Introduction
Renal cell carcinoma (RCC) has the highest mortality
rate of the genitourinary cancers, and accounts for 2% of all
cancers in the world. In China, RCC is the second most frequent
genitourinary malignancy, with a steady increase in the incidence
in recent decades. One-third of patients are initially diagnosed
with disease that is locally invasive or that has already reached
stage IV. The only potentially effective treatment for primary and
metastatic RCC is surgical resection (1), although recurrence occurs in ~25% of
patients following nephrectomy (2).
The disease remains one of the most treatment-resistant
malignancies and is associated with a poor prognosis. It has
previously been found that the prognosis for patients with distant
disease is generally poor without systemic therapy, including
immunotherapy, radiation and targeted treatment, with a 5-year
survival rate of ≤10% (1).
RCC is unique in that it has the propensity for
vascular invasion, namely invasion into the renal vein, and
subsequently the inferior vena cava (IVC) or intracardially
(3), forming a tumor thrombus.
However, the isolated recurrence of RCC in IVC following surgical
resection is a rare event. A previous study indicated that the
recurrence can happen locally, with an incidence of 1.61% following
surgery (4). The resection of a
recurrent tumor thrombus and reconstruction of the IVC often makes
clinical management challenging. However, aggressive surgical
management in patients with tumor recurrence may offer an
opportunity for cure or palliation. The current study presents our
experience in the surgical management of a patient with an isolated
local recurrence of RCC in the IVC following radical
nephrectomy.
Case report
Radical nephrectomy
A 59-year-old male was admitted to the Department of
Urology, First Affiliated Hospital of Anhui Medical University, on
February 25, 2003, due to a 3-month history of severe gross
hematuria. The patient had a medical history of hypertension
controlled by oral medication for 3 years. The patient's body
weight and height were 80 kg and 172 cm, respectively, with a body
mass index of 27.04. Vital signs were stable following a physical
examination, and no superficial lymph nodes were found. The results
from an electrocardiogram, pulmonary function test, stool analysis
and other routine laboratory tests were all within normal limits,
with the exception of the level of fibrinogen [5.2 g/l (normal
range, ~2.0–4.0g/l)]. Computed tomography (CT) of the abdomen
revealed an enhancing well-defined heterogeneous large mass (6.5 cm
in maximal diameter) in the lower pole of the right kidney and
extending through Gerota's fascia. No para-aortic lymph nodes or
lung metastatic lesions were detected by CT scan. Magnetic
resonance imaging (MRI) showed no evidence of renal vein or caval
thrombi or emboli.
The patient underwent a radical nephrectomy,
including lymphadenectomy. Histopathology of the surgical specimen
diagnosed a grade 2 clear cell carcinoma, with a negative surgical
margin. The pathological stage of the tumor was pT3aN0M0.
Surgical management of the recurrent
tumor
On January 5, 2007, 4 years after the radical
nephrectomy, the patient presented to the Department of Urology,
First Affiliated Hospital of Anhui Medical University, with
bilateral lower extremity edema. Upon analysis with Doppler
ultrasonography, a hypoechoic mass, 3.86×3.70 cm in size, was
located in the IVC. MRI clearly showed a tumor thrombus within the
IVC, 4.10×2.80 cm in size, extending from the infrarenal level to
the level of the left renal vessels, with no adherence within the
vena cava (Fig. 1).
Under general anesthesia, a chevron incision
provided excellent exposure of the IVC, the left kidney and the
liver. A thrombectomy and partial IVC resection were performed, and
the defect was reconstructed with a polytetrafluoroethylene (PTFE)
prosthetic graft. In order to prevent potential hemodynamic
instability, the superior and IVC were clamped to the tumor
thrombus during the surgery. Initially, am attempt was made to
remove the thrombus down to the level of the left renal vessels,
however, this was not successful, as adherence of the thrombus with
the vena caval wall was subsequently observed. Therefore, the tumor
thrombus and the wall of the IVC (~7 cm in length) were excised,
and reconstruction of the IVC was performed using a PTFE prosthetic
graft (Fig. 2). The surgical duration
was ~4 h. The total amount of blood loss was ~600 ml and the amount
of blood transfused was ~400 ml. The patient was discharged home
without severe complications after an 11-day hospital stay.
