Introduction
In western countries, the reported incidence of
uveal melanoma is ~7 cases per million population per year. In
China, the incidence of the disease is much lower (1,2). Due to
its unique biological properties and the lack of diagnostic
techniques in its early stage, 40% of choroidal melanoma cases
present with metastases at diagnosis. The most frequently affected
organ is the liver (93–95% of cases), due to the higher blood flow
and a paucity of lymphatics in the choroid (3). Metastasis usually occurs in the majority
of patients within 5 years of treatment of a primary lesion. Upon
the occurrence of liver metastases, the lesions are frequently
multiple, distributed and difficult to resect surgically (4–6). More than
90% of patients succumb within 1 year, and even with aggressive
treatment strategies, the median survival time of patients with
liver metastases is less than 13 months (7–9).
Furthermore, a few cases of spontaneous rupture of metastatic
hepatic melanoma have been reported (10–12).
However, surgeons do not often attach significance to the risk of
bleeding caused by spontaneous rupture of metastatic melanoma,
which causes difficulties in the timely rescue if the tumor
ruptures.
Here, we report an extremely rare case of metastatic
melanoma following the treatment of primary ocular lesions, which
is relatively unusual for two reasons: firstly, a solitary hepatic
metastasis with spontaneous rupture is extremely rare; secondly,
the exceptionally long disease-free period (10 years) with this
mode of metastasis is also rare. This case emphasizes the need for
clinicians to pay attention to the presence of ruptured hepatic
metastatic melanoma and the characteristics of metastatic
melanoma.
Case report
Patient characteristics
On December 3, 2013, a 45-year-old Chinese male was
referred to the First Affiliated Hospital of Zhejiang University
(Hangzhou, China), with complaints of bloating for a month. Ten
years earlier, the patient had undergone left ocular enucleation
and artificial eye implantation at a different hospital.
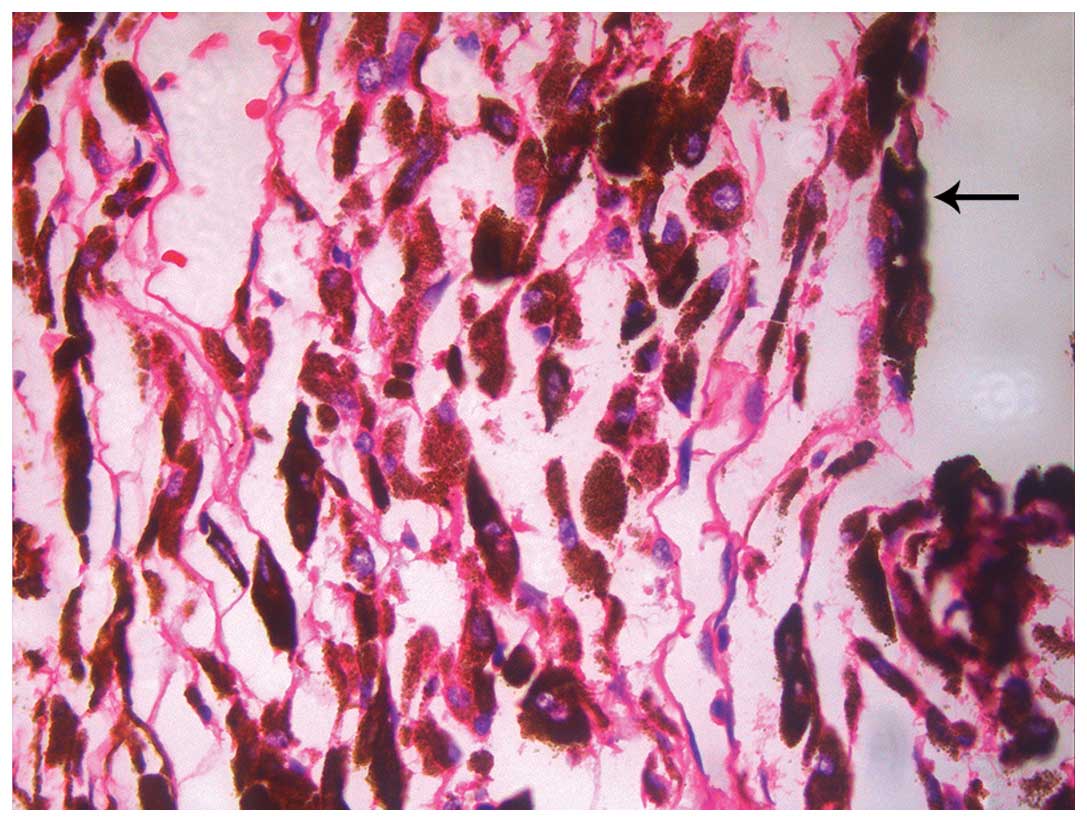
Postoperative pathology revealed choroidal melanoma without
intrascleral or vascular involvement (Fig. 1). On December 22, 2013, right
hepatectomy was performed for the purposes of diagnosis and cure.
