Introduction
Thyroid cancer is one of the most prevalent
malignancies of the endocrine system. Papillary thyroid carcinoma
(PTC), the most common histological type of thyroid cancer, has
demonstrated the fastest rising incidence in previous years when
compared with other endocrine cancers (1,2). According
to the SEER 9 database of the National Cancer Institute (Bethesda,
MD, USA), the gender ratio of PTC in female and male patients
declines from >5 at 20–24 years old to 3.4 at 35–44 years old,
and reaches almost 1 at >80 years old, indicating that PTC
occurs predominantly during the reproductive years (3). The predominance of PTC in females has
been observed in all geographical areas and ethnic groups (1,4).
As epidemiological data report a strong female
predilection for thyroid cancer, the gender hormones estrogen and
progesterone may play vital roles in the pathogenesis of thyroid
neoplasm (1,4). Estrogen and progesterone act through the
estrogen receptor (ER) and progesterone receptor (PR),
respectively, which belong to the nuclear hormone receptor
superfamily and are expressed in benign and malignant thyroid
tissues. There are two ER isoforms, ERα, which is expressed in the
endometrium, breasts, ovarian stroma and hypothalamus, and ERβ,
which is expressed in the kidneys, brain, bones, heart, lungs,
intestinal mucosa, prostate and endothelium. PR has two isoforms,
PR-A and PR-B, which are expressed in the breasts, uterus and brain
(5).
Notably, it has been found that estrogen regulates
the transcription of numerous cell proliferation-associated genes
(6–8).
In addition, accumulating evidence has revealed that estrogen
exerts direct effects on thyroid cell lines originating from normal
thyroid gland tissue and thyroid carcinoma by ER-dependent
mechanisms, such as enhancement of proliferation, modulation of
sodium-iodide symporter and thyroglobulin gene expression, and
upregulation of matrix metalloproteinase (MMP) 9 production
(8–10).
In PTC cells, RET/PTC re-arrangement is a common
genetic event (11). A previous study
has indicated that RET/PTC-induced cell growth is mediated in part
by epidermal growth factor receptor (EGFR) (12). Increased expression of EGFR has been
revealed to play an important role in thyroid tumor progression.
Additionally, PTC stromal invasion may be regulated through
EGFR-dependent activation of MMP-2/gelatinase A (13).
Generally, PTC is associated with a favorable
prognosis (14–16). However, up to 10% of patients with PTC
succumb as a direct result of this carcinoma, and 22–30% experience
recurrent disease (14,16). Thus, it is important to be able to
identify the PTC patients that possess a poor prognosis, through
which an appropriate treatment may be chosen. The present study
examined the association between immunohistochemical (IHC) factors,
consisting of ERα, PR and EGFR expression, and gender and tumor
size in patients with PTC.
Materials and methods
Subjects
A total of 78 paraffin-embedded PTC tissue specimens
were obtained from the Department of Pathology at the First
Affiliated Hospital of Dalian Medical University (Dalian, Liaoning,
China), with the specimens being excised between January 2011 and
December 2013. The tissues consisted of 64 PTC tissue specimens,
obtained from 27 male and 37 female patients, and 14 nodular
thyroid goiter (NTG) tissue specimens, obtained from 4 male and 10
female patients. Slides stained with hematoxylin and eosin were
examined initially and optimal slides were selected for IHC
staining. The present study was approved by the Ethics Committee of
the First Affiliated Hospital of Dalian Medical University. Written
informed consent was obtained from the patients.
Immunohistochemistry
Serial tissue sections were sliced at a width of 4
µm from formalin-fixed and paraffin-embedded tissue blocks, and the
slices were mounted onto poly-L-lysine-coated glass slides (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The sections were
deparaffinized and rehydrated through a graded series of
xylene-ethanol, and then incubated for 7 min in 3% hydrogen
peroxide to inhibit endogenous peroxidases. Antigen retrieval was
performed by boiling the slides for 30 min in EDTA (pH 8.9–9.1).
The tissue sections were incubated with primary rabbit anti-human
monoclonal ERα (clone, 1D5; dilution, 1:200), rabbit anti-human
monoclonal PR (clone, PgR 636; dilution, 1:250) and mouse
anti-human monoclonal EGFR (clone, UMAB 95; dilution, 1:200; Dako
North America, Inc., Carpinteria, CA, USA) antibodies.
Immunoreactivity was assessed using the chromogen
3,3′-diamino-benzidine (Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
Fujian, China). The slides were then counterstained with
hematoxylin, washed three times with phosphate-buffered saline for
5 min, dehydrated with graded alcohol and xylene, and mounted onto
coverslips. Appropriate positive and negative controls were
performed simultaneously with the patient specimen; breast
carcinoma specimens were used as positive controls for ERα and PR,
colon cancer specimens were used as the positive control for EGFR
and PBS was used as the negative control.
IHC analysis
The slides were immunostained for ERα, PR and EGFR
and the staining was then assessed using the scoring system
reported by Allred et al (17). Briefly, the proportion score (PS)
expressed the estimated proportion of tumor cells that stained
positive for ERα, PR and EGFR, as follows: 0, none; 1, 1/100; 2,
1/100 to 1/10; 3, 1/10 to 1/3; 4, 1/3 to 2/3; and 5, >2/3. The
intensity score (IS) expressed the average intensity of the
staining, as follows: 0, none; 1, weak; 2, intermediate; and 3,
strong. The PS and IS were then added to obtain a total score (TS),
which ranged between 0 and 8. Tumors were classed as positive for
the expression of ERα, PR or EGFR if they possessed a TS ≥3.
Statistical analysis
The data were analyzed using SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). The differences between groups
were tested using the χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of ERα, PR and EGFR is
elevated in PTC tissues
To examine whether benign and malignant thyroid
cells express ERα, PR and EGFR, IHC analysis was performed on the
PTC and NTG tissue sections. Tumor cells with a score ≥3 were
considered to be positive for the expression of ERα, PR or EGFR.
ERα, PR and EGFR were found to be expressed more frequently in the
PTC tissues compared with NTG tissues (Fig. 1). Briefly, 38 out of 64 (59.4%) PTC
tissues demonstrated a strong immunopositivity for ERα (P<0.05),
29 out of 64 (45.3%) PTC tissues demonstrated an elevated
expression of PR (P<0.05), and 62 out of 64 (96.9%) PTC tissues
demonstrated a significantly increased expression of EGFR
(P<0.05) (Table I).
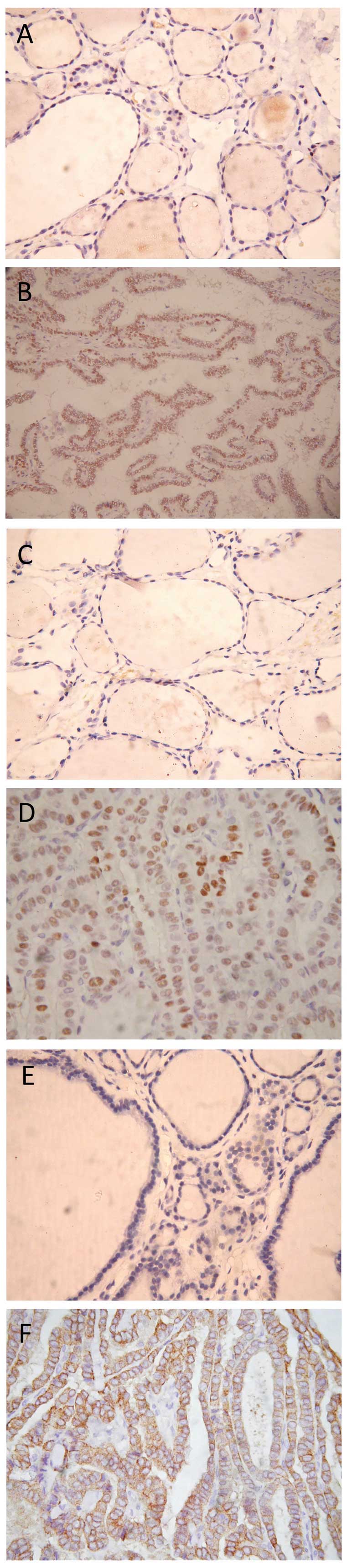 | Figure 1.Immunohistochemical staining for ERα,
PR and EGFR in PTC and NTG tissues. (A) NTG tissue without ERα
staining (magnification, x400). (B) PTC tissue with nuclear ERα
staining (magnification, x400). (C) NTG tissue without PR staining
(magnification, x400). (D) PTC tissue with nuclear PR staining
(magnification, x400). (E) NTG tissue without EGFR staining
(magnification, x400). (F) PTC tissue. ERα, estrogen receptor α;
PR, progesterone receptor; EGFR, epidermal growth factor receptor;
PTC, papillary thyroid carcinoma; NTG, nodular thyroid goiter. |
 | Table I.Increased ERα, PR and EGFR expression
by PTC tissue compared with NTG (n=78). |
Table I.
Increased ERα, PR and EGFR expression
by PTC tissue compared with NTG (n=78).
|
| Tissue type, n
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Variable | NTG | PTC | χ2 | P-value |
|---|
| Total | 14 (100.0) | 64 (100.0) |
|
|
| ERα | 2 (14.3) | 38 (59.4) | 9.348 | 0.002 |
| PR | 1 (7.1) | 29 (45.3) | 7.071 | 0.008 |
| EGFR | 11 (78.6) | 62 (96.9) | 6.415 | 0.011 |
In addition, the expression of ERα and PR was
detected in the nucleus, while the expression of EGFR was examined
in the cytoplasm and cell membrane.
ERα expression is increased in PTC
patients with a larger tumor size
It has been reported that the tumor size is strongly
associated with the therapy administered to patients with PTC and
the prognosis of the patients (18).
To investigate whether the expression of ERα, PR and EGFR is also
associated with the risk of PTC, the IHC data was categorized
according to the size of the tumor. It was found that ERα
expression is markedly associated with the size of PTC tumors
(P<0.05) (Table II), indicating
that ERα may be used as a marker to predict the risk of PTC.
 | Table II.Elevated ERα expression in papillary
thyroid carcinoma patients with larger size of tumor (n=64). |
Table II.
Elevated ERα expression in papillary
thyroid carcinoma patients with larger size of tumor (n=64).
|
| Tumor size, n
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Variable | <1 cm | >1 cm | χ2 | P-value |
|---|
| Total | 33 (100.0) | 31 (100.0) |
|
|
| ERα | 14 (42.4) | 19 (77.4) | 4.904 | 0.02 |
| PR | 13 (39.4) | 16 (51.6) | 2.509 | 0.113 |
| EGFR | 32 (97.0) | 30 (96.8) | 4.799 | 0.091 |
Expression of ERα, PR, and EGFR in
male and female patients with PTC is not different
An increasing number of studies have revealed that
the incidence of PTC is significantly increased in females compared
with males (1,3,4). To
determine whether the expression of ERα, PR, and EGFR is consistent
with this observation, the IHC data was analyzed based on the
gender of the patients. However, it was found that there was no
significant difference between the expression level of ERα, PR, and
EGFR in male and female patients with PTC (Table III). These data suggest that the
expression level of ERα, PR and EGFR may not be a direct causative
factor for the high incidence of PTC in females.
 | Table III.No difference in ERα, PR and EGFR
expression by males or females of papillary thyroid carcinoma
(n=64). |
Table III.
No difference in ERα, PR and EGFR
expression by males or females of papillary thyroid carcinoma
(n=64).
|
| Gender, n (%) |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Male | Female | χ2 | P-value |
|---|
| Total | 27 (100.0) | 37 (100.0) |
|
|
| ERα | 17 (63.0) | 21 (56.8) | 0.249 | 0.618 |
| PR | 15 (55.6) | 14 (37.8) | 1.977 | 0.160 |
| EGFR | 27 (100.0) | 35 (94.6) | 1.507 | 0.220 |
Discussion
There are an increasing number of studies indicating
that estrogen may exert a direct effect on tumorigenesis in human
thyroid cells by ER-dependent or ER-independent mechanisms, through
modulating cell proliferation and regulating the function of the
thyroid (8,19,20). The
mechanistic evidence for these effects on thyroid function and
growth regulation was reviewed by Santin and Furlanetto (8).
Estrogen regulates cell proliferation by binding to
specific receptors, including ER. There are two isoforms of ER, ERα
and ERβ. These isoforms are coded for by distinct genes and are
expressed differently in human tissues during morphogenesis and in
adult life (21,22). The expression pattern of ER isoforms
has been demonstrated in neoplastic and non-cancerous human thyroid
tissues; however, the results are not consistent (19,20).
Experimental studies have revealed that estrogen affects PTC
development by interacting with ER at the level of target thyroid
cells, thereby promoting the proliferation of mutated follicular
cells (23). In addition, several
different thyroid cancer cell lines have been revealed to express
ER (24–26), and the proliferation of these cells
was stimulated by ERα agonists, and downregulated by ERβ agonists
(23). Consistently, the present
study revealed that the expression of ERα is increased in PTC
tissues, compared with the expression in NTG tissues. Notably,
significantly elevated expression of ERα was identified in PTC
patients with a larger tumor size, indicating that the expression
of ERα may be used as a predictor for an increased risk of PTC.
EGFRs are monomer cell-surface receptors that belong
to the ErbB family of receptor tyrosine kinases. Mutations leading
to EGFR overexpression have been associated with a variety of
malignancies, including head and neck, esophageal, ovarian,
cervical, lung and bladder cancers (27). In the present study, it was found that
the expression of EGFR is also increased in PTC, suggesting that
overexpression of EGFR may be a factor in the development of
PTC.
Numerous studies have reported that the
overexpression of EGFR in an anaplastic, undifferentiated subtype
of PTC is associated with a high mortality rate (28–31).
However, little is known about the value of EGFR expression in
predicting the prognosis of thyroid carcinoma. In the present
study, the clinical value of EGFR for the prediction of the risk of
PTC was evaluated, although no significant association was
identified between EGFR expression and the size of the PTC
tumor.
In conclusion, the present study revealed that the
expression levels of ERα, PR and EGFR were significantly elevated
in PTC tissues compared with NTG tissues. In addition, the
expression of ERα was found to correlate with the size of PTC
tumors. Therefore, the results of this study indicate that
immunohistochemical analyses of ERα, PR and EGFR expression in
patients with PTC may present a potential prognostic marker.
References
|
1
|
Rahbari R, Zhang L and Kebebew E: Thyroid
cancer gender disparity. Future Oncol. 6:1771–1779. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kilfoy BA, Devesa SS, Ward MH, et al:
Gender is an age-specific effect modifier for papillary cancers of
the thyroid gland. Cancer Epidemiol Biomarkers Prev. 18:1092–1100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajoria S, Suriano R, George AL, et al:
Estrogen activity as a preventive and therapeutic target in thyroid
cancer. Biomed Pharmacother. 66:151–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kansakar E, Chang YJ, Mehrabi M and Mittal
V: Expression of estrogen receptor, progesterone receptor, and
vascular endothelial growth factor-A in thyroid cancer. Am Surg.
75:785–789. 2009.PubMed/NCBI
|
|
6
|
Renoir JM, Marsaud V and Lazennec G:
Estrogen receptor signaling as a target for novel breast cancer
therapeutics. Biochem Pharmacol. 85:449–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koos RD: Minireview: Putting physiology
back into estrogens' mechanism of action. Endocrinology.
152:4481–4488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santin AP and Furlanetto TW: Role of
estrogen in thyroid function and growth regulation. J Thyroid Res.
2011:8751252011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamat A, Rajoria S, George A, et al:
Estrogen-mediated angiogenesis in thyroid tumor microenvironment is
mediated through VEGF signaling pathways. Arch Otolaryngol Head
Neck Surg. 137:1146–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong W, Zhang H, Li J, et al: Estrogen
induces metastatic potential of papillary thyroid cancer cells
through estrogen receptor alpha and beta. Int J Endocrinol.
2013:9415682013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam KY, Lo CY and Leung PS: High
prevalence of RET protooncogene activation (RET/PTC) in papillary
thyroid carcinomas. Eur J Endocrinol. 147:741–745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croyle M, Akeno N, Knauf JA, et al:
RET/PTC-induced cell growth is mediated in part by epidermal growth
factor receptor (EGFR) activation: evidence for molecular and
functional interactions between RET and EGFR. Cancer Res.
68:4183–4191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wells A: EGF receptor. Int J Biochem Cell
Biol. 31:637–643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang BH, Lo CY, Chan WF, et al: Prognostic
factors in papillary and follicular thyroid carcinoma: their
implications for cancer staging. Ann Surg Oncol. 14:730–738. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eustatia-Rutten CF, Corssmit EP, Biermasz
NR, et al: Survival and death causes in differentiated thyroid
carcinoma. J Clin Endocrinol Metab. 91:313–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
18
|
DeLellis RA and Williams ED: Tumours of
the thyroid and parathyroidWorld Health Organization Classification
of Tumours: Pathology and Genetics of Tumours of Endocrine Organs.
DeLellis RA, Lloyd RV, Heitz PU and Eng C: 3rd. IARC Press; Lyon:
pp. 562004
|
|
19
|
Huang Y, Dong W, Li J, Zhang H, Shan Z and
Teng W: Differential expression patterns and clinical significance
of estrogen receptor-α and β in papillary thyroid carcinoma. BMC
Cancer. 14:3832014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kavanagh DO, Mcllroy M, Myers E, et al:
The role of oestrogen receptor {alpha} in human thyroid cancer:
Contributions from coregulatory proteins and tyrosine kinase
receptor HER2. Endocr Relat Cancer. 17:255–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gorski J and Hou Q: Embryonic estrogen
receptors: do they have a physiological function? Environ Health
Perspect. 103 (Suppl 7):69–72. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weihua Z, Warner M and Gustafsson JA:
Estrogen receptor beta in the prostate. Mol Cell Endocrinol.
193:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen GG, Vlantis AC, Zeng Q, et al:
Regulation of cell growth by estrogen signaling and potential
targets in thyroid cancer. Curr Cancer Drug Targets. 8:367–377.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeng Q, Chen G, Vlantis A, et al: The
contributions of oestrogen receptor isoforms to the development of
papillary and anaplastic thyroid carcinomas. J Pathol. 214:425–433.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng Q, Chen GG, Vlantis AC, et al:
Oestrogen mediates the growth of human thyroid carcinoma cells via
an oestrogen receptor-ERK pathway. Cell Prolif. 40:921–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee ML, Chen GG, Vlantis AC, et al:
Induction of thyroid papillary carcinoma cell proliferation by
estrogen is associated with an altered expression of Bcl-xL. Cancer
J. 11:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):9–15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Laan BF, Freeman JL and Asa SL:
Expression of growth factors and growth factor receptors in normal
and tumorous human thyroid tissues. Thyroid. 5:67–73. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duh QY, Siperstein AE, Miller RA, et al:
Epidermal growth factor receptors and adenylate cyclase activity in
human thyroid tissues. World J Surg. 14:410–417. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizukami Y, Nonomura A, Hashimoto T, et
al: Immunohistochemical demonstration of epidermal growth factor
and c-myc oncogene product in normal, benign and malignant thyroid
tissues. Histopathology. 18:11–18. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akslen LA, Myking AO, Salvesen H and
Varhaug JE: Prognostic impact of EGF-receptor in papillary thyroid
carcinoma. Br J Cancer. 68:808–812. 1993. View Article : Google Scholar : PubMed/NCBI
|