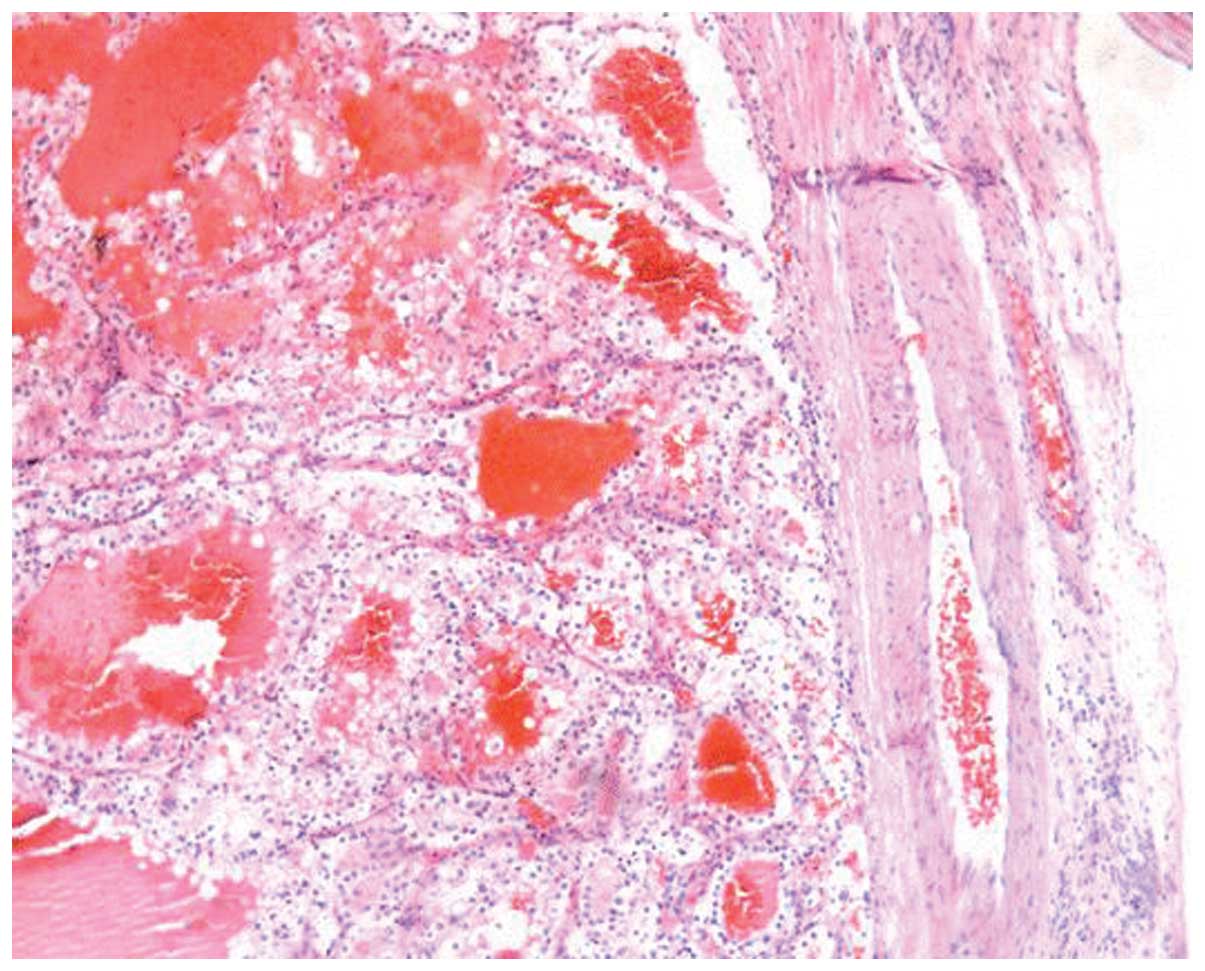
Macroscopic evaluation of the specimen showed that
the tumor thrombus was 4.0×3.0×1.5 cm in size. Histopathological
examination demonstrated that the thrombus consisted of clear
cancer cells. The pathological diagnosis was of clear cell RCC
(Fig. 3), Fuhrman grade 2. Therefore,
the overall diagnosis of the patient was the local recurrence of
RCC in the IVC with caval wall infiltration. Adjuvant interferon
therapy was performed in the post-operative course (Interferon, 3
MIU/iH, 3 times/week for a duration of 12 weeks). The patient was
in good general health; proteinuria was observed 3 years
post-surgery, however, further treatment was not required. Routine
follow-up has demonstrated no signs of reoccurrence as local or
distant metastasis for 72 months.
