (EOC) accounts
for 85–90% of all ovarian carcinomas (3). EOC is often asymptomatic and, currently,
no well-established strategies exist for its early detection. The
disease spreads readily by direct exfoliation of cells throughout
the peritoneal cavity and often recurs at the surface of the
peritoneum. Furthermore, it may spread via the lymphatic and
hematogenous routes prior to causing any symptoms (4). The majority of patients with ovarian
carcinoma are diagnosed with advanced-stage disease. Although
optimal cytoreductive surgery and improved chemotherapy have
increased the five-year survival rates, the overall survival gains
have been limited due to an inability to eradicate all the cancer
cells. Therefore, out of all the gynecological cancer types,
ovarian carcinoma remains the leading cause of mortality (5). The precise mechanisms underlying
invasion and metastasis remain poorly understood. Therefore, it is
important to clarify the mechanism of dissemination in order to
increase survival and prevent the metastasis and dissemination of
ovarian carcinoma cells.
Degradation of the extracellular matrix is required
for tumor growth and metastasis (6,7). It is
primarily regulated by a variety of proteinases, which are known to
influence cellular activities, migration and invasion (8,9).
Dipeptidyl peptidase IV (DPPIV) and seprase/fibroblast activation
protein α (FAPα) are membranous serine-type membrane peptidase
(SIMP), type II transmembrane proteins with multiple functions,
including dipeptidase activities (10). A number of studies have revealed that
DPPIV is expressed in cancer cells and is involved in tumor
progression and invasion (11,12).
Although ovarian carcinoma is a malignancy of the female genital
tract with one of the highest mortality rates, studies concerning
seprase and DPPIV expression in ovarian carcinoma tissues are
lacking. The practical significance of these genes in patients with
ovarian cancer remains largely unknown and, therefore, requires
further investigation. The aim of the present study was to
investigate the expression of DPPIV [also known as cluster of
differentiation (CD)26] and seprase/FAPα in EOC cells at the
protein and mRNA levels. In addition, the association between the
DPPIV and seprase expression levels and known clinicopathological
prognosticators, including the tumor International Federation of
Gynecology and Obstetrics (FIGO) stage, tumor grade and disease
outcome, were assessed in order to determine their clinical
value.
Materials and methods
Ovarian carcinoma cell lines
The commercial ovarian carcinoma cell lines, OVCA-3
and SKOV-3, were provided by the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China). Immunohistochemical analysis (IHC)
and reverse transcription-polymerase chain reaction (RT-PCR) were
used to determine the mRNA and protein expression levels of DPPIV
and seprase in the cell lines.
Patients and samples
The present study included 199 patients with
epithelial ovarian tumors who underwent surgery at the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between 2005 and 2010. A total of 199 formalin-fixed and
paraffin-embedded tumor specimens were collected. The study was
approved by the Institutional Ethics Review Board of Zhengzhou
University (Zhengzhou, China). In addition, 31 fresh ovarian cancer
tissue samples were frozen and stored at −80°C. The tissue sections
were stained with antibodies and hematoxylin and eosin
(Sigma-Aldrich, St. Louis, MO, USA) for morphological evaluation.
Clinicopathological information was retrieved from the medical
records of the patients. Staging was performed according to the
FIGO guidelines (FIGO, 2000) (13).
The clinical features of the patients are summarized in Table I. This study was approved by the
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University and was performed according to the Declaration of
Helsinki. Written informed consent was obtained from each patient's
family.
 | Table I.Clinicopathological features and
expression levels of seprase and DPPIV proteins in patients with
epithelial ovarian cancer. |
Table I.
Clinicopathological features and
expression levels of seprase and DPPIV proteins in patients with
epithelial ovarian cancer.
|
|
| DPPIV proteins | Seprase proteins |
|---|
|
|
|
|
|
|---|
| Parameters | Case number (n=128),
n | Positive (%) |
χ2-value | P-value | Positive (%) |
χ2-value | P-value |
|---|
| FIGO stage |
|
| 9.311 | 0.01a |
| 6.865 | 0.032a |
| I | 31 | 21 (67.7) |
|
| 21 (72.4) |
|
|
| II | 40 | 32 (80.0) |
|
| 35 (85.4) |
|
|
| III | 57 | 53 (90.0) |
|
| 54 (93.1) |
|
|
| Histology |
|
| 2.326 | 0.313 |
| 3.188 | 0.203 |
|
Serous | 78 | 67 (85.9) |
|
| 69 (88.5) |
|
|
|
Mucous | 41 | 33 (80.5) |
|
| 35 (85.4) |
|
|
|
Others | 9 | 6 (66.7) |
|
| 6 (66.7) |
|
|
| Grade |
|
| 2.889 | 0.236 |
| 0.74 | 0.691 |
| G1 | 27 | 20 (74.1) |
|
| 22 (81.5) |
|
|
| G2 | 41 | 33 (80.5) |
|
| 35 (85.4) |
|
|
| G3 | 60 | 53 (88.3) |
|
| 53 (88.3) |
|
|
| Age/year |
|
| 0.003 | 0.959 |
| 0.08 | 0.778 |
|
≥60 | 75 | 62 (82.7) |
|
| 65 (86.7) |
|
|
|
≤60 | 53 | 44 (83.0) |
|
| 45 (84.9) |
|
|
| LN |
|
| 4.23 | 0.04a |
| 6.223 | 0.013a |
|
Positive | 48 | 44 (91.7) |
|
| 46 (95.8) |
|
|
|
Negative | 80 | 62 (77.5) |
|
| 64 (80.0) |
|
|
Characteristics of antibodies
used
The rat monoclonal antibodies against DPPIV and
seprase were obtained from Abcam (cat. nos. ab119346 and ab53066,
respectively; Cambridge, MA, USA). Subsequent to optimizing the
antibody dilutions, IHC was performed using a LabVision Autostainer
720 (Thermo Fisher Scientific, Waltham, MA, USA). The
deparaffinized sections were microwaved in 10 mM citrate buffer (pH
6.0) in order to expose the epitopes. Following a 5-min incubation
with 0.03% hydrogen peroxide to block endogenous peroxidase
activity, the slides were washed with Tris-buffered saline (TBS)
and incubated with the primary anti-DPPIV and anti-seprase rat
monoclonal antibodies (dilution, 1:75) at 4°C overnight. Next, the
slides were incubated with the rabbit anti-rat immunoglobulin G
(IgG; dilution, 1:200; cat. no. ab6728) secondary antibody (Abcam)
for 30 min. The slides were then incubated with a
peroxidase-labeled polymer and conjugated to a goat anti-rabbit
antibody (dilution 1:200; cat. no. ab150081; Abcam) for 30 min.
Next, the slides were stained with 3,3′-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich) for 10 min, counterstained with
hematoxylin, dehydrated and then mounted in Diatex mounting medium
(Diatex S.A., Lyon, France).
The ovarian carcinoma DPPIV-positive and
seprase-positive cell lines, OVCAR-3 and SKOV-3, were used as
positive controls in the present study. The negative controls
consisted of the replacement of the primary antibody with IgG at
the same concentration and from the same source. All the controls
provided satisfactory results.
The IHC results were evaluated according to the
pattern, intensity and extent of staining as follows: i) Staining
pattern, demonstrating the presence of membranous, cytoplasmic or
nuclear staining; ii) extent of staining, represented with the
following scale: 0, no staining; 1, staining of <10% of cancer
cells; 2, staining of 11–50% of cancer cells; and 3, staining of
>50% of cancer cells. Overall, at least 300 cells were analyzed;
and iii) staining intensity, represented with the following scale:
0, negative; 1, weak; 2, moderate; and 3, strong. After setting the
aforementioned scoring criteria of the tumor cells, the IHC results
were scored according to the intensity and extent of staining by
two independent senior pathologists who were blinded to the
clinicopathological data. The discordant scores were re-evaluated,
and the consensus score was used for further analysis. Based on the
intensity and extent of staining, the IHC results were scored
between 0 and 3 as follows: 0, negative; 1, weak; 2, moderate; and
3, strong.
In situ mRNA hybridization
Specific primers (listed in Table II), including the T7 RNA polymerase
promoter sequence at the 5′ end, were designed using Primer Premier
version 5.0 and synthesized. PA15His (full length seprase cDNA) and
pcD26His (DPPIV) were obtained from Dr Wen-Tian Chen (State
University of New York at Stony Brook, Stony Brook, NY, USA) and
used to prepare the DNA templates. The PCR-produced DNA templates
and the primers, which included the T7 RNA polymerase promoter site
at their 5′ end, were used for in vitro transcription
according to the In vitro Transcription T7 Kit (Ambion Life
Technologies; Austin, TX, USA) manufacturer's instruction.
Subsequently, anti-sense and sense fluorescein isothiocyanate
(FITC)-cRNA probes (Ambion Life Technologies) were synthesized
against DPPIV and seprase.
 | Table II.Polymerase chain reaction primers
containing T7 RNA polymerase promoter sequence at the 5′ end. |
Table II.
Polymerase chain reaction primers
containing T7 RNA polymerase promoter sequence at the 5′ end.
| Primers | Primer
sequences | Product length | Annealing
temperature |
|---|
| 1-DPPIV |
|
|
|
|
Antisense probes | F: (T7) 5′-TAA TAC
GAC TCA CTA TAGG-ACT-GAA-CTG-GGC-CAC-TTA-CC-3′ |
|
|
|
| R:
5′-GTT-ACG-TAC-CCT-CCA-TAT-GAC-C-3′ | 247bp | 58°C |
| 2-DPPIV |
|
|
|
| Sense
probes | F:
5′-ACT-GAA-CTG-GGC-CAC-TTA-CC-3′ |
|
|
|
| R: (T7) 5′- TAA TAC
GAC TCA CTA TAGG-GTT-ACG-TAC-CCT-CCA-TAT-GAC-C 3′ | 247bp |
|
| 3-Seprase |
|
|
|
|
Anti-sense probes | F: (T7) 5′-TAA TAC
GAC TCA CTA TAGG-GAT-TCT-TCC-TCC-TCA-ATT-TG-3′ |
|
|
|
| R:
5′-GTC-ACC-TTG-GAA-AGC-TGT-TC-3′ | 190bp | 58°C |
| 4-Seprase |
|
|
|
| Sense
probes | F:
5’-GAT-TCT-TCC-TCC-TCA-ATT-TG-3′ |
|
|
|
| R: (T7) 5′-TAA TAC
GAC TCA CTA TAGG -GTC-ACC-TTG-GAA-AGC-TGT-TC-3′ | 190bp |
|
Sections measuring 5 µm were cut, mounted onto
poly-L-lysine coated slides and air-dried. Following dewaxing and
rehydration, the sections were treated with 0.3% Triton X-100
(Sigma-Aldrich) for 15 min at room temperature, followed by a
20-min treatment with 500 µg/µl proteinase K (Takara, Dalian,
China) at 37°C. Next, the samples were incubated with 0.1 M glycine
in phosphate-buffered saline (PBS) for 5 min and then 4%
paraformaldehyde in 0.1 M PBS for an additional 5 min at room
temperature for fixation.
Prehybridization was performed in 20 µl of the
prehybridization solution (Ambion Life Technologies) at 37°C for 1
h, following a 10-min treatment with 0.25% acetic anhydride in 0.1
M triethanolamine-HCl buffer (pH 8.0) at room temperature. The
tissues were incubated overnight in 10 µl hybridization solution
along with FITC-cRNA-probes coated with Sigmacote (Sigma-Aldrich)
at 37°C. The hybridization tissues were incubated with normal
rabbit serum (dilution, 1:25; cat. no. ab7487; Abcam), anti-FITC
antibodies (dilution, 1:300; cat. no. ab19224; Abcam), rabbit
anti-mouse IgG (dilution, 1:50; cat. no. ab6728; Abcam) and
alkaline phosphatase-anti-alkaline phosphatase (dilution, 1:50;
cat. no. ab95462; Abcam) antibodies. The sections were stained with
a NBT/BCIP mixture (Sigma-Aldrich) and then counterstained with
fast red (Sigma-Aldrich). A positive reaction in this assay was
indicated by blue staining. The aforementioned positive controls
and sense probes were used as positive and negative controls for
each hybridization. The scoring criteria were similar to those used
for the IHC.
Microselection
A microselection method (14,15) was
used to avoid normal elements and areas with prominent infiltration
of lymphoid cells. In total, 5-µm frozen sections were cut and
stained with hematoxylin and eosin. Next, a region of tumor cells
in the sections was selected using the Nikon Eclipse 50i microscope
(Nikon Inc; Melville, NY, USA). The corresponding area on the
frozen blocks was then marked and oriented. Next, the block was
trimmed in order to calculate the tumor cell area. The trimmed
frozen block was re-embedded with O.C.T.™ compound (Tissue-Tek,
Torrance, CA, USA), and a new 5-µm frozen section was created from
the re-embedded block to ensure that the cancer cells occupied
>80% of the selected area. Next, 20-µm frozen sections were cut
and transferred into cooled Eppendorf tubes. Subsequent to section
collection, an additional 5-µm frozen section was cut and stained
with hematoxylin and eosin for morphological analysis. If normal
elements were apparent, the sections were not used, and a different
region was selected for microselection.
RT-PCR
Total RNA was extracted from microselected carcinoma
tissues of patients with ovarian carcinoma using the RNeasy Mini
kit (Qiagen, Valencia, CA, USA), according to the manufacturer's
instructions.
The primer pairs for seprase and DPPIV mRNA
(previously mentioned) were used in the present study without the
T7 RNA polymerase promoter sequence at the 5′ end. The PCR
reactions were performed in a 25-µl reaction mixture using the
Qiagen OneStep RT-PCR kit (Qiagen), according to the manufacturer's
instructions (Table II). GAPDH
primers were used as an internal control to generate an amplified
105 bp PCR fragment. The program included steps for reverse
transcription and PCR, starting with reverse transcription of RNA
at 50°C for 30 min. PCR amplification consisted of an initial
heating step at 95°C for 15 min in order to activate the HotStar
Taq DNA polymerase, deactivate the reverse transcriptases and
denature the cDNA. The cDNA was then subjected to 35 cycles of
denaturation at 94°C for 40 sec, 56°C for 40 sec and 72°C for 1 min
for primer extension. The PCR products were visualized by
electrophoresis on a 7.5% polyacrylamide gel. The GAPDH primers
were used as loading controls for each sample. For the negative
control, water was used instead of the template RNA. The OVCAR-3
and SKOV-3 samples were used as the positive controls as they are
known to be seprase- and DPPIIV-positive. The positive and negative
controls produced satisfactory results in all the series. The
images were then semi-quantitatively analyzed by comparing the
intensity of the amplified seprase and DPPIV bands with the control
GAPDH band. Subsequently, the ratio of the intensities of the
DPPIV, seprase and GAPDH bands was recorded and divided into the
following three grades: low, +; moderate, ++; and high, +++.
Western blot analysis
The 20-µm frozen sections obtained from the
microselected tissues were homogenized in the lysis buffer
(Beyotime, Shanghai, China) to preserve the integrity and
phosphorylation activity of the proteins. The lysis buffer
consisted of 1% NP40, 10% glycerol, 20 mM Tris-HCl (pH 7.5), 137 mM
NaCl, 100 mM NaF, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl
fluoride and 10 µg/ml each of leupeptin, pepstatin and aprotinin.
Next, the lysates were sonicated at a power setting of 50 W for a
duration of 5 s, with intervals of 15 s, repeated six times, and
clarified by centrifugation (10,000 × g, 15min, 4°C), followed by
protein quantification using Bradford analysis.
The samples (20 µg proteins/each lane) were
separated by 7.5% SDS-PAGE and then blotted onto Millipore
immobilon-P membranes (EMD Millipore, Billerica, MA, USA). Next,
the membranes were blocked for 1 h at room temperature with 5%
non-fat dry milk in TBS/Tween-20 (TBST). The membranes were
incubated overnight at 4°C with mAb D8 (dilution, 1:800) and E26
(dilution, 1:800). A mouse monoclonal anti-β-actin antibody (mouse
IgG1 isotype; Sigma-Aldrich) was used as the loading control. The
reactions with the anti-rat and anti-mouse IgG (heavy + light
chains) horseradish peroxidase conjugates (Promega Corporation,
Madison, WI, USA) diluted with TBST (dilution, 1:5000), in addition
to the washing color reaction, were assessed using the ECL Plus
Western Blotting Detection Reagent kit (GE Healthcare Life
Sciences, Little Chalfont, UK). Subsequently, the blots were
exposed in an X-ray film cassette (Dskar Health Care Co., Ltd.;
Guangzhou, Guangdong, China) and immediately developed.
The negative controls consisted of antibodies in the
absence of lysate, whereas the positive controls consisted of
samples from the OVCAR-3 and SKOV-3 cell lines.
The images were semi-quantitatively analyzed by
determining the ratio of DPPIV and seprase to actin. The intensity
ratio of the DPPIV or seprase bands and the β-actin band was
recorded and divided into three grades, as follows: low, +;
moderate, ++; and high, +++.
Statistical analysis
Statistical analyses were performed using the SPSS
version 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
The statistical significance of the intergroup differences was
evaluated using the χ2 test. Survival analyses were
performed using the Kaplan-Meier product-limit method, and the
differences were evaluated using the log-rank test. The
disease-free survival period accounted for the time between surgery
and first recurrence, metastasis or mortality. To further
investigate the relationship between overall survival and the
prognostic factors, a Cox proportional hazard model was applied.
All the statistical tests were two-sided. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
IHC of seprase and DPPIV
IHC identified positive staining for the DPPIV
protein in 106 of the 128 patients (85.94%) with ovarian cancer.
This was significantly higher compared with the results observed
for the borderline ovarian tumor (56.09%) and benign ovarian tumor
(16.66%) patients. In total, 22 (17.19%), 28 (21.88%), 40 (31.25%)
and 38 (29.69%) of the 128 tissue samples from ovarian cancer
patients demonstrated negative, weak, moderate and strong DPPIV
protein expression, respectively. Furthermore, 116 of the 128 cases
(85.94%) demonstrated positive staining for seprase, while 18
(14.06%), 35 (27.34%), 25 (19.53%) and 50 (39.06%) of the 128
patients were negative, weak, moderate or strong for seprase
protein expression, respectively.
Although both the cancer and reactive mesothelial
cells expressed seprase and DPPIV, the levels were consistently
higher in the carcinoma cells. Protein expression was cytoplasmic
and/or membranous in the tumor cells, whereas it was exclusively
cytoplasmic in the mesothelial cells. Furthermore, DPPIV and
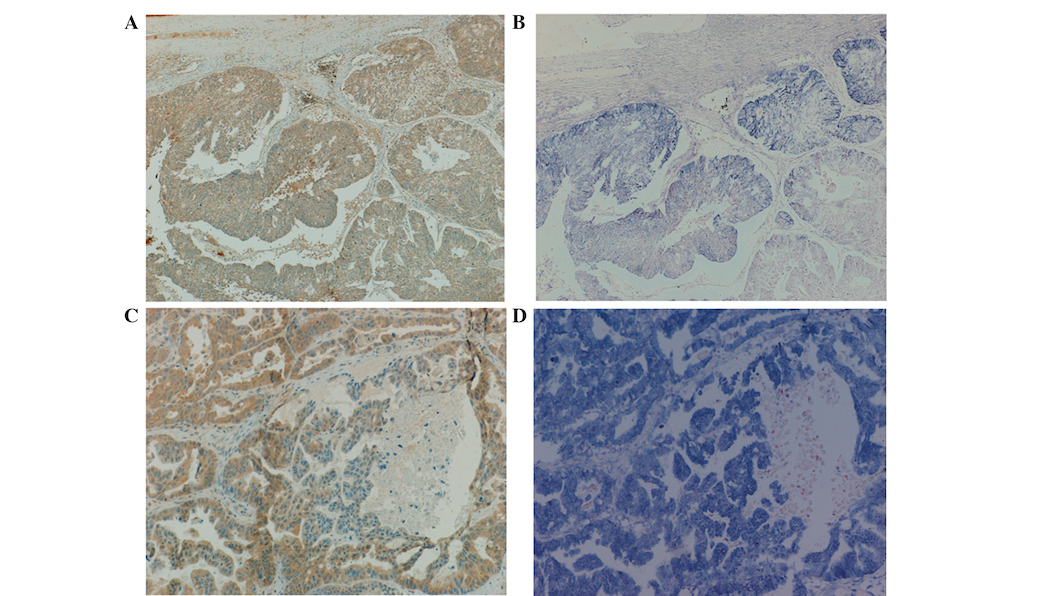
seprase were found to often co-localized in the same tumor regions
(Fig. 1), and a positive correlation
was identified between DPPIV and seprase protein expression
(rs=0.504, P=0.001). Higher expression levels of
the proteins were identified in the cancer tissues when compared
with the levels in borderline ovarian tumors (56.09%) or benign
ovarian tumors (16.66%; Table
III).
 | Table III.DPPIV and seprase expression levels
in different ovarian tissues. |
Table III.
DPPIV and seprase expression levels
in different ovarian tissues.
|
|
| DPPIV | Seprase |
|---|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ | % | − | + | ++ | +++ | % |
|---|
| Benign tumor | 30 | 25 | 3 | 2 | 0 | 16.66 | 24 | 4 | 2 | 0 | 20.00 |
| Borderline
tumor | 41 | 18 | 14 | 7 | 2 | 56.09 | 16 | 12 | 12 | 1 | 60.97 |
| Malignant
tumor | 128 | 22 | 28 | 40 | 38 | 82.81a | 18 | 35 | 25 | 50 | 85.93a |
Association between seprase and DPPIV
immunoreactivity and clinical pathological features
In order to examine the clinicopathological
significance of seprase and DPPIV expression, their association
with a number of clinicopathological factors was investigated. The
expression levels of the seprase and DPPIV proteins increased with
increasing FIGO stage (P=0.013 and P=0.023). Furthermore, the
expression levels of seprase and DPPIV were significantly higher in
the EOC patients with lymph node metastasis compared with those
without lymph node metastasis. However, this observation did not
correlate with the histological grade (P>0.05). In addition, no
significant difference was detected among various age groups and
histological types for seprase or DPPIV (Table I).
Seprase and DPPIV mRNA expression
levels by in situ hybridization (ISH)
ISH using FITC-labeled cRNA probes was used to
detect signals in the cancer and stromal cells. In the control
experiments, which used sense probes, signals were not detectable
in any tissue region. Of the 86 EOC samples, 96.5% (83/86) stained
positive for seprase mRNA by ISH. Based on the results of the
semi-quantitative RT-PCR, 3 (3.49%), 21 (24.42%), 36 (41.86%) and
26 (30.23%) of the tumors exhibited negative, weak, moderate or
strong seprase mRNA expression, respectively. Seprase mRNA
expression was correlated with the corresponding protein as
demonstrated by the IHC results (rs=0.48,
P=0.001). As for DPPIV, 2 (2.33%), 18 (20.93%), 40 (46.51%) and 26
(30.23%) of the cases demonstrated negative, weak, moderate or
strong mRNA expression, respectively. A significant association was
observed between the protein and mRNA expression of DPPIV and
seprase (rs=0.66, P=0.001). In addition, the mRNA
expression profiles were similar to the protein levels.
RT-PCR
Variable levels of seprase mRNA were observed among
the 31 tumors that were analyzed. Of those, 29 cases demonstrated a
degree of DPPIV expression following microselection-assisted RT-PCR
(Fig. 2).
 | Figure 2.DPPIV and seprase reverse
transcription-polymerase chain reaction products in ovarian tumors
and carcinoma cell lines. Seprase (top panel): Lane 1, ovarian
carcinoma OVCA-3 cell line (+++); lanes 2 and 7, two representative
samples for the borderline tumor tissue (+); lanes 3, 5 and 6,
three representative samples for the ovarian carcinoma tissue (++
and +++); lane 4, benign ovarian tumor tissue (+); lane 8, ovarian
carcinoma SKOV-3 cell line (++). DPPIV (bottom panel): Lane 1,
ovarian carcinoma OVCA-3 cell line (+++); lanes 2 and 4, two
representative samples for the borderline tumor tissue (++, +);
lanes 3, 5 and 7, three representative samples for the ovarian
carcinoma tissue (+++); and lanes 6 and 8, two representative
samples for the benign ovarian tumors (–). DDPIV, dipeptidyl
peptidase IV. |
Immunoblotting
In total, 25 out of the 31 tumor samples were
detected to present the dimeric (170-kDa) and monomeric (97-kDa)
forms of seprase. However, following western blot analysis, only
one case exhibited the 170-kDa dimeric form alone. A 200–220 kDa
DPPIV form was identified in 29 out of the 31 samples (Fig. 3). The DPPIV and seprase protein were
detected in the human EOC cell lines, SKOV3 and OVCAR3.
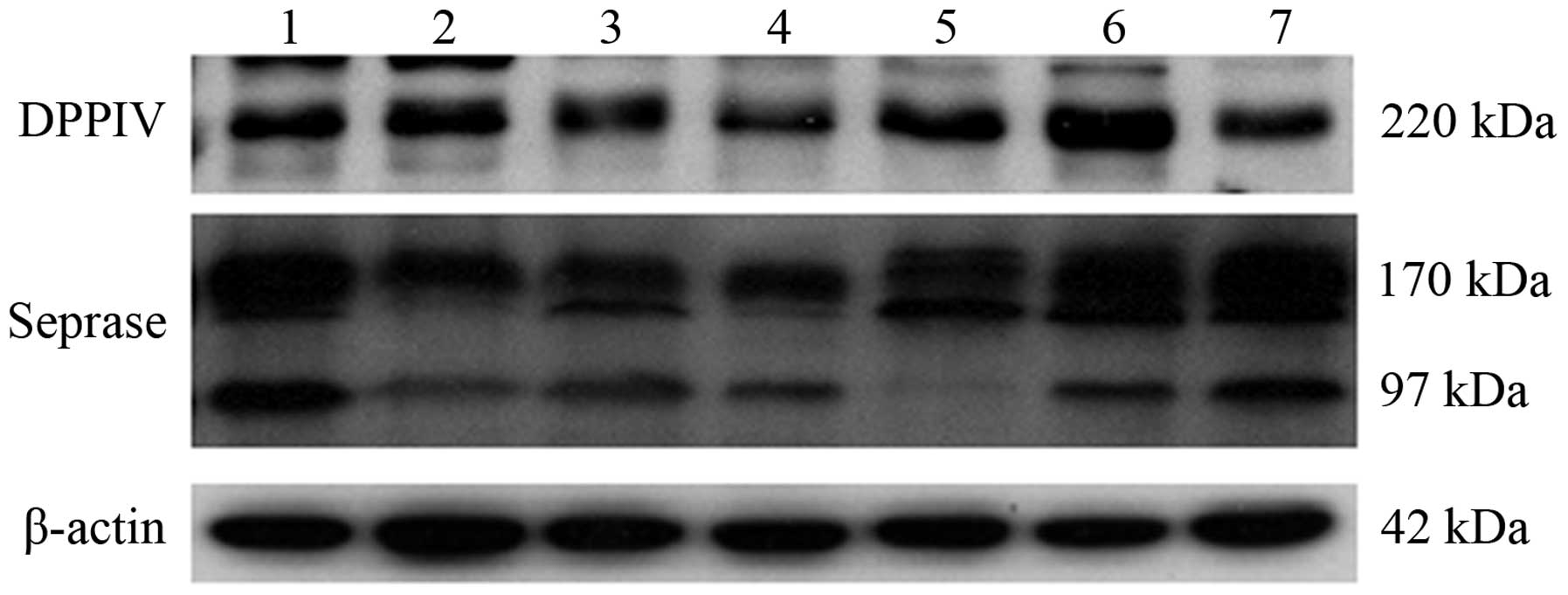 | Figure 3.Western blotting results for the
ovarian carcinoma samples and cell lines. DPPIV (top panel): Lane
1, ovarian carcinoma OVCAR3 cell line (++); lane 2, ovarian
carcinoma SKOV3 cell line (++); lane 3, borderline ovarian tumor
tissue (+); lane 4, benign ovarian tumor tissue (+); lanes 5 and 7,
two representative samples for the ovarian carcinoma tissue (++);
lane 6, ovarian carcinoma tissue (+++). Seprase (middle panel):
Lane 1, ovarian carcinoma OVCAR3 cell line (++); lane 2, ovarian
carcinoma SKOV3 cell line (++); lane 3, borderline ovarian tumor
tissue (++); lane 4, benign ovarian tumor tissue (+); lanes 5–7,
three representative samples for the ovarian carcinoma tissues
(+++). β-actin (bottom panel). DDPIV, dipeptidyl peptidase IV. |
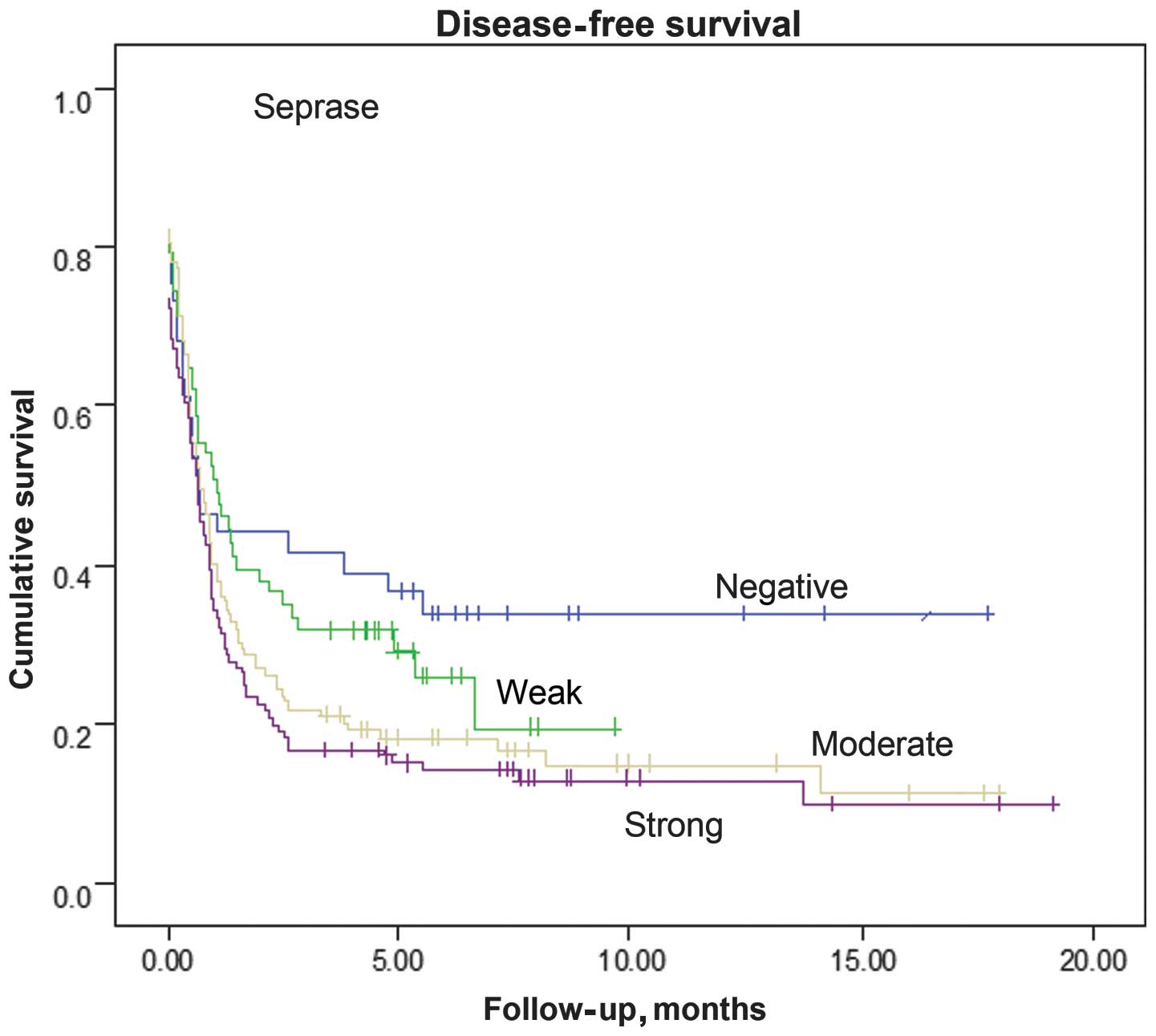
Survival
Univariate analysis of the 128 patients included in
this cohort revealed that increased levels of seprase, but not
DPPIV, were significantly associated with a decreased probability
of disease-free survival (P=0.03 and P=0.52, respectively; Fig. 4). The survival rates of the patients
within each histological subgroup did not differ between the
seprase-positive and -negative tumors (data not shown). Therefore,
several variables were investigated in order to evaluate whether
they had an impact on survival. In the Cox multivariate analysis of
the FIGO stage, age groups and histological grade were included,
with seprase being an independent risk factor for poor outcome.
Discussion
DPPIV, seprase/FAPα and other associated prolyl
serine peptidases are serine-type integral membrane peptidases
(SIMPs) (10). SIMPs exhibit high
structural homology, with a cytoplasmic tail that contains six
amino acids (a.a.), a 20-a.a. (seprase) or 22-a.a. (DPPIV)
transmembrane domain at the N terminus, an N-glycosylated and
cysteine-rich substrate-binding domain and a 200-a.a. region at the
C-terminus, which contains the catalytic region consisting of a
catalytic serine in a non-classical orientation (16). DPPIV and seprase/FAPα share 68% of
their identity at the catalytic region and the conserved serine
protease motif, Gly-Trp-Ser-Tyr-Gly (15). A previous study has revealed that
DPPIV and seprase/FAPα cleave prolyl peptide bonds (17). Although it is unclear how they are
activated, dimerization is required for prolyl peptidase and
gelatinase activities (18). The
N-glycosylated and cysteine-rich substrate-binding domains may be
important in the recognition of and binding to of substrates. The
substrates are proline-containing peptides, including certain
growth factors, such as vasoactive peptides, neuropeptides and
chemokines (19). Following digestion
of the bioactive peptides, DPPIV and seprase/FAPα are able to
regulate a number of cellular functions at the cell surface. They
function as adhesion molecules and, due to their enzymatic
activities, are involved in cell-extracellular matrix interactions
and bioactive peptide/cytokine/growth factor metabolism by reducing
the activity of chemokines and other peptide mediators (10). DPPIV and seprase exhibit a range of
cellular roles, since they are able to form complexes alone or with
each other and then interact with other membrane-associated
molecules. The localization of protease complexes at cell surface
invadopodia is important in the processing of soluble factors, such
as neuropeptide Y and certain chemokines (20). Furthermore, this process degrades
local extracellular matrix components that are required for cell
migration and matrix invasion during tumor invasion, angiogenesis
and metastasis (21).
Previous studies have revealed that seprase
overexpression was evident in stromal fibroblasts and tumor cells
in invasive breast, gastric, colonic and cervical carcinomas;
however, it was absent or undetectable in all normal tissue cells,
with the exception of cells involved in the early stages of wound
healing (22–25). In addition, an in vivo study
using a mouse model of human breast cancer, demonstrated that
seprase increased microvessel density and promoted rapid tumor
growth (26). Another study revealed
that the stromal expression of seprase was associated with
prolonged survival in patients with invasive ductal carcinoma of
the breast (27).
A number of previous studies have reported that
DPPIV mRNA and protein are abnormally expressed in a variety of
human carcinomas, including prostate, thyroid and colon cancer, as
well as endometrial adenocarcinoma; in addition, they were found to
be involved in the processes of tumor progression and metastasis
(28–32). However, Kajiyama et al
(33) identified that nude mice
inoculated with DPPIV-transfected SKOV3 cells exhibited
significantly less peritoneal dissemination and increased survival
times compared with those without transfection (33). This conflicting finding may be the
result of short follow-up periods or a lack of samples. Therefore,
further studies are required in order to clarify this result.
To the best of our knowledge, the study of DPPIV and
seprase in ovarian carcinomas has so far been confined to cell
lines (34–36). Furthermore, no large-scale comparative
studies concerning seprase expression in specimens of ovarian
carcinoma have been conducted. Based on a large series of patients
with EOC, to the best of our knowledge, the present study provided
the first immunohistochemical evidence that an overexpression of
seprase and DPPIV is more often and strongly observed in malignant
tissues compared with borderline and benign counterparts (data not
shown). In cancerous tissues, positive staining was not only
observed in cancer cells, but also in certain stromal spindle cells
(fibroblasts) and microvessel endothelial cells adjacent to the
cancer cells. However, the immunoreactivity of the cancer cells was
consistently stronger compared with that of the stromal spindle and
microvessel endothelial cells. Immunoreactivity for seprase or
DPPIV protein was not evident in the fibroblasts from normal
tissues remote from the cancer cells. The pattern of protein
expression in the tumor cells was mainly cytoplasmic, although
membranous and nuclear expression was occasionally observed. By
contrast, the expression was exclusively cytoplasmic in the stromal
spindle and microvessel endothelial cells. In certain cases,
stronger staining of DPPIV and seprase was present in the malignant
cells located at the infiltration front, rather than the central
region of the cancer tissue.
In addition, the tumor nests demonstrated diffuse
and heterogeneous immunostaining. The DPPIV and seprase proteins
often colocalized in the same tumor regions (Fig. 1). Furthermore, a positive correlation
was detected between DPPIV and seprase proteins
(rs=0.504, P=0.001). These observations suggest
that ovarian carcinoma cells produce DPPIV and seprase. This
supports the hypothesis that DPPIV and seprase are the primary
cell-surface enzymes responsible for cellular invasion and are
important in ovarian cancer.
Generally, patients with advanced ovarian carcinoma
exhibit a poorer survival rate compared with patients at earlier
stages. In the present study, the protein expression of seprase and
DPPIV was correlated with the FIGO stage and the presence or
absence of lymph node metastasis. By contrast, no significant
correlation was observed between the two proteins and the patient
age, or the histological grade or type of the tumor. In addition,
it was revealed that increased seprase protein expression was
negatively associated with disease-free survival (P=0.033)
(Fig. 4). However, no association was
detected between DPPIV protein expression and disease-free survival
(P=0.521).
In the present study, seprase and DPPIV mRNA
transcripts were detected in ovarian carcinomas. IHC established
that seprase mRNA expression was correlated with its corresponding
protein (rs=0.48, P=0.001). With respect to
DPPIV, a significant correlation was observed between DPPIV mRNA
and protein (rs=0.66, P=0.001). Although seprase
and DPPIV mRNA transcripts were detected, protein immunoreactivity
was negative in certain tumors. This discrepancy in the protein
expression of seprase and DDPIV in certain tumors may be the result
of post-transcriptional regulation by factors such as estrogen.
In conclusion, the results of the present study
indicated that seprase and DPPIV are involved in the progression of
ovarian cancer. The results assisted the identification of a cohort
of patients with EOC that exhibit poorer prognoses. Therefore,
DDPIV and seprase may serve as potential prognostic markers for
this type of tumor.
Acknowledgements
This study was supported by grants from the
Excellent Youth Foundation of Henan Scientific Committee (no.
104100510007), and the Medical Science and Technique Foundation of
Henan Province (no. 201001005).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aletti GD, Gallenberg MM, Cliby WA, Jatoi
A and Hartmann LC: Current management strategies for ovarian
cancer. Mayo Clin Proc. 82:751–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morrison J: Advances in the understanding
and treatment of ovarian cancer. J Br Menopause Soc. 11:66–71.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bakrin N, Bereder JM, Decullier E, Classe
JM, et al: FROGHI (French Oncologic and Gynecologic HIPEC) Group:
Peritoneal carcinomatosis treated with cytoreductive surgery and
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced
ovarian carcinoma: a French multicentre retrospective cohort study
of 566 patients. Eur J Surg Oncol. 39:1435–1443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Werb Z: ECM and cell surface proteolysis:
regulating cellular ecology. Cell. 91:439–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talvensaari-Mattila A, Santala M, Soini Y
and Turpeenniemi-Hujanen T: Prognostic value of matrix
metalloproteinase-2 (MMP-2) expression in endometrial endometrioid
adenocarcinoma. Anticancer Res. 25:4101–4105. 2005.PubMed/NCBI
|
|
8
|
Rowe RG and Weiss SJ: Navigating ECM
barriers at the invasive front: the cancer cell-stroma interface.
Annu Rev Cell Dev Biol. 25:567–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deakin NE and Chaplain MA: Mathematical
modeling of cancer invasion: the role of membrane-bound matrix
metalloproteinases. Front Oncol. 3:702013.PubMed/NCBI
|
|
10
|
Chen WT, Kelly T and Ghersi G: DPPIV,
seprase and related serine peptidases in multiple cellular
functions. Curr Top Dev Biol. 54:207–232. 2003.PubMed/NCBI
|
|
11
|
Goscinski MA, Suo ZH, Nesland JM, Flørenes
VA and Giercksky KE: Dipeptidyl peptidase IV expression in cancer
and stromal cells of human esophageal squamous cell carcinomas,
adenocarcinomas and squamous cell carcinoma cell lines. APMIS.
116:823–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mentlein R, Hattermann K, Hemion C,
Jungbluth AA and Held-Feindt J: Expression and role of the cell
surface protease seprase/fibroblast activation protein-α (FAP-α) in
astroglial tumors. Biol Chem. 392:199–207. 2011.PubMed/NCBI
|
|
13
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Suo Z, Risbegr B, Karlsson MG,
Villman K and Nesland JM: Expression of EPhb2 And Ephb4 in Berast
Caerinoma. Phatol oneol Res. 10:26–33. 2004.
|
|
15
|
Lawrie LC, Curran S, MeLeod HL, Fothergill
JE and Murray GI: Applieation of laser capture microdissection and
porteomics in colon cancer. Mol Pathol. 54:253–258. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monsky WL, Lin CY, Aoyama A, Kelly T,
Akiyama SK, Mueller SC and Chen WT: A potential marker protease of
invasiveness, seprase, is localized on invadopodia of human
malignant melanoma cells. Cancer Res. 54:5702–5710. 1994.PubMed/NCBI
|
|
17
|
Chen WT and Kelly T: Seprase complexes in
cellular invasiveness. Cancer Metastasis Rev. 22:259–269. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kotacková L, Baláziová E and Sedo A:
Expression pattern of dipeptidyl peptidase IV activity and/or
structure homologues in cancer. Folia Biol (Praha). 55:77–84.
2009.PubMed/NCBI
|
|
19
|
Pro B and Dang NH: CD26/dipeptidyl
peptidase IV and its role in cancer. Histol Histopathol.
19:1345–1351. 2004.PubMed/NCBI
|
|
20
|
Mueller SC, Ghersi G, Akiyama SK, Sang QX,
Howard L, Pineiro-Sanchez M, et al: A novel protease-docking
function of integrin at invadopodia. J Biol Chem. 274:24947–24952.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Brien P and O'Connor BF: Seprase: an
overview of an important matrix serine protease. Biochim Biophys
Acta. 1784:1130–1145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelly T, Kechelava S, Rozypal TL, West KW
and Korourian S: Seprase, a membrane-bound protease, is
overexpressed by invasive ductal carcinoma cells of human breast
cancers. Mod Pathol. 11:855–863. 1998.PubMed/NCBI
|
|
23
|
Jin X, Iwasa S, Okada K, Mitsumata M and
Ooi A: Expression patterns of seprase, a membrane serine protease,
in cervical carcinoma and cervical intraepithelial neoplasm.
Anticancer Res. 23:3195–3198. 2003.PubMed/NCBI
|
|
24
|
Okada K, Chen WT, Iwasa S, Jin X, Yamane
T, Ooi A and Mitsumata M: Seprase, a membrane-type serine protease,
has different expression patterns in intestinal- and diffuse-type
gastric cancer. Oncology. 65:363–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wikberg ML, Edin S, Lundberg IV, Van
Guelpen B, Dahlin AM, Rutegård J, et al: High intratumoral
expression of fibroblast activation protein (FAP) in colon cancer
is associated with poorer patient prognosis. Tumour Biol.
34:1013–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Wang S and Kelly T: Seprase
promotes rapid tumor growth and increased microvessel density in a
mouse model of human breast cancer. Cancer Res. 64:2712–2716. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ariga N, Sato E, Ohuchi N, Nagura H and
Ohtani H: Stromal expression of fibroblast activation
protein/seprase, a cell membrane serine proteinase and gelatinase,
is associated with longer survival in patients with invasive ductal
carcinoma of breast. Int J Cancer. 95:67–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilson MJ, Ruhland AR, Quast BJ, Reddy PK,
Ewing SL and Sinha AA: Dipeptidylpeptidase IV activities are
elevated in prostate cancers and adjacent benign hyperplastic
glands. J Androl. 21:220–226. 2000.PubMed/NCBI
|
|
29
|
Khin EE, Kikkawa F, Ino K, Kajiyama H,
Suzuki T, Shibata K, et al: Dipeptidyl peptidase IV expression in
endometrial endometrioid adenocarcinoma and its inverse correlation
with tumor grade. Am J Obstet Gynecol. 188:670–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizokami Y, Kajiyama H, Shibata K, Ino K,
Kikkawa F and Mizutani S: Stromal cell-derived
factor-1alpha-induced cell proliferation and its possible
regulation by CD26/dipeptidyl peptidase IV in endometrial
adenocarcinoma. Int J Cancer. 110:652–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abe M, Havre PA, Urasaki Y, Ohnuma K,
Morimoto C, Dang LH and Dang NH: Mechanisms of confluence-dependent
expression of CD26 in colon cancer cell lines. BMC Cancer.
11:512011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyake Y, Aratake Y, Sakaguchi T, Kiyoya
K, Kuribayashi T, Marutsuka K and Ohno E: Examination of
CD26/DPPIV, p53 and PTEN expression in thyroid follicular adenoma.
Diagn Cytopathol. 40:1047–1053. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kajiyama H, Kikkawa F, Suzuki T, Shibata
K, Ino K and Mizutani S: Prolonged survival and decreased invasive
activity attributable to dipeptidyl peptidase IV overexpression in
ovarian carcinoma. Cancer Res. 62:2753–2757. 2002.PubMed/NCBI
|
|
34
|
Kennedy A, Dong H, Chen D and Chen WT:
Elevation of seprase expression and promotion of an invasive
phenotype by collagenous matrices in ovarian tumor cells. Int J
Cancer. 124:27–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lai D, Ma L and Wang F: Fibroblast
activation protein regulates tumor-associated fibroblasts and
epithelial ovarian cancer cells. Int J Oncol. 41:541–550.
2012.PubMed/NCBI
|
|
36
|
Yang L, Ma L and Lai D: Over-expression of
fibroblast activation protein alpha increases tumor growth in
xenografts of ovarian cancer cells. Acta Biochim Biophys Sin
(Shanghai). 45:928–937. 2013. View Article : Google Scholar : PubMed/NCBI
|