Introduction
Glioma is the most lethal primary brain tumor, with
a median survival time of only 12 months. This tumor is incurable
due to the aggressive proliferation and rapid infiltration of glioma
cells. Despite advances in surgery and novel modalities in
radiotherapy and chemotherapy, the prognosis for patients suffering
from this disease remains poor, and even the etiology of glioma
remains unclear (1,2). Ionizing radiation is the primary form of
therapy subsequent to an optimal surgical resection or biopsy,
prolonging median survival for a maximum of 6–8 months (3). However, curative treatment remains poor
in spite of the fact that novel methods have increased the
therapeutic potential of radiation in tumor therapy. The
application of sub-lethal doses of radiation may result in local
failure, and may promote the migration and invasion of glioma cells
(4).
Tamoxifen (TAM) is a well-known non-steroidal
anti-estrogen agent with low toxicity that is widely used to treat
estrogen-dependent breast cancer. There have been an increasing
number of studies reporting that this agent may also inhibit the
growth of estrogen receptor-negative tumors, such as melanoma,
malignant peripheral nerve sheath tumors, bladder cancer and glioma
(5–8).
This indicates that TAM may exert anti-tumor effects in an estrogen
receptor-independent manner. Furthermore, the ability of TAM to
penetrate the blood-brain barrier facilitates its utilization in
treatment of malignant diseases in the central nervous system
(9). Although certain intracellular
signal transduction pathways, such as protein kinase C (PKC),
transforming growth factor-β, calmodulin, transcription factor
c-Myc, mitogen-activated protein kinase p38 and c-Jun NH2-terminal
kinase, have been implicated in TAM-induced apoptosis, the exact
molecular mechanism remains elusive (10–13). It
has been reported that TAM interferes with the activity of the
catalytic subunit of the PKC (14–16), and
the activity of PKC is associated with the growth rate of malignant
gliomas in vitro (17,18). Therefore, TAM may exert a synergistic
effect with radiotherapy and the mechanism may be associated with
the activity of PKC.
Studies have revealed that the activity of PKC is
significantly upregulated in glioma, and this increase is
concordant with the malignant growth rates (19–21). The
PKC family contains 12 subtypes classified in three classes based on
their requirement for specific activators and cofactors (22–24).
PKC-ι, a member of the atypical PKC family, was of particular
interest as this kinase is involved in cell growth, proliferation,
survival and apoptosis. PKC-ι has been demonstrated to promote
survival and prevent apoptosis in non-small-cell lung cancer and
gastric carcinoma (25,26), and is highly activated and
overexpressed in glioma. The kinase also plays a key role in cell
cycle progression and proliferation (27,28). These
findings indicated that PKC-ι is overexpressed in the
hyper-proliferative state of gliomas, and is also associated with
the resistance of gliomas to apoptosis in response to treatment
with cytotoxic agents or ionizing radiation. Therefore,
pharmacological manipulation of PKC-ι activity may restrain tumor
cell proliferation and restore susceptibility to apoptosis,
presenting a promising method for the treatment of glioma.
In the present study, the radiosensitizing effects
of TAM in human glioma A172 and U251 cells were examined in
vitro and the mechanisms of TAM-enhanced radiosensitization
were also investigated. The present results demonstrated that the
inhibition of PKC-ι activity by TAM may, at least in part,
radiosensitize glioma cells.
Materials and methods
Cell culture and irradiation
Human glioma A172 and U251 cells (Cell Bank of
Chinese Academy of Sciences, Shanghai, China) were cultured as a
monolayer in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum, 100 µg/ml streptomycin and 100 units/ml
penicillin at 37°C under a humidified atmosphere containing 5%
CO2. TAM was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and was dissolved in dimethyl sulfoxide at a concentration
of 10 mM. The cells were irradiated by 160 kV X-rays at a dose rate
of 1.15 Gy/min using a RS-2000 Pro Biological Irradiator (Rad
Source Technologies, Inc., Alpharetta, GA, USA).
Colony-forming assay
The A172 and U251 cells were plated in triplicate
into six-well plates and irradiated with various doses of X-rays 24
h subsequent to plating. The cells were treated with TAM
immediately following irradiation. After 48 h, the TAM-containing
medium was replaced with fresh growth medium. Subsequent to being
cultured for 12 days, the cells were fixed and stained with 1%
crystal violet and colonies containing >50 cells were counted.
The survival fraction was calculated as the fraction of colonies
divided by that of the control group. The cell survival curves were
then fitted using single hit multi-target radiobiological
models.
Flow cytometry analysis
A172 or U251 cells were irradiated with 4 Gy X-ray
and treated with TAM immediately following irradiation. After 24 or
48 h, the cells were harvested and fixed with 70% ice-cold ethanol.
Subsequent to incubation with RNase A, the cells were stained with
25 µg/ml propidium iodide (PI) for 30 min on ice. The distribution
of the cell cycle was analyzed using a FACSCalibur flow cytometer
(BD Biosciences, San Jose, CA, USA). For apoptosis analysis, cells
were washed with ice-cold phosphate-buffered saline (PBS) and
stained using an Annexin V-phycoerythrin/7-aminoactinomycin D
apoptosis detection kit (BD Biosciences). The percentage of
apoptotic cells was measured by flow cytometry. At least 10,000
cells were measured for each sample.
Western blotting
The cells were rinsed with ice-cold PBS and lysed by
RIPA lysis buffer with protease and phosphatase inhibitors for 20
min on ice. The cells were then centrifuged at 12,000 × g
for 10 min at 4°C. Cell lysates containing equal amount of protein
were resolved on 10% SDS-PAGE, and electrically transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories,