Discussion
To the best of our knowledge, RCC has a biological
behavioral tendency for venous system invasion, with extension into
the renal vein (50%), IVC (4–10%) or right atrium (~1%) of new
cases diagnosed as RCC (5,6). Although local recurrence following
radical nephrectomy is another biological behavior, which may recur
at numerous locations and occurs in 2–3% of patients (7), the late local recurrence of an RCC
thrombus extending from the IVC is rare; a thorough review of the
published English literature revealed <10 cases, including the
present study (8–12). In the present study, a patient with
local recurrence of a tumor thrombus extending from the IVC 4 years
after a right radical nephrectomy for advanced RCC is reported.
From the recorded cases, patients with RCC extending
into the IVC primarily presented with clinical signs or symptoms of
venous obstruction (1), such as
dilated superficial abdominal wall veins, caput medusae, pulmonary
embolus or proteinuria (8,9). Owing to a slow-growing tumor thrombus
and progressive IVC obstruction, up to 40% of patients reported
were asymptomatic. However, the symptoms of venous obstruction
appeared in another 40% of reported cases. In the present case, the
patient exhibited significant lower extremity edema.
In total, >30% of patients with non-metastatic
RCC developed local or systemic tumor recurrence following a
radical surgery (13). The recurrent
tumor appeared in nearly all organs of the body, but most commonly
in the lungs, bone, brain and liver, although IVC recurred rarely.
Surgical treatment for recurrent patients who have no signs of
local or distant metastasis is considered to be beneficial.
Systemic adjuvant medical therapy conferred certain benefits in
managing the patients with distant metastasis, however, appeared to
be of limited benefit in cases of local recurrence (7). In the present case, the patient
underwent successful surgical resection of the tumor thrombus and
part of the IVC, with defect reconstruction of the IVC. There were
no local or distant metastasis signs within 6 years of follow-up.
The results indicated that radical surgery may be a potential
treatment for the local recurrence of RCC.
The long-term survival of patients with locally
recurrent RCC is poor, with a 5-year survival rate of 28% (7). The early detection of tumor recurrence
can provide the best chance for the long-term survival of patients
with RCC. Risk factors associated with the local recurrence of RCC
include the size of the primary tumor, a high-grade, invasion of
the IVC and involvement of regional lymph nodes (14). The reasons for local recurrence in the
IVC may be similar. However, a reasonable explanation for local
recurrence in the IVC is unclear. Associated studies have indicated
that local recurrence may be associated with the involvement of the
IVC wall, tumor seeding (8,9) or skip metastasis, which had occurred
prior to radical nephrectomy. Moreover, the right renal vein is
shorter than the left side, which may be more convenient for the
migration of tumor cells. A review of the literature and the
present case indicated ten published cases of RCC recurring in the
IVC with no local recurrence or distant metastases, 9 right and 1
left. Therefore, 90% of local recurrences in reported cases were of
right RCC (8–12). In the present case, the tumor
recurrence in the IVC was confirmed in the patient following
radical nephrectomy for right RCC. At a 72-month follow-up with
strict surveillance strategies following the second surgery on the
tumor recurrence, there were no signs of recurrence as local or
distant metastasis.