The patient was scheduled for abdominal ultrasonography and a liver
function test 1 month after surgery; however, the patient's
treatment was delayed until 2 weeks after the scheduled date due to
the Chinese Spring Festival. On examination, isolated pancreatic
recurrence was observed. Distal pancreatic resection and
splenectomy were performed on February 5, 2014. Until now, no
symptoms of recurrence have been observed in our patient. The study
was approved by the Ethics Committee of the First Affiliated
Hospital of Zhejiang University. Written informed consent was
obtained from the patient's family.
Imaging examinations
Abdominal magnetic resonance imaging (MRI) was
performed using Magneton Impact 3.0T operating on the Numaris VB33D
imaging system (Philips, Veenpluis, Netherlands), and transverse
and sagittal T1-weighted and T2-weighted images were examined.
Gadopentetate dimeglumine (Magnevist; Shering, Berlin, Germany) was
intravenously administered at a dose of 0.1 mmol/kg of body weight,
and contrast-enhanced images were obtained immediately. An
abdominal computed tomography (CT) scan was performed using a
Brilliance 64-channel scanner (Philips). For enhanced CT, 30 ml
iohexol (GE Healthcare, Carrigtwohill, Ireland) was administered
via intravenous drip infusion, and images were obtained. For
computed tomography angiography (CTA) images, 50 ml iobitridol
(Guerbet, Villepinte, France) was administered via intravenous drip
infusion. Positron emission tomography (PET-CT) was performed by
injection of F-18-fluoro-2-deoxyglucose (FDG) at a dose of 7 mCi
(calculated by 0.1 mCi/kg). Full-body PET-CT scans were performed
from the top of the head to the bottom of the feet. The CT portion
was carried out using a multi-detector helical computed tomography
scanner (Philips).
Histological examination
The paraffin-embedded tumor tissue sections were
stained immunohistochemically with optimally diluted primary
antibodies and appropriate antigen retrieval solutions. The
Histofine SAB-PO (M) or (R) immunohistochemical staining kit
(Nichirei, Tokyo, Japan) was used. The primary antibodies used were
anti-S100 protein (rabbit polyclonal; Dako, Glostrup, Denmark),
anti-HMB45 protein (rabbit polyclonal; Dako), anti-Melan A protein
(mouse polyclonal; Dako) and anti-α-fetoprotein (AFP; mouse
polyclonal; Dako).
Surgery
The protocol of surgical resection was decided
together by the patient and the treatment team in various
departments including those of Hepatobiliary Surgery, Radiology and
Chemotherapy. The effect of surgery was assessed using the
following standards: R0, negative microscopic margins; R1, positive
microscopic margin; and R2, gross residual disease. To begin, the
surgical team made a roof-shaped incision under the bilateral
costal margin from the front tip of the 11th rib in order to fully
expose the visual operative field. In the next step, the ligamentum
teres hepatic and falciform ligament was disconnected, and the
whole liver and the lesion were exposed clearly. Then R0 lesion
resection was performed following careful dissociation of the
common bile duct, hepatic artery and portal vein. Finally, bleeding
was stopped by using the meticulous hemostatic technique, a
drainage catheter was put in place and the abdominal cavity was
closed.
Imaging findings
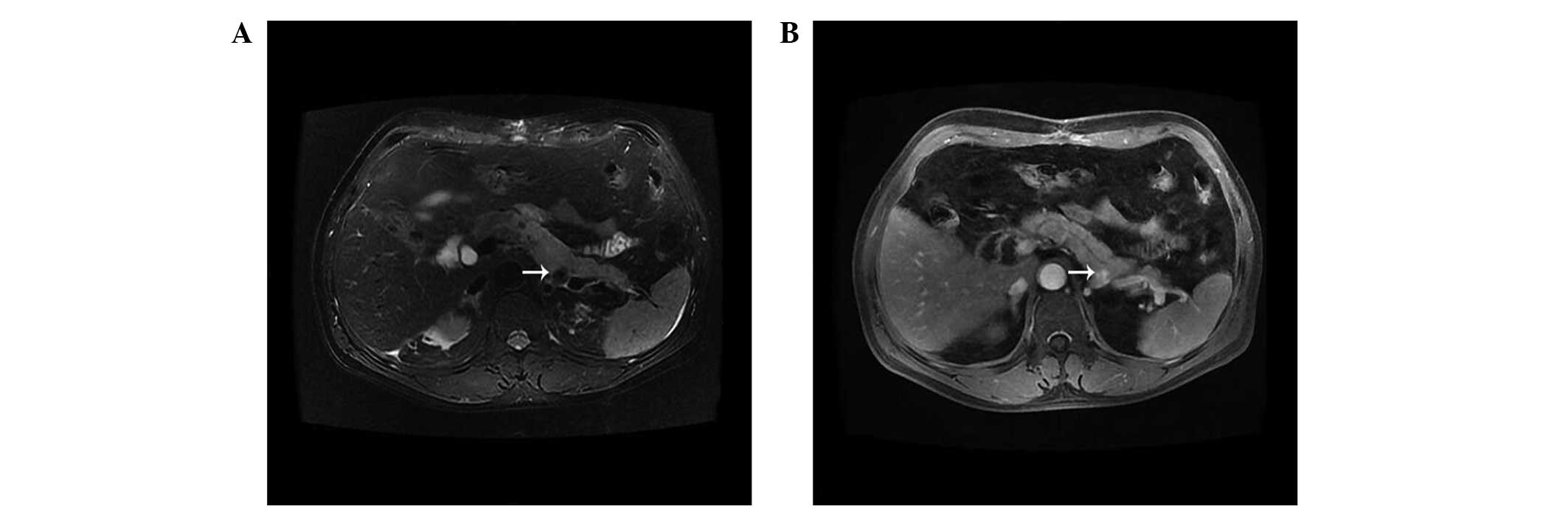
Abdominal CT scan and MRI (Fig. 2) revealed a 12×13 cm mass in the right
liver. In the CT arterial phase, a mild heterogeneous enhanced
signal revealed that the mass in the right liver may be a hepatic
tumor. T1-weighted imaging revealed a peripheral high signal and a
low heterogeneous signal. T2-weighted imaging revealed a
center-enhanced mass with a low signal edge. Hepatic CTA revealed
no abnormal vessels that might be considered as possible tumor
vessels, and the huge mass had partially ruptured and was bleeding
spontaneously. The whole liver volume was 2,428 cm3, and
the left liver volume was 450 cm3. PET-CT revealed a
solitary huge nodule with increased FDG uptake in the right
liver.
Surgery and immunohistochemical
findings
Radical right hepatectomy was performed on December
22, 2013. Rapid histological examination during surgery revealed
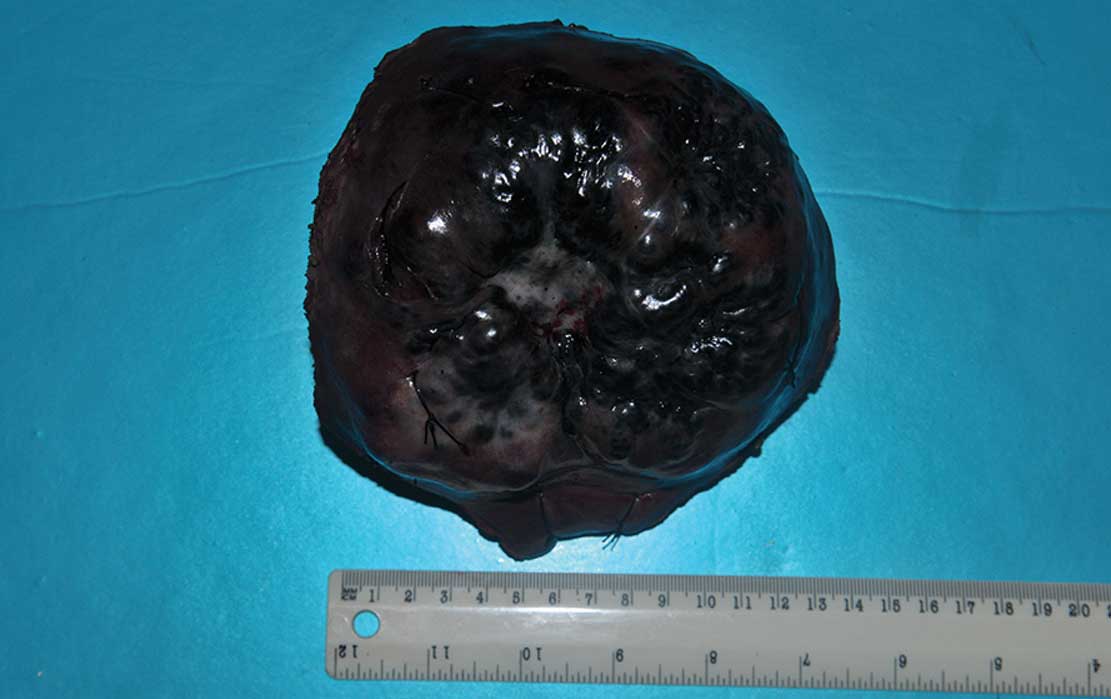
malignant hepatic metastatic melanoma. A macroscopic view of the
tumor is shown in Fig. 3. The hard
protruding mass on the liver has a size of ~11×10 cm and an
irregular boundary with a sunken central white area, which was
regarded as an indication of spontaneous intratumoral hemorrhage
and necrosis. The diagnosis of malignant hepatic metastatic
melanoma was made after the results of detailed histopathological
examination were confirmed: HMB45(+), S100(+), AFP(–) and Melan
A(+) (Fig. 4). One-and-a-half months
after surgery, an isolated pancreatic lesion was identified by
abdominal MRI scan (Fig. 5). Distal
pancreatic resection and splenectomy were performed. Postoperative
immunohistochemistry also confirmed melanoma metastasis: HMB45(+),
S100(–), AFP(–) and Melan A(+).
Discussion
In recent years, melanoma has caused wide public
concern due to the rapid increase of its incidence, in addition to
its highly malignant and metastatic characteristics. Although the
incidence of melanoma in China is currently relatively low compared
with that in European and American countries, new cases of melanoma
have been reported at a notable rate of almost 20,000 new cases a
year (1). In western countries, the
reported incidence of uveal melanoma is ~7 cases per million
population per year. Most patients present with localized disease
and can expect a 5-year survival rate of 82–87% if they are younger
than 60 years (2,13). Kivelä et al (3) suggested that tumor micrometastases
develop as long as 5 years before the local ocular therapy, and the
most frequently affected organ is the liver (93–95% of cases) due
to the higher blood flow and paucity of lymphatics in the choroid.
However, Miyamoto et al (14)
considered that if the dissemination of the primary tumor occurs
through a hematogenous route, then the cells that escape from the
primary tumor should first encounter the capillary beds of the
lungs. These authors therefore concluded that the reason why the
liver is the main site of uveal melanoma metastasis is a
combination of two factors: the reflection of the homing of tumor
cells to this organ and the survival of these uveal melanoma cells
in the liver microenvironment. In recent years, literature has
indicated that histological, genetic and demographic factors are
associated with metastases in uveal melanoma, including mutations
in genes GNAQ, GNA11 and PTEN, the helix-loop-helix inhibitor ID2,
chromosome 3, 6 and 8 alteration, miRNA-21, and mutations in c-Kit
and BRAF (15–17).
Rajpal et al (18) revealed that approximately two-thirds
of patients present with metastatic disease less than 5 years after
initial diagnosis by analyzing the samples of 35 patients. In a
retrospective study at Duke University, only 168 patients out of
7,104 patients with diagnosed uveal melanoma had recurrence 10 or
more years after initial diagnosis, indicating an incidence of 2.4%
(19). Pons et al (20) analyzed a total of 58 patients and
demonstrated that the median time for metastases development was
25.63 months. A systematic review revealed that metastases to the
liver occurred at a median interval of 54 months (21). Moreover, Yang et al (4) identified that 75% of Chinese patients
presented with liver metastases at the time the primary tumor was
diagnosed in a retrospective single-center analysis. In our
patient, melanoma recurrence occurred a decade after treatment of
the original tumor, which can be counted as a relatively rare
case.
Once liver metastasis occurs, the lesions are often
multiple, distributed and difficult to resect surgically (6). More than 90% of patients succumb within
1 year, and patients are considered resistant to commonly available
systemic chemotherapy or chemoimmunotherapy, with single-agent
response rates below 10%. Even with aggressive treatment
strategies, the median survival time of patients with liver
metastases is less than 13 months (7–9). A
detailed review of uveal melanoma has revealed that liver function
tests, serum markers including alkaline phosphatase and lactate
dehydrogenase, and imaging screening including abdominal
ultrasound, CT, MRI and PET-CT contribute to the early detection of
melanoma metastasis (13). Triozzi
and Singh (22) also reviewed blood
biomarkers of uveal melanoma metastasis covering vascular
endothelial growth factor, hepatocyte growth factor, epidermal
growth factor and insulin-like growth factor-1. However, Augsburger
et al (23) reported that
there is no significant decisive evidence that early detection of
metastatic disease impacts survival. Active surveillance might
become widely accepted if these interventions are associated with a
lower disease burden (15).
The modern multidisciplinary management of melanoma
metastases to the liver has evolved greatly in recent years.
Vahrmeijer et al (24)
described the various treatment modalities of uveal melanoma
metastases confined to the liver in a review, suggesting that
regional treatment modalities including hepatic artery infusion,
isolated hepatic perfusion and hepatic arterial chemoembolization
may be considered in order to increase the response rate. Mariani
et al (25) undertook a
detailed retrospective review of 3,873 patients with uveal melanoma
and concluded that surgical resection is able to offer a greatly
improved long-term survival and appears at present to be the
optimal way of improving the prognosis in metastatic uveal
melanoma. A further meta-analysis further elaborates that radical
resection (R0 resection) of liver metastases from melanoma appears
to improve overall survival compared with non-operative management
or incomplete resection. Data from this review indicates that the
presence of multiple lesions involving one viscera is not a
contraindication to surgical therapy if R0 resection is achievable
(26). However, in the majority of
patients, hepatectomy is impossible due to the extent or location
of metastatic melanoma (4). A
noteworthy study by Zhao et al (27) reported a case of adult-to-adult living
donor liver transplantation as a treatment option for metastatic
melanoma. Although the high recurrence rate and probable poor
prognosis of this malignant tumor make transplantation
controversial, we remain of the opinion that this new treatment
option should be provided.
In addition, a few cases of spontaneous rupture of
metastatic hepatic melanoma have been reported (10–12), but
the pathogenesis and treatment modalities have not been determined.
You et al (28) considered
that tumor protrusion >1 cm above the liver surface is a
significant independent risk factor for spontaneous rupture of
hepatocellular carcinoma by retrospectively analyzing a sample of
34 patients. We considered that the situation in our case was
likely to be similar to that of ruptured hepatocellular carcinoma.
Chun et al (10) suggested
that transcatheter arterial embolization would be an effective,
safe treatment in patients with rupture of metastatic hepatic
melanoma. In our case, the CTA image of the patient revealed that
the huge mass had partially ruptured and was bleeding
spontaneously; hemostatic drugs were given to relieve bleeding.
However, surgeons do not often attach significance to the risk of
bleeding caused by spontaneous rupture of metastatic melanoma,
which naturally makes timely rescue more complicated.
In conclusion, surgery is a feasible and effective
method for only a small number of patients. When surgery cannot be
performed to remove the lesion, it remains unclear which type of
adjuvant therapy is the most conducive to the patient. Since the
total number of cases of melanoma with liver metastases is low, and
large-sample multi-center double-blind randomized controlled trials
are lacking, opinions on the optimal treatment of liver metastatic
melanoma are not yet unified. Our case is quite unusual in two
respects: firstly, a solitary hepatic metastasis with spontaneous
rupture is extremely rare; secondly, the exceptionally long
disease-free period (10 years) with this mode of metastasis is also
rare. This case emphasizes the need for clinicians to pay attention
to the presence of ruptured hepatic metastatic melanoma and the
characteristics of metastatic melanoma.
Acknowledgements
The authors would like to thank the staff of the
Departments of Hepato-Biliary-Pancreatic Surgery, Pathology and
Radiology in the First Affiliated Hospital of Zhejiang University
Medicine School for their helpful assistance.
References
|
1
|
Committee of Experts of the Chinese
Society of Clinical Oncology for Melanoma, . China melanoma
treatment guidelines (2011). Chin Clin Oncol. 159–171. 2012.
|
|
2
|
Balch CM, Soong SJ, Gershenwald JE, et al:
Prognostic factors analysis of 17,600 melanoma patients: validation
of the American Joint Committee on Cancer melanoma staging system.