Hercules, CA, USA). Non-specific binding was blocked with
Tris-buffered saline containing 5% (w/v) skim milk for 2 h at room
temperature. The membranes were subsequently incubated with the
following antibodies: Rabbit anti-caspase-3 (1:1,000), rabbit
anti-poly(ADP-ribose) polymerase (PARP; 1:1,000; Proteintech Group,
Chicago, IL, USA), rabbit anti-B-cell lymphoma 2-associated death
promoter (Bad; 1:1,000; Epitomics, Burlingame, CA, USA), rabbit
anti-phosphorylated PKC-ι (p-PKC-ι; 1:1,000), mouse anti-PKC-ι
(1:1,000), rabbit anti-cyclin-dependent kinase 7 (cdk7; 1:2,000),
rabbit anti-phosphorylated cdk7 (p-cdk7; 1:500) (Abcam, Cambridge,
MA, USA) and mouse anti-β-actin (1:2,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, CA). The membranes were then incubated with a
horseradish peroxidase-conjugated secondary antibody.
Immunoblotting signals were detected using by using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc., Foster City,
CA, USA).
Statistical analysis
The data were expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance subsequent to multiple comparisons using the
S-N-K method using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with TAM enhances the
radiosensitivity of A172 and U251 cells
TAM has been reported to increase the
radiosensitivity of several glioma cell lines (18,29). In
the present study, the radiosensitizing effects of TAM on the human
malignant glioma A172 and U251 cells were explored. The cells were
irradiated with various doses of X-rays, and treated with 10 µM TAM
for 2 days subsequent to irradiation. A colony-forming assay was
performed to examine the effects of TAM on the radiosensitivity of
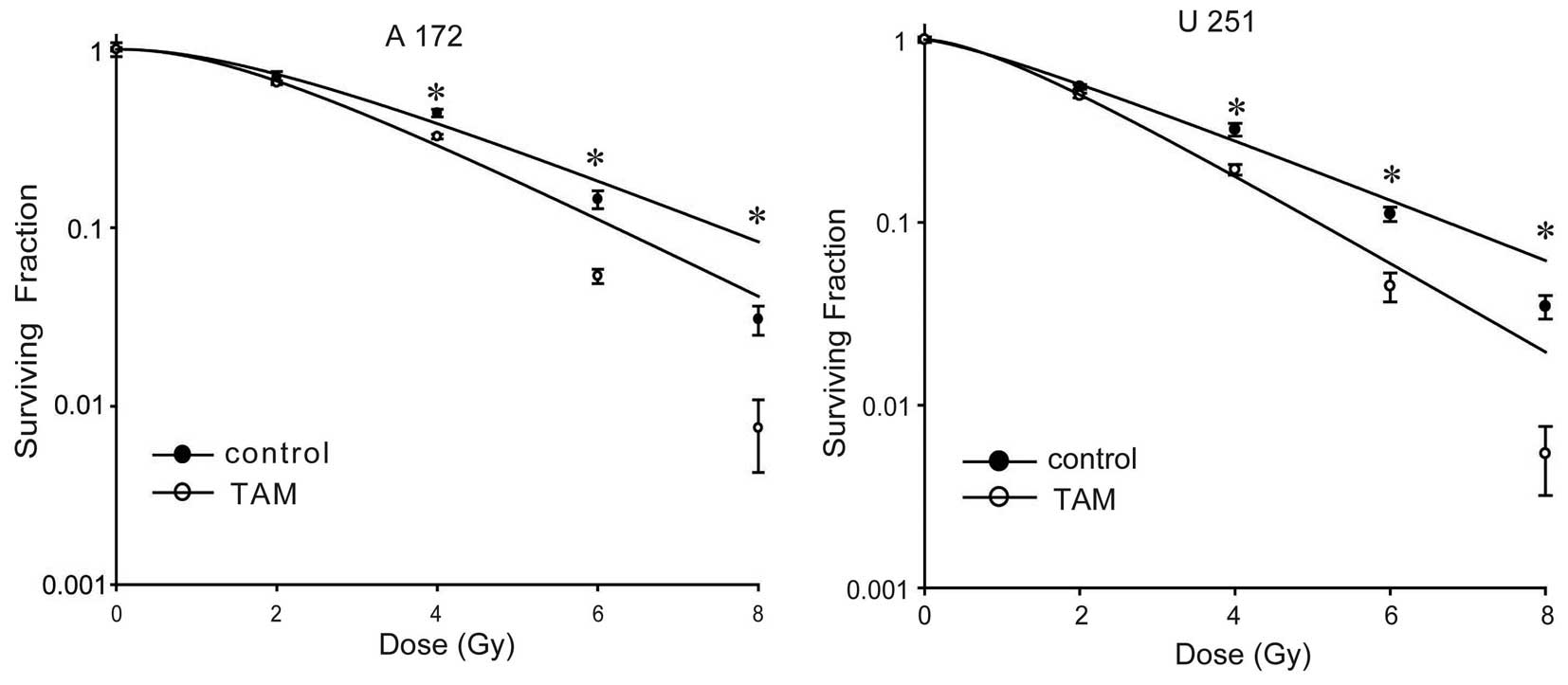
A172 and U251 cells. As shown in Fig.
1, treatment with TAM radiosensitized A172 and U251 cells. The
sensitivity enhancement ratios were 1.24 and 1.46 in A172 and U251
cells, respectively.
TAM increased radiation-induced
apoptosis
In order to investigate the roles of apoptosis in
the radiosensitizing effects of TAM, the A172 or U251 cells were
stained with phycoerythrin-conjugated Annexin V and flow cytometry
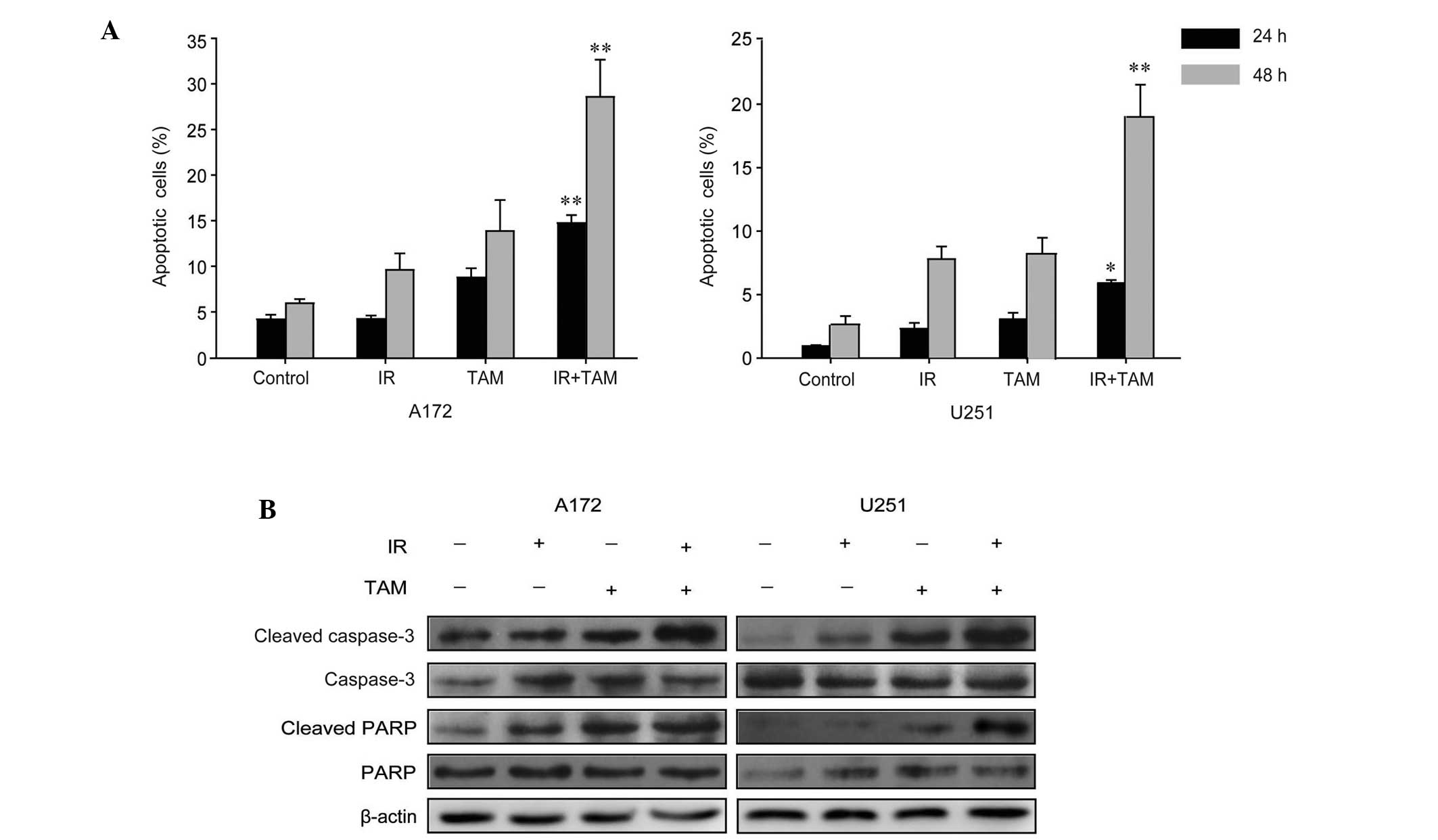
analysis was performed. As shown in Fig.
2A, combined treatment consisting of radiation and TAM induced
a substantial increase in the apoptotic rate compared to the group
treated with radiation alone. Subsequently, western blotting was
performed to assess the activation of the apoptosis signaling
pathway. It was found that TAM upregulated the expression levels of
cleaved caspase-3 and cleaved PARP in A172 and U251 cells (Fig. 2B).
TAM enhanced radiation-induced G2/M
arrest
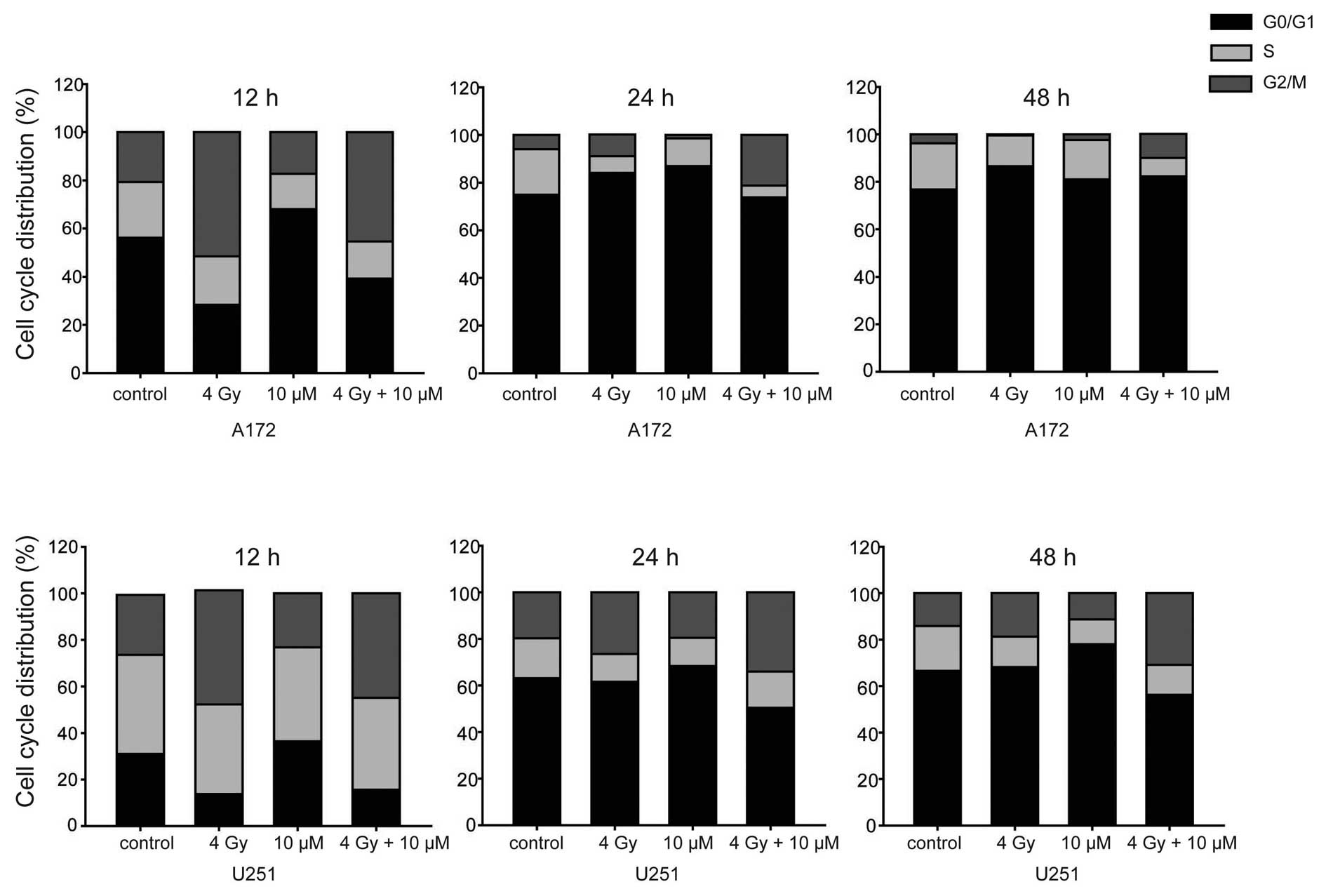
In order to investigate the effect of TAM or TAM
combined with radiation on cell cycle progression, flow cytometry
analysis was performed to reveal the cell cycle distribution at
various time points. G2/M phase arrest was induced by radiation and
the percentage of G2/M phase cells was gradually reduced at 24 h
(Fig. 3), indicating the completion
of DNA damage repair and reentering of cell cycle progression.
Treatment with TAM did not increase radiation-induced G2/M phase
arrest, but did maintain G2/M phase arrest, therefore postponing
cell cycle progress. Notably, treatment with TAM alone induced
G0/G1 phase arrest, suggesting the inhibition of DNA synthesis.
TAM suppressed the activation of PKC-ι
signaling
The estrogen-independent antitumor activity of TAM
may be partly due to the inhibitory effects of TAM on PKC (11). It has been reported that the
expression and function of atypical PKC-ι is highly upregulated in
glioma cells (28). In the present
study, western blot analysis revealed that the expression levels of
p-PKC-ι (T555) were decreased by TAM treatment in A172 and U251
cells (Fig. 4). In glioma T98 G and
U87MG cells, PKC-ι may act as a Bad kinase, thereby phosphorylating
and negatively regulating the pro-apoptotic function of Bad.
Similarly, combined administration of TAM and radiation induced a
significant increase in the levels of Bad protein in A172 and U251
cells (Fig. 4). In addition, PKC-ι
may phosphorylate Cdk7 in a cell cycle-dependent manner (27). As shown in Fig. 4, the expression of p-cdk7 was
significantly decreased in glioma cell lines subsequent to
treatment with TAM. These results suggested that the increased
radiation-induced cell apoptosis, as well as the prolonged cell
cycle arrest, may be in part due to the downregulation of PKC-ι
signaling by TAM treatment.
Discussion
In the present study, the colony-forming assay
revealed the radiosensitizing effects of TAM on the human malignant
glioma A172 and U251 cells. The number of apoptotic cells was
considerably increased by treatment with TAM and radiation. In
addition, TAM treatment did not induce an increase in the number of
cells that underwent G2/M arrest subsequent to radiation exposure,
but delayed the recovery of cell cycle progression. The activity of
PKC-ι signaling, which plays an important role in the
proliferation, apoptosis and cell cycle regulation of glioma cells
(27,28,30), was
markedly suppressed by TAM.
Ionizing radiation created DNA double strand breaks
and activated an integrated DNA damage response, including DNA
lesion sensing, signal transduction and activation of functional
proteins (31). The cellular outcome
of the DNA damage response is dependent upon the success of DNA
repair. Cells may maintain genome integration if the DNA damage is
well repaired; however, by contrast, the cell cycle may arrest at
the G2/M checkpoint to allow DNA damage repair, and cells may enter
the process of programmed cell death if the DNA damage repair fails
(32). Radiation treatment alone
induces apoptosis in glioma cells, and TAM enhances the
pro-apoptotic effects of radiation, suggesting that TAM interferes
with the DNA damage response. Similarly, TAM treatment maintains
G2/M phase arrest and prevents cells entering mitosis subsequent to
the administration of ionizing radiation. Since the
peroxidase-mediated metabolism of TAM was revealed to produce DNA
adducts and contribute to the formation of DNA damage (33), treatment with TAM alone induced G0/G1
phase arrest in human glioma cells. Additionally, A172 cells and
U251 cells express wild-type p53 and mutant p53 respectively,
whereas TAM treatment resulted in an equivalent increase of
radiosensitivity in the two cell lines, indicating the
radiosensitizing effects of TAM may be independent of p53
status.
At present, the mechanism by which TAM increases the
radiosensitivity of glioma cells is not completely clear. A
synergistic effect of TAM with radiation in C6 glioma cells has
been demonstrated, and may partially be due to the inhibition of
PKC activation (18). The atypical
PKC family member, PKC-ι was of particular interest as it is a key
regulator of cell survival, proliferation, invasion and
chemoresistance in glioma (27,30,34,35).
The present results revealed an inhibitory role of TAM in the
expression levels of p-PKC-ι, which may participate in the
radioresistance of glioma cells. In the human glioma T98G and U87MG
cell lines, PKC-ι is able to directly phosphorylate Bad, and
restrain its pro-apoptotic function (30). PKC-ι may play an equivalent role in
A172 and U251 cells, since inhibition of PKC-ι by TAM was revealed
to increase the expression of Bad. In metazoans, the only known
CDK-activating kinase is Cdk7 (36),
which was revealed to be phosphorylated by PKC-ι in a cell cycle
dependent manner (27,37). Inhibiting Cdk7 in the G2 phase blocks
entry into mitosis and disrupts Cdk1/cyclin B complex assembly
(38). It has been reported that
decreased Cdk7 activity led to inactivation of Cdk1 and caused an
irreversible G2/M phase arrest in human gastric carcinoma cells
(39). In A172 and U251 cells, the
sustained G2/M phase arrest induced by radiation and TAM treatment
may be the result of PKC-ι-mediated inactivation of Cdk7.
In summary, the present data revealed that TAM
enhanced the radiosensitivity of human glioma cells, accompanied by
increased cell apoptosis and sustained G2/M phase arrest.
Mechanistically, the capability of TAM to repress the activation of
PKC-ι, as well as its downstream targets Bad and Cdk7, may play an
vital role in the radiosensitizing effects on A172 and U251
cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos., 31270897, 81271682 and
30870585) and the Graduate Innovation Foundation of Medical College
of Soochow University and the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
References
|
1
|
Parlato C, Barbarisi M, Moraci M and
Moraci A: Surgery, radiotherapy and temozolomide in treating
high-grade gliomas. Front Biosci. 11:1280–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laperriere N, Zuraw L and Cairncross G:
Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology
Disease Site Group: Radiotherapy for newly diagnosed malignant
glioma in adults: a systematic review. Radiother Oncol. 64:259–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wild-Bode C, Weller M, Rimner A, Dichgans
J and Wick W: Sublethal irradiation promotes migration and
invasiveness of glioma cells: implications for radiotherapy of
human glioblastoma. Cancer Res. 61:2744–2750. 2001.PubMed/NCBI
|
|
5
|
Pu YS, Hsieh TS, Tsai TC, et al: Tamoxifen
enhances the chemosensitivity of bladder carcinoma cells. J Urol.
154:601–605. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Kokunai T and Tamaki N: Tamoxifen
interacts with NEU/C-ERBB-2 receptor and inhibits growth of human
malignant glioma cell lines. Kobe J Med Sci. 47:131–140.
2001.PubMed/NCBI
|
|
7
|
Kohli L, Kaza N, Coric T, et al:
4-Hydroxytamoxifen induces autophagic death through K-Ras
degradation. Cancer Res. 73:4395–4405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribeiro MP, Silva FS, Paixão J, Santos AE
and Custódio JB: The combination of the antiestrogen endoxifen with
all-trans-retinoic acid has anti-proliferative and anti-migration
effects on melanoma cells without inducing significant toxicity in
non-neoplasic cells. Eur J Pharmacol. 715:354–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilking N, Appelgren LE, Carlstrom K,
Pousette A and Theve NO: The distribution and metabolism of
14C-labelled tamoxifen in spayed female mice. Acta Pharmacol
Toxicol (Copenh). 50:161–168. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gundimeda U, Chen ZH and Gopalakrishna R:
Tamoxifen modulates protein kinase C via oxidative stress in
estrogen receptor-negative breast cancer cells. J Biol Chem.
271:13504–13514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horgan K, Cooke E, Hallett MB and Mansel
RE: Inhibition of protein kinase C mediated signal transduction by
tamoxifen. Importance for antitumour activity. Biochem Pharmacol.
35:4463–4465. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang Y, Cortina R and Perry RR: Role of
c-myc in tamoxifen-induced apoptosis estrogen-independent breast
cancer cells. J Natl Cancer Inst. 88:279–284. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandlekar S and Kong AN: Mechanisms of
tamoxifen-induced apoptosis. Apoptosis. 6:469–477. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakadate T, Jeng AY and Blumberg PM:
Comparison of protein kinase C functional assays to clarify
mechanisms of inhibitor action. Biochem Pharmacol. 37:1541–1545.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran C, Khatib Z, Petkarou A, et
al: Tamoxifen modulation of etoposide cytotoxicity involves
inhibition of protein kinase C activity and insulin-like growth
factor II expression in brain tumor cells. J Neurooncol. 67:19–28.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Brian CA, Liskamp RM, Solomon DH and
Weinstein IB: Inhibition of protein kinase C by tamoxifen. Cancer
Res. 45:2462–2465. 1985.PubMed/NCBI
|
|
17
|
Xiao H, Goldthwait DA and Mapstone T: The
identification of four protein kinase C isoforms in human
glioblastoma cell lines: PKC α, γ, ε and ζ. J Neurosurg.
81:734–740. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Yamada H, Sakai N, Niikawa S and
Nozawa Y: Enhancement of radiosensitivity by tamoxifen in C6 glioma
cells. Neurosurgery. 31:725–729; discussion 729–730. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Couldwell WT, Uhm JH, Antel JP and Yong
VW: Enhanced protein kinase C activity correlates with the growth
rate of malignant gliomas in vitro. Neurosurgery. 29:880–886;
discussion 886–887. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Couldwell WT, Antel JP and Yong VW:
Protein kinase C activity correlates with the growth rate of
malignant gliomas: Part II. Effects of glioma mitogens and
modulators of protein kinase C. Neurosurgery. 31:717–724,
discussion 724. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baltuch GH and Yong VW: Signal
transduction for proliferation of glioma cells in vitro occurs
predominantly through a protein kinase C-mediated pathway. Brain
Res. 710:143–149. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue M, Kishimoto A, Takai Y and
Nishizuka Y: Studies on a cyclic nucleotide-independent protein
kinase and its proenzyme in mammalian tissues. II. Proenzyme and
its activation by calcium-dependent protease from rat brain. J Biol
Chem. 252:7610–7616. 1977.PubMed/NCBI
|
|
23
|
Kishimoto A, Takai Y, Mori T, Kikkawa U
and Nishizuka Y: Activation of calcium and phospholipid-dependent
protein kinase by diacylglycerol, its possible relation to
phosphatidylinositol turnover. J Biol Chem. 255:2273–2276.
1980.PubMed/NCBI
|
|
24
|
Castagna M, Takai Y, Kaibuchi K, Sano K,
Kikkawa U and Nishizuka Y: Direct activation of calcium-activated,
phospholipid-dependent protein kinase by tumor-promoting phorbol
esters. J Biol Chem. 257:7847–7851. 1982.PubMed/NCBI
|
|
25
|
Regala RP, Weems C, Jamieson L, Copland
JA, Thompson EA and Fields AP: Atypical protein kinase Cι plays a
critical role in human lung cancer cell growth and tumorigenicity.
J Biol Chem. 280:31109–31115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takagawa R, Akimoto K, Ichikawa Y, et al:
High expression of atypical protein kinase C λ/ι in gastric cancer
as a prognostic factor for recurrence. Ann Surg Oncol. 17:81–88.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desai SR, Pillai PP, Patel RS, McCray AN,
Win-Piazza HY and Acevedo-Duncan ME: Regulation of Cdk7 activity
through a phosphatidylinositol (3)-kinase/PKC-ι-mediated signaling
cascade in glioblastoma. Carcinogenesis. 33:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel R, Win H, Desai S, Patel K, Matthews
JA and Acevedo-Duncan M: Involvement of PKC-ι in glioma
proliferation. Cell Prolif. 41:122–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donson AM, Weil MD and Foreman NK:
Tamoxifen radiosensitization in human glioblastoma cell lines. J
Neurosurg. 90:533–536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Desai S, Pillai P, Win-Piazza H and
Acevedo-Duncan M: PKC-ι promotes glioblastoma cell survival by
phosphorylating and inhibiting BAD through a phosphatidylinositol
3-kinase pathway. Biochim Biophys Acta. 1813:1190–1197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He J, Li J, Ye C, et al: Cell cycle
suspension: a novel process lurking in G2 arrest. Cell Cycle.
10:1468–1476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaikwad NW and Bodell WJ:
Peroxidase-mediated dealkylation of tamoxifen, detected by
electrospray ionization-mass spectrometry and activation to form
DNA adducts. Free Radic Biol Med. 52:340–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldwin RM, Garratt-Lalonde M, Parolin DA,
Krzyzanowski PM, Andrade MA and Lorimer IA: Protection of
glioblastoma cells from cisplatin cytotoxicity via protein kinase
Cι-mediated attenuation of p38 MAP kinase signaling. Oncogene.
25:2909–2919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baldwin RM, Parolin DA and Lorimer IA:
Regulation of glioblastoma cell invasion by PKCι and RhoB.
Oncogene. 27:3587–3595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schachter MM and Fisher RP: The
CDK-activating kinase Cdk7: taking yes for an answer. Cell Cycle.
12:3239–3240. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bicaku E, Patel R and Acevedo-Duncan M:
Cyclin-dependent kinase activating kinase/Cdk7 co-localizes with
PKC-ι in human glioma cells. Tissue Cell. 37:53–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Larochelle S, Merrick KA, Terret ME, et
al: Requirements for Cdk7 in the assembly of Cdk1/cyclin B and
activation of Cdk2 revealed by chemical genetics in human cells.
Mol Cell. 25:839–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu J, Guo QL, You QD, et al: Gambogic
acid-induced G2/M phase cell-cycle arrest via disturbing
CDK7-mediated phosphorylation of CDC2/p34 in human gastric
carcinoma BGC-823 cells. Carcinogenesis. 28:632–638. 2007.
View Article : Google Scholar : PubMed/NCBI
|