The prognostic impact of the level of the tumor
thrombus is controversial. One study reported that the level of
tumor thrombus was an independent prognostic factor for survival.
Lambert et al reported that the survival rate decreased with
a higher level of tumor thrombus (15). By contrast, other studies proposed
that the presence of a tumor thrombus did not reduce the survival
rate and increase the risk of further metastasis (5,16,17). IVC invasion and a friable thrombus
were reported as independent prognostic factors in two studies
(18,19). In general, non-metastatic renal tumors
with venous thrombi have a better prognosis following nephrectomy
and tumor thrombectomy compared with patients with metastatic
disease. Unfortunately, the presence of a thrombus is often
associated with a larger tumor and lymph node or distant
metastases, which means a poor prognosis for patients (1). However, there are limited studies on the
recurrence of RCC extending from the IVC, thus there is little
available information with regard to the treatment and
prognosis.
To the urologist, the treatment of the local
recurrence of RCC in the IVC is the real challenge. At present,
thrombectomy with a partial IVC resection is the potential
treatment for the local recurrence of RCC in the IVC. The surgical
approach for the removal of a caval thrombus depends on the level
of the thrombus and the adherence with the vena cava (19). Therefore, accurate determination of
the extent of IVC involvement is crucial. Although a number of
techniques can be used to assess the presence and level of the
tumor thrombus, such as abdominal ultrasonography, CT and MRI, at
present, MRI is the gold standard for assessing the level of an IVC
thrombus (20,21). MRI provides better delineation of the
association of the caval wall and the tumor embolus (22).
It is widely agreed that open surgery is essential
for the removal of a thrombus that extends into the IVC or atrium.
The use of hand-assisted and pure laparoscopic radical nephrectomy
have been reported, which may be safe and effective (23,24),
although the methods can only be applied in selected cases with low
level thrombi, and require high levels of experience and skill.
However, no such studies have been reported for recurrence of an
RCC thrombus. If a tumor thrombus invades the wall of the cava,
resection of the caval wall is inevitable, which ensures negative
surgical margins and reduces the risk of late recurrence from the
venous wall (11,25). Controversy remains with regard to the
reconstruction of the IVC. A previous study proposed that the
reconstruction of the IVC may damage the venous collaterals and
reduce the collateral venous return (26). In the present case, owing to the
adherence of the tumor thrombus to the venous wall and the partial
IVC resection, it was necessary to reconstruct the IVC with a PTFE
prosthetic graft.
In order to diagnose tumor recurrence early,
intensive surveillance plays a key role in all tumor patients even
when complete surgical resection of all tumors has been performed.
No firm conclusions can be reached from the limited and tenuous
information available, although the present study suggests that IVC
thrombectomy may be the only potential treatment for the local
recurrence of RCC in the IVC. Further studies are required for the
management of RCC recurrence and progression in the IVC.
Acknowledgements
This study was supported by the Project of Cultivate
Scientific Research Foundation (grant no. F1407D).
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patard JJ, Pignot G, Escudier B, et al:
ICUD-EAU International Consultation on Kidney Cancer 2010:
treatment of metastatic disease. Eur Urol. 60:684–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karnes RJ and Blute ML: Surgery insight:
management of renal cell carcinoma with associated inferior vena
cava thrombus. Nat Clin Pract Urol. 5:329–339. 2008.PubMed/NCBI
|
|
4
|
Chow JJ, Ahmed K, Fazili Z, Sheikh M and
Sheriff M: Solitary renal fossa recurrence of renal cell carcinoma
after nephrectomy. Rev Urol. 16:76–82. 2014.PubMed/NCBI
|
|
5
|
Manassero F, Mogorovich A, Di Paola G, et
al: Renal cell carcinoma with caval involvement: contemporary
strategies of surgical treatment. Urol Oncol. 29:745–750. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HL, Zisman A, Han KR, et al:
Prognostic significance of venous thrombus in renal cell carcinoma.
Are renal vein and inferior vena cava involvement different? J
Urol. 171:588–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itano NB, Blute Ml, Spotts B and Zincke H:
Outcome of isolated renal cell carcinoma fossa recurrence after
nephrectomy. J Urol. 164:322–325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finkelstein MP, Drinis S, Tortorelis DG,
et al: Recurrence of renal cell carcinoma with extensive vena caval
thrombus three years after radical nephrectomy. Urol Int.
68:199–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minervini A, Salinitri G, Lera J, et al:
Solitary floating vena caval thrombus as a late recurrence of renal
cell carcinoma. Int J Urol. 11:239–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horger DC, Bissada NK, Curry NS and
Chaudhary UB: Isolated late recurrence of renal cell carcinoma in
the inferior vena cava. Can J Urol. 11:2467–2469. 2004.PubMed/NCBI
|
|
11
|
Smaldone MC, Cannon GM Jr and Hrebinko RL:
Resection of recurrent inferior vena cava tumor after radical
nephrectomy for renal cell carcinoma. Urology. 67:1084.e5–1084.e7.
2006.
|
|
12
|
Smith RB: Long-term survival of a vena
caval recurrence of renal cell carcinoma. J Urol. 125:575–578.
1981.PubMed/NCBI
|
|
13
|
Zisman A, Pantuck AJ, Wieder J, et al:
Risk group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klatte T, Lam JS, Shuch B, et al:
Surveillance for renal cell carcinoma: why and how? When and how
often? Urol Oncol. 26:550–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lambert EH, Pierorazio PM, Shabsigh A, et
al: Prognostic risk stratification and clinical outcomes in patients
undergoing surgical treatment for renal cell carcinoma with
vascular tumor thrombus. Urology. 69:1054–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sweeney P, Wood CG, Pisters LL, et al:
Surgical management of renal cell carcinoma associated with complex
inferior vena caval thrombi. Urol Oncol. 21:327–333. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka M, Fujimoto K, Okajima E, et al:
Prognostic factors of renal cell carcinoma with extension into
inferior vena cava. Int J Urol. 15:394–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ljungberg B, Stenling R, Osterdahl B, et
al: Vein invasion in renal cell carcinoma: Impact on metastatic
behavior and survival. J Urol. 154:1681–1684. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertini R, Roscigno M, Freschi M, et al:
Impact of venous tumour thrombus consistency (solid vs friable) on
cancer-specific survival in patients with renal cell carcinoma. Eur
Urol. 60:358–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kallman DA, King BF, Hattery RR, et al:
Renal vein and inferior vena tumor thrombus in renal cell
carcinoma: CT, US, MRI and venacavography. J Comput Assist Tomogr.
16:240–247. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kirkali Z and Van Poppel H: A critical
analysis of surgery for kidney cancer with vena cava invasion. Eur
Urol. 52:658–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pouliot F, Shuch B, Larochelle JC, et al:
Contemporary management of renal tumors with venous tumor thrombus.
J Urol. 184:833–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guzzo TJ, Schaeffer EM, McNeil BK, et al:
Laparoscopic radical nephrectomy for patients with pathologic T3b
renal-cell carcinoma: the Johns Hopkins experience. J Endourol.
23:63–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Henderson A, Murphy D, Jaganathan K, et
al: Hand-assisted laparoscopic nephrectomy for renal cell cancer
with renal vein tumor thrombus. Urology. 72:268–272. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hardwigsen J, Baqué P, Crespy B, et al:
Resection of the inferior vena cava for neoplasms with or without
prosthetic replacement: a 14-patient series. Ann Surg. 233:242–249.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarkar R, Eilber FR, Gelabert HA, et al:
Prosthetic replacement of the inferior vena cava for malignancy. J
Vasc Surg. 28:75–83. 1998. View Article : Google Scholar : PubMed/NCBI
|