J Clin Oncol. 19:3622–3634. 2001.PubMed/NCBI
|
|
3
|
Kivelä T, Eskelin S, Mäkitie T and
Summanen P: Exudative retinal detachment from malignant uveal
melanoma: predictors and prognostic significance. Invest Ophthalmol
Vis Sci. 42:2085–2093. 2001.PubMed/NCBI
|
|
4
|
Yang XY, Xie F, Tao R, Li AJ and Wu MC:
Treatment of liver metastases from uveal melanoma: a retrospective
single-center analysis. Hepatobiliary Pancreat Dis Int. 12:602–606.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivoire M, Kodjikian L, Baldo S,
Kaemmerlen P, Négrier S and Grange JD: Treatment of liver
metastases from uveal melanoma. Ann Surg Oncol. 12:422–428. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rietschel P, Panageas KS, Hanlon C, Patel
A, Abramson DH and Chapman PB: Variates of survival in metastatic
uveal melanoma. J Clin Oncol. 23:8076–8080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varghese S, Xu H, Bartlett D, et al:
Isolated hepatic perfusion with high-dose melphalan results in
immediate alterations in tumor gene expression in patients with
metastatic ocular melanoma. Ann Surg Oncol. 17:1870–1877. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters S, Voelter V, Zografos L, et al:
Intra-arterial hepatic fotemustine for the treatment of liver
metastases from uveal melanoma: experience in 101 patients. Ann
Oncol. 17:578–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubio S, Barbero-Villares A, Reina T,
Nieto S, Mendoza J and García-Buey L: Rapidly-progressive liver
failure secondary to melanoma infiltration. Gastroenterol Hepatol.
28:619–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chun HJ, Osuga K, Fahrni M and Nakamura H:
Massive bleeding of ruptured metastatic hepatic melanoma treated by
transarterial embolization. Jpn J Radiol. 28:395–397. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagner WH, Lundell CJ and Donovan AJ:
Percutaneous angiographic embolization for hepatic arterial
hemorrhage. Arch Surg. 120:1241–1249. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooperman AM, Weiland LH and Welch JS:
Massive bleeding from a ruptured metastatic hepatic melanoma
treated by hepatic lobectomy. Case report and review of the
literature. Mayo Clin Proc. 51:167–170. 1976.PubMed/NCBI
|
|
13
|
Papastefanou VP and Cohen VM: Uveal
melanoma. J Skin Cancer. 2011:5739742011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamoto C, Balazsi M, Bakalian S,
Fernandes BF and Burnier MN Jr: Uveal melanoma: Ocular and systemic
disease. Saudi J Ophthalmol. 26:145–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Materin MA, Faries M and Kluger HM:
Molecular alternations in uveal melanoma. Curr Probl Cancer.
35:211–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C and Wei W: The miRNA expression
profile of the uveal melanoma. Sci China Life Sci. 54:351–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sisley K, Doherty R and Cross NA: What
hope for the future? GNAQ and uveal melanoma. Br J Ophthalmol.
95:620–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajpal S, Moore R and Karakousis CP:
Survival in metastatic ocular melanoma. Cancer. 52:334–336. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crowley NJ and Seigler HF: Late recurrence
of malignant melanoma. Analysis of 168 patients. Ann Surg.
212:173–177. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pons F, Plana M, Caminal JM, et al:
Metastatic uveal melanoma: is there a role for conventional
chemotherapy? - A single center study based on 58 patients.
Melanoma Res. 21:217–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hameed AM, Ng EE, Johnston E, et al:
Hepatic resection for metastatic melanoma: a systematic review.
Melanoma Res. 24:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Triozzi PL and Singh AD: Blood biomarkers
of uveal melanoma metastasis. Br J Ophthalmol. 95:3–4. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Augsburger JJ, Corrêa ZM and Shaikh AH:
Effectiveness of treatments for metastatic uveal melanoma. Am J
Ophthalmol. 148:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vahrmeijer AL, van de Velde CJ, Hartgrink
HH and Tollenaar RA: Treatment of melanoma metastases confined to
the liver and future perspectives. Dig Surg. 25:467–472. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mariani P, Piperno-Neumann S, Servois V,
et al: Surgical management of liver metastases from uveal melanoma:
16 years' experience at the Institut Curie. Eur J Surg Oncol.
35:1192–1197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aubin JM, Rekman J, Vandenbroucke-Menu F,
et al: Systematic review and meta-analysis of liver resection for
metastatic melanoma. Br J Surg. 100:1138–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Yan LN and Li B: Adult-to-adult
living donor liver transplantation for malignant metastatic
melanoma to the liver. Hepatobiliary Pancreat Dis Int. 9:329–332.
2010.PubMed/NCBI
|
|
28
|
You MX, Yu XX, Wu K, Lin YS, Zhu GQ and
Shi CS: Analysis of risk factors for spontaneous rupture of
hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 35:217–220.
2013.(In Chinese). PubMed/NCBI
|