Introduction
Gastric cancer is the fourth most common cancer
worldwide, and the second leading cause of cancer-associated
mortalities (1). Important factors
underlying the poor prognosis of gastric cancer are recurrence and
distant metastases, which may be a result of free cancer cells
being shed into the peritoneal cavity during surgical manipulation
(2). Therefore, reducing the
incidence of recurrence and metastasis is crucial for improving the
survival of patients. At present, laparoscopic resection is the
first choice of treatment for gastric cancer. This involves the
percutaneous injection of CO2 into the region
surrounding the target tumor in order to physically separate the
lesion from adjacent structures; however, this approach enhances
the spread of free cancer cells into the peritoneal cavity
(3).
Emerging treatments for gastric cancer with
peritoneal carcinomatosis include the use of regional chemotherapy,
specifically hyperthermic intraperitoneal chemotherapy, which may
improve the prognosis of patients (4). The exposure of tumors to hyperthermic
conditions has been revealed to be an effective adjuvant therapy to
radiotherapy and chemotherapy for a range of cancers, including
locally advanced head and neck cancer (1), melanoma (2), esophageal cancer (3,4), locally
advanced cervical cancer (5) and
gliomas (6). These results suggest
that hyperthermic conditions confer a beneficial effect during
laparoscopic resection of gastric cancer (5). CO2 pneumoperitoneum is used
in laparoscopic surgery for the creation of an operative field.
Previous data has revealed that compared with ambient temperature
CO2, hyperthermic CO2 (HT-CO2)
pneumoperitoneum directly inhibits cell proliferation and induces
cell apoptosis in gastric and colorectal cancers (5,6). However,
the oncological effect and underlying mechanism of
HT-CO2 on tumor immune responses remains unclear.
Dendritic cells (DCs) act as specialized accessories
to induce immunity and tolerance (7,8). A number
of previous studies have established that DC-derived exosomes (Dex)
are able to modulate tumor-associated immune responses (9). Exosomes are small, membrane-bound
vesicles measuring ~100 nm in diameter that are involved in the
endocytic pathway and externalized by a variety of cell types.
Exosomes are formed by a fusion between multivesicular bodies and
the plasma membrane, which is followed by exocytosis (10,11). It
has been demonstrated that Dex pulsed with tumor antigens induce
potent T cell-dependent antitumor effects in tumor-bearing hosts
(12).
HT-CO2 pneumoperitoneum has been
identified to exert an efficacious cytotoxic effect on cancer
cells, and may also enhance immune function by improving the
antitumor activity of DCs, which inhibit free gastric cancer cell
proliferation and disrupt gastric cancer recurrence. This
hypothesis encourages the investigation of the mechanisms for
HT-CO2 pneumoperitoneum on DC immune responses.
The present study examined the effects of
HT-CO2 on the antitumor activity of Dex in gastric
cancer cells. It was identified that Dex treated with
HT-CO2 induced a significant decrease in AGS cell
proliferation in vitro and in vivo. In addition, Dex
treated with HT-CO2 increased the apoptosis of AGS
cells.
Materials and methods
In vitro HT-CO2 model
An in vitro HT-CO2 model was
constructed in order to simulate the HT-CO2
pneumoperitoneum in the human body. The model enabled the exposure
of cells to HT-CO2 at a constant temperature, pressure,
flow rate and humidity, with an accurate stability of ±0.3°C, ±1
mmHg, ±0.5 l/min and ±5% CO2, respectively. The model
was designed by the present research group and has approved patent
protection from the State Intellectual Property Office of China
(patent no., ZL201220519625.5).
Preparation and treatment of DCs
DCs were prepared as primary spleen cultures from
inbred mice, as previously described (13). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen, Guangzhou, Guangdong,
China) containing 10% fetal calf serum (Invitrogen) in 5%
CO2 in air at 37°C. The medium was changed as required.
After several weeks, the splenic cells consisted of a stromal cell
monolayer of fibroblastic and endothelial cells that continuously
supported the proliferation and differentiation of hemopoietic
cells into non-adherent dendritic-like cells. The supernatant was
then transferred to a Falcon® 100 mm Cell Culture Dish (BD
Biosciences, Franklin Lakes, NJ, USA) for the next step of
purification. The cells in the supernatant were cultured in DMEM
containing 10% fetal calf serum in 5% CO2 in air at
37°C. On day 8 of culture, DCs were harvested at medium as
non-adherent cells released into the culture supernatant. Cell
viability was assessed using the Trypan Blue exclusion method
(Sigma-Aldrich, St. Louis, MO, USA). In total, 106 cells
were cultured under normal conditions or at 43°C, in a 95%
CO2 atmosphere or at 43°C with a 95% CO2
atmosphere for 4 h prior to exosome preparation.
Exosome preparation and
characterization
Exosomes in the cell culture supernatant were
prepared using a total exosome isolation kit (Invitrogen) according
to the manufacturer's instructions. The isolated products were then
examined by electron microscopy (JEM-2010; JEOL Ltd., Tokyo, Japan)
in order to confirm the morphology, and then cluster of
differentiation (CD)63 detection was performed through western
blotting. Next, the purified exosomes were fixed for 1 h in 4%
paraformaldehyde and washed once with phosphate-buffered saline.
The pellets were then fixed in 2.5% glutaraldehyde, loaded on
Formvar/carbon-coated electron microscopy grids, post-fixed in 1%
glutaraldehyde, and contrasted successively in 2%
methycellulose/0.4% uranyl acetate (pH 4.0). Observations were made
using a JEM-2010HR electron microscope (JEOL, Tokyo, Japan). The
purified exosomes underwent sterile filtration through a 0.22-µm
membrane and stored at −80°C until use.
Cell culture and cell proliferation
assays
The gastric cancer AGS cell line was used in the
present study. The cells were cultured in RPMI-1640 (Invitrogen)
containing 10% fetal bovine serum (FBS) at 37°C under a humidified
atmosphere containing 5% CO2. For the cell proliferation
assay, AGS cells were plated into 96-well plates (Corning
Incorporated, Corning, New York, NY, USA) containing a medium
supplemented with 10% FBS at a density of ~1,000 cells per well 24
h after exosome treatment. For the quantification of cell
viability, cultures were stained using the cell counting kit-8
(CCK-8; Beyotime Institute of Biotechnology, Haimen, Jiangsu,
China) at various time points. In brief, 20 µl CCK-8 solution was
added to each well and incubated for 4 h at 37°C. Each solution was
then measured spectrophotometrically at 450 nm using a Multiskan
Ascent microplate reader (ELx800; Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Analysis of cell apoptosis
Subsequent to a 24-h incubation with Dex, an Annexin
V-fluorescein isothiocyanate apoptosis detection kit was used to
assess cellular apoptosis (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Apoptosis was
analyzed using a FACSCanto flow cytometer (BD Biosciences). For
each sample, at least 104 cells were analyzed using FCAP
Array™ v3.0.1 software (BD Biosciences). For the Hoechst 33258
(Sigma-Aldrich) fluorescence staining, AGS cells were seeded into
24-well plates (Corning Incorporated) and treated with 5 µl of 20
µg/ml Hoechst 33258 for 30 min in the dark. Morphological changes
in the nuclei of AGS cells were analyzed and counted using a
fluorescence microscope (Zeiss, Oberkochen, Germany) in five
different fields in order to discriminate between normal and
apoptotic cells. Images of the cells were captured and then
processed using Adobe Photoshop software version 7.0 (Adobe
Systems, Inc., San Jose, CA, USA). Caspase-3 activity was
determined using a caspase assay kit, according to the
manufacturer's instructions (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). A spectrofluorometer with an excitation
wavelength of 400 nm and an emission wavelength of 505 nm was used
to measure the level of free 7-amino-4-trifluoromethyl
coumarin.
Western blot analysis
The exosome protein concentration was assessed using
Bradford reagent (Bio-Rad, Guangzhou, Guangdong, China). Overall,
100 µM total protein was separated using SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane (Millipore
China Ltd., Guangzhou, Guangdong, China). The membrane was then
incubated with the primary antibodies at 4°C overnight. The primary
polyclonal rabbit anti-mouse heat shock protein (HSP)70 (1:1,000;
cat. no. 4876) and CD63 (1:1,000; cat. no. 14023) antibodies, and
the horseradish peroxidase-conjugated secondary goat anti-rabbit
IgG antibody (1:3,000; cat. no. 7074P2) were obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA). The β-actin primary
antibody was purchased from Bioworld Technology (catalog no.
BSAP0060; Shanghai, China). An enhanced chemiluminescence substrate
kit (Thermo Fisher Scientific, Guangzhou, Guangdong, China) was
used to visualize the protein bands. The percentage reduction in
band intensity was calculated based on the untreated samples and
then normalized to β-actin.
In vivo carcinogenesis assay
In total, 20 specific pathogen-free grade BALB/c
nude mice aged 6–8 weeks old were purchased from the Animal
Experimental Center of the Guangdong Medical College (Foshan,
Guangdong, China). The AGS cells, at a density of 2×106
cells per 100 µl serum-free medium, were injected into the right
flank of each mouse. After 4 weeks, the animals were sacrificed by
sodium barbital injection (50 mg/kg) and the tumors were weighed.
The animal experiments were approved by the Experimental Animal
Care and Use Committee of Guangdong Medical College.
Statistical analysis
The data from at least three separate experiments
are expressed as the mean ± standard deviation. Unless stated
otherwise, the Student's t-test was used for comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Generation of DCs
The DCs were prepared as primary spleen cultures
from inbred mice. The cells were harvested at medium as
non-adherent cells released into culture supernatant. The cell
viability was determined using the Trypan Blue exclusion method and
the cell viability was >90% in all experiments. In total,
106 cells were collected prior to exosome
preparation.
Exosome preparation and
characterization
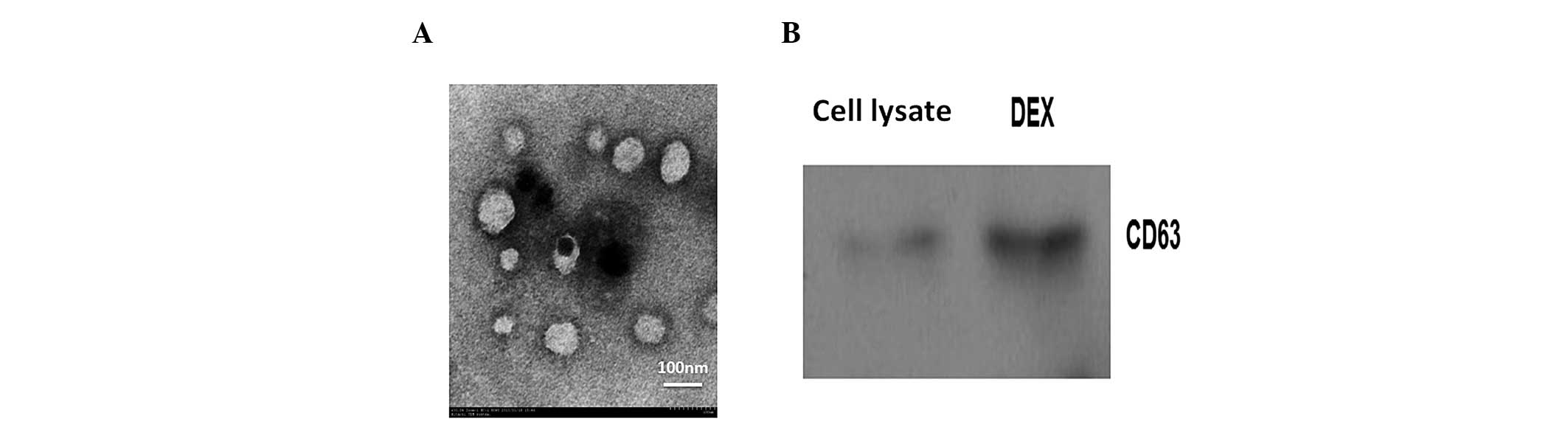
The exosomes were successfully prepared from DCs.
Electron microscopy was performed in order to confirm the
morphology of the isolated products. It was revealed that the
isolated exosomes were universal membrane vesicles with a diameter
of 60–100 nm (Fig. 1A). Western blot
analysis of Dex revealed the presence of the exosome marker CD63
(Fig. 1B).
Dex inhibit gastric cancer cell
proliferation
The present study analyzed the effect of Dex
repression on the growth of AGS cells. The proliferation rate was
determined by the CCK-8 assay. It was established that treatment
with Dex resulted in a significant inhibition of the proliferation
of AGS cells (45.3±9.1%; Fig. 2B).
This finding demonstrates that HT-CO2-treated Dex
downregulated the growth of gastric cancer cells.
Induction of apoptosis following Dex
treatments
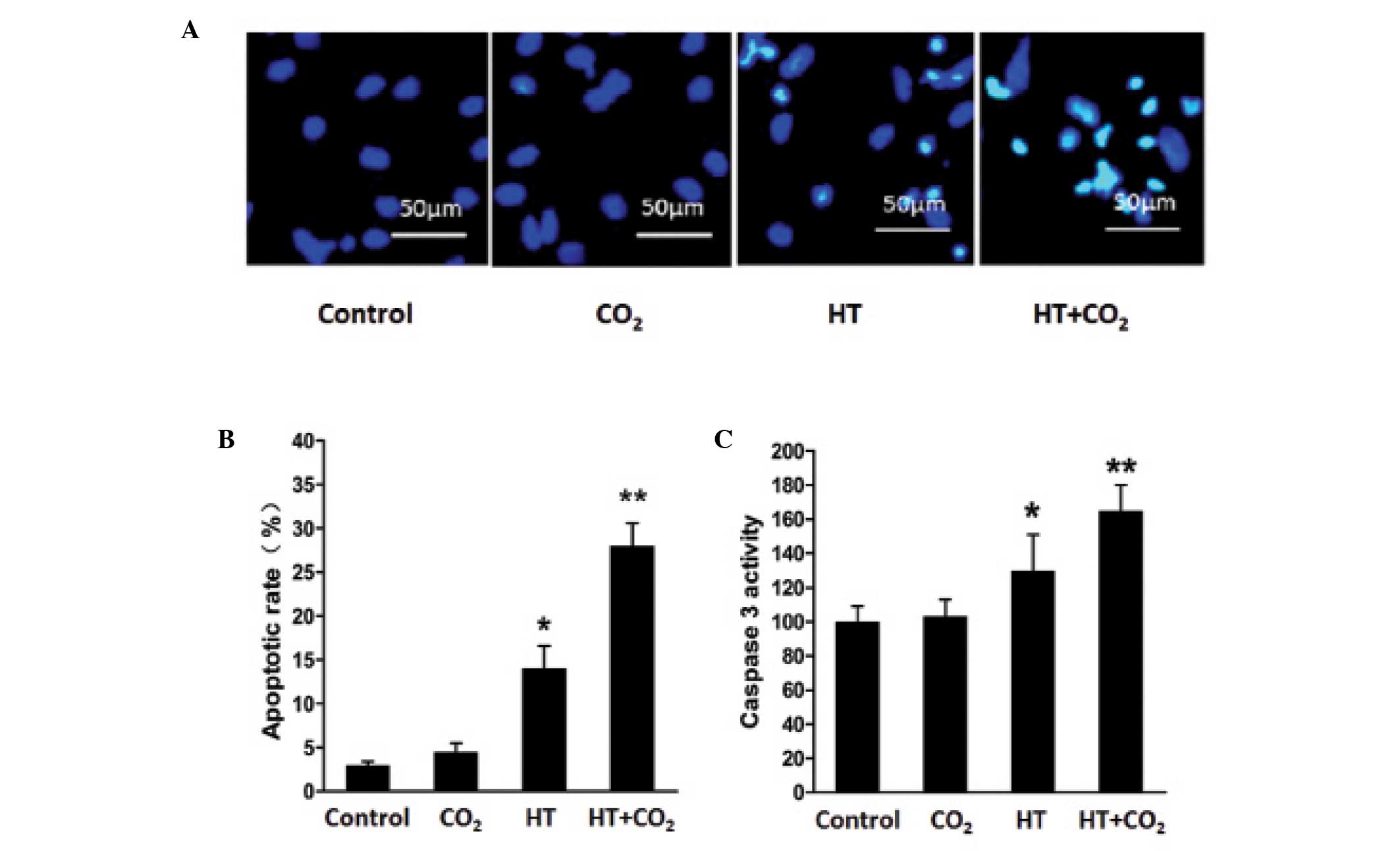
AGS cells that had been treated with Dex for 48 h
were evaluated for apoptosis. The apoptotic rate of the
CO2-, heat- and HT-CO2-treated Dex groups
were 5.10±1.40, 13.30±1.32 and 27.70±1.87%, respectively (Fig. 3B). These values were significantly
higher compared with the control group (3.1±0.44%). Compared with
the group treated with heat alone, the HT-CO2 group
exhibited an increased apoptotic rate. These results indicate that
the heat and CO2-treated Dex are more effective than a
single treatment for inducing AGS cell apoptosis. Consistent
results were obtained from Hoechst 33258 fluorescence staining and
the analysis of caspase-3 activity (Fig.
3A and C). The caspase-3 activity (OD405) of the
heat- and HT-CO2-treated Dex groups was markedly higher
than that of the control group. However, no significant difference
was observed between the CO2 alone-treated group and the
control group.
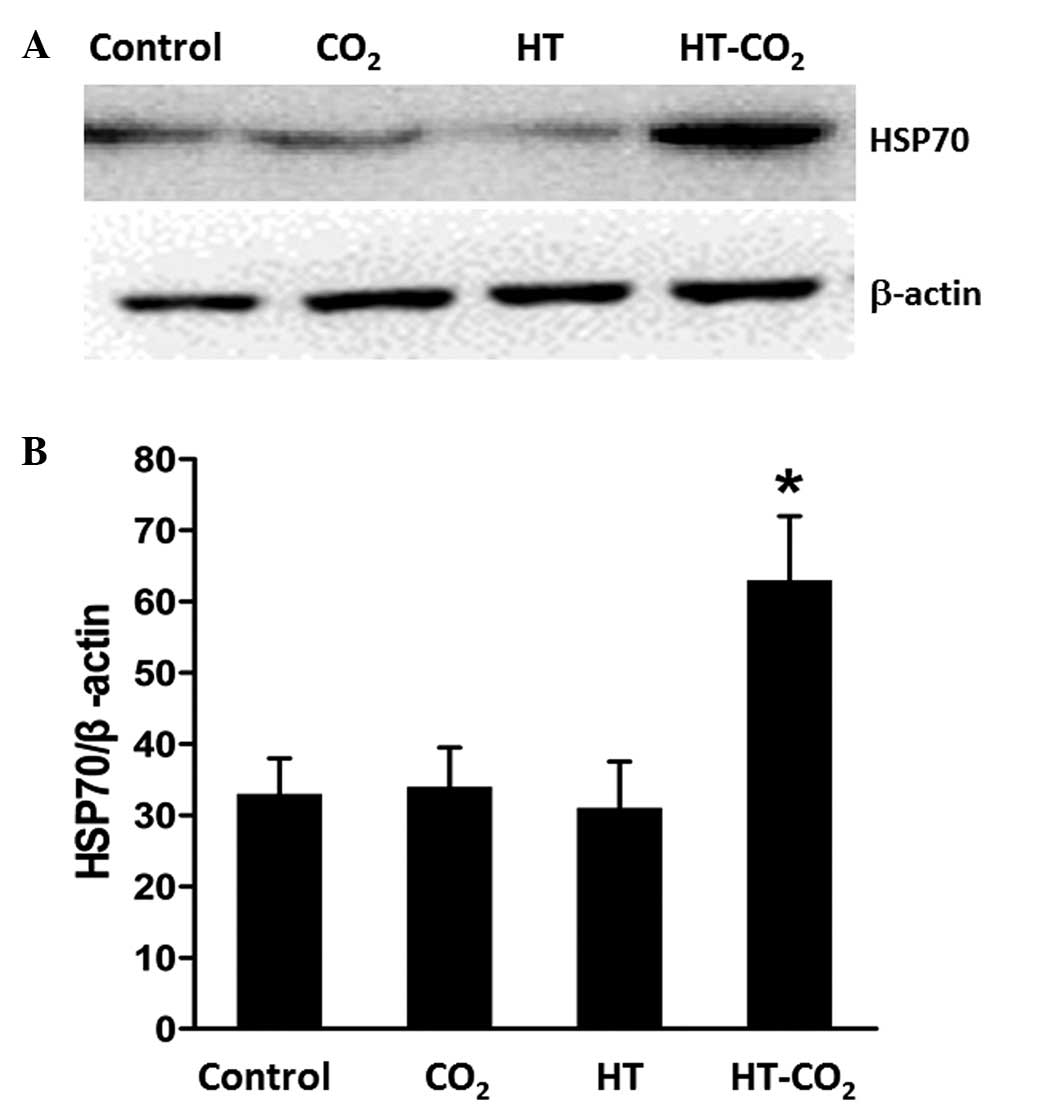
Western blot analysis
The protein composition of the DC-derived exosomes
was analyzed by SDS-PAGE and then compared with that of the DC
lysate. As shown in Fig. 4, the
expression of CD63 was enriched in the exosome preparations
compared with the DC lysate, which indicated a successful exosome
extraction. The analysis of HSP70 expression levels following
treatment revealed that HT-CO2-treated Dex induced an
increase in the level of HSP70. This suggests that HSP70 may have a
key role in the antitumor effect of Dex.
Tumor growth
For the in vivo experiments performed in
mice, the antitumor effects of Dex were investigated in the gastric
cancer AGS cell line. Tumor growth was significantly reduced in the
heat and CO2-treated Dex group. By contrast, no marked
effects were observed in the control group, and heat- and
CO2-treated Dex groups (Fig.
5A). The tumor volumes are shown in Fig. 5B, with a mean tumor volume of
823.9±330.2 mm3 in the control group, and 809.4±241.5
and 793.5±231.1 mm3 in the heat- and
CO2-treated Dex groups, respectively. Treatment with
HT-CO2-treated Dex reduced the tumor volume to
533.4±286.6 mm3 (P<0.05).
Discussion
The tumor-free principle is a primary rule for the
radical resection of tumors. The no-touch isolation technique has
been developed in order to prevent cancer cells from being shed
into the peritoneal cavity during the surgical resection of gastric
cancers. However, free cancer cells have been detected in the blood
and peritoneal cavity following radical resection (14). Prognosis is particularly unfavorable
for patients with peritoneal carcinomatosis. Establishing how to
control free cancer cells is important in order to prevent gastric
cancer recurrence and metastasis (4).
DCs are antigen-presenting cells that have an
important role in the initiation of antitumor immune responses. DCs
acquire antigens from apoptotic tumor cells to induce the
activation of major histocompatibility complex (MHC) class
I-restricted cytotoxic T lymphocytes, and also initiate antitumor
immunity (15). Exosomes are
nanovesicles that originate from late endosomal compartments and
are secreted by the majority of living cells in ex vivo cell
cultures. Dex are able to modulate immune responses, either
directly, by exposing MHC and costimulatory molecules, or
indirectly, by conveying internal components to surrounding cells
(9). A previous study revealed that
Dex are able to stimulate the proliferation of T lymphocytes in
vitro and generate antitumor immune responses in vivo
(16). A further study revealed that,
in patients with malignant glioma, tumor-derived exosome-leaded DCs
elicited a specific CD8(+) cytotoxic T-lymphocyte response against
autologous tumor cells (17). The
functional role of exosomes has been characterized according to
their protein composition (18).
HSP70 is a major host component in exosomes. Previous evidence has
indicated that hematopoietic and tumor cells may secrete HSPs into
the circulation through exosome-mediated, granule-mediated or lipid
raft-mediated exocytosis (19). Such
extracellular HSPs have been identified to activate innate immune
responses through the use of Toll-like receptors (20). The analysis of the specific actions of
HSPs should lead to the identification of effective HSP-based
immunotherapies. It is known that HSP70 is involved in the
regulation of immune cells (21). A
previous study revealed that when peripheral blood mononuclear
cells were treated at 40°C for 1 h and then recovered at 37°C for 4
h, the level of HSP70 in the exosomal vesicles increased (22). The exposure of tumors to hyperthermic
conditions has been established to be an effective adjuvant therapy
to radiotherapy and chemotherapy. It has been hypothesized that
elevated temperatures may have the effect of enhancing antitumor
immunity (23).
In the present study, DCs were treated with
HT-CO2, or with heat or CO2 alone. Dex from
the HT-CO2-treated group significantly decreased AGS
cell proliferation. In addition, flow cytometry, Hoechst 33258
fluorescence staining and the analysis of caspase-3 activity
revealed that it enhanced the apoptosis of AGS cells. Furthermore,
an effective suppression of tumor growth was observed in mice
treated with HT-CO2 Dex.
To the best of our knowledge, the present study was
the first to demonstrate that HT-CO2-treated Dex reduced
the proliferation and induced the apoptosis of the gastric cancer
AGS cell line in vitro and in vivo. Therefore, Dex
may confer an effective free tumor cell ablation benefit in
induction of antitumor immunity.
Acknowledgements
This study was supported by grants from the Science
and Technological Program for Dongguan's Higher Education (grant
nos. 201010515000081, 201010815213, 2011105102016 and
2012105102005).
References
|
1
|
Flanagan MA and Leitman IM: Radical
gastrectomy with para-aortic lymphadenectomy for carcinoma? The
controversy continues. Commentary on risk factors for metastasis to
para-aortic lymph nodes in gastric cancer: a single institution
study in China. Journal of Surgical Research. J Surg Res.
185:e11–13. 2013. View Article : Google Scholar
|
|
2
|
Tong JH, Sun Z, Wang ZN, et al: Early
gastric cancer with signet-ring cell histologic type: risk factors
of lymph node metastasis and indications of endoscopic surgery.
Surgery. 149:356–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kariya S, Tanigawa N, Kojima H, et al:
Radiofrequency ablation combined with CO2 injection for
treatment of retroperitoneal tumor: protecting surrounding organs
against thermal injury. AJR Am J Roentgenol. 185:890–893. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KW, Chow O, Parikh K, et al:
Peritoneal carcinomatosis in patients with gastric cancer, and the
role for surgical resection, cytoreductive surgery and hyperthermic
intraperitoneal chemotherapy. Am J Surg. 207:78–83. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou HM, Feng B, Zhao HC and Zheng MH:
Antitumor effects of hyperthermic CO2 pneumoperitoneum
on human gastric cancer cells. Asian Pac J Cancer Prev. 13:117–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Y, Zheng M, Feng B, et al:
Hyperthermic CO2 pneumoperitoneum induces apoptosis in
human colon cancer cells through Bax-associated mitochondrial
pathway. Oncol Rep. 19:73–79. 2008.PubMed/NCBI
|
|
7
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inaba K, Inaba M, Deguchi M, et al:
Granulocytes, macrophages and dendritic cells arise from a common
major histocompatibility complex class II-negative progenitor in
mouse bone marrow. In: Proc Natl Acad Sci USA. 90. pp. 3038–3042.
1993; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viaud S, Théry C, Ploix S, et al:
Dendritic cell-derived exosomes for cancer immunotherapy: what's
next? Cancer Res. 70:1281–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002.PubMed/NCBI
|
|
11
|
Denzer K, Kleijmeer MJ, Heijnen HF,
Stoorvogel W and Geuze HJ: Exosome: from internal vesicle of the
multivesicular body to intercellular signaling device. J Cell Sci.
113:3365–3374. 2000.PubMed/NCBI
|
|
12
|
André F, Chaput N, Schartz NE, et al:
Exosomes as potent cell-free peptide-based vaccine I Dendritic
cell-derived exosomes transfer functional MHC class I/peptide
complexes to dendritic cells. J Immunol. 172:2126–2136. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quah BJ and O'Neill HC: Mycoplasma
contaminants present in exosome preparations induce polyclonal B
cell responses. J Leukoc Biol. 82:1070–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Mao X, Guo F, et al: An isolation
technique to prevent the spread of tumor cells during radical
gastrectomy for gastric carcinoma located on the anterior wall of
the gastric antrum. Eur J Surg Oncol. 39:1136–1143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Moyana T, Saxena A, Warrington R,
Jia Z and Xiang J: Efficient antitumor immunity derived from
maturation of dendritic cells that had phagocytosed
apoptotic/necrotic tumor cells. Int J Cancer. 93:539–548. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quah BJ and O'Neill HC: The immunogenicity
of dendritic cell-derived exosomes. Blood Cells Mol Dis. 35:94–110.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bu N, Wu H, Sun B, et al: Exosome-loaded
dendritic cells elicit tumor-specific CD8+cytotoxic T cells in
patients with glioma. J Neurooncol. 104:659–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anand PK: Exosomal membrane molecules are
potent immune response modulators. Commun Integr Biol. 3:405–408.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HP, Morse D and Choi AM: Heat-shock
proteins: new keys to the development of cytoprotective therapies.
Expert Opin Ther Targets. 10:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamura Y, Torigoe T, Kukita K, et al:
Heat-shock proteins as endogenous ligands building a bridge between
innate and adaptive immunity. Immunotherapy. 4:841–852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pockley AG: Heat shock proteins as
regulators of the immune response. Lancet. 362:469–476. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lancaster GI and Febbraio MA:
Exosome-dependent trafficking of HSP70: a novel secretory pathway
for cellular stress proteins. J Biol Chem. 280:23349–23355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peer AJ, Grimm MJ, Zynda ER and Repasky
EA: Diverse immune mechanisms may contribute to the survival
benefit seen in cancer patients receiving hyperthermia. Immunol
Res. 46:137–154. 2010. View Article : Google Scholar : PubMed/NCBI